Abstract
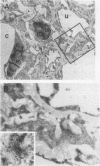
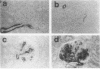
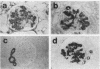
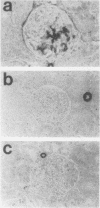
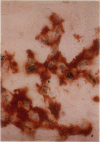
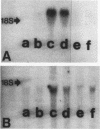
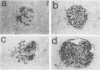
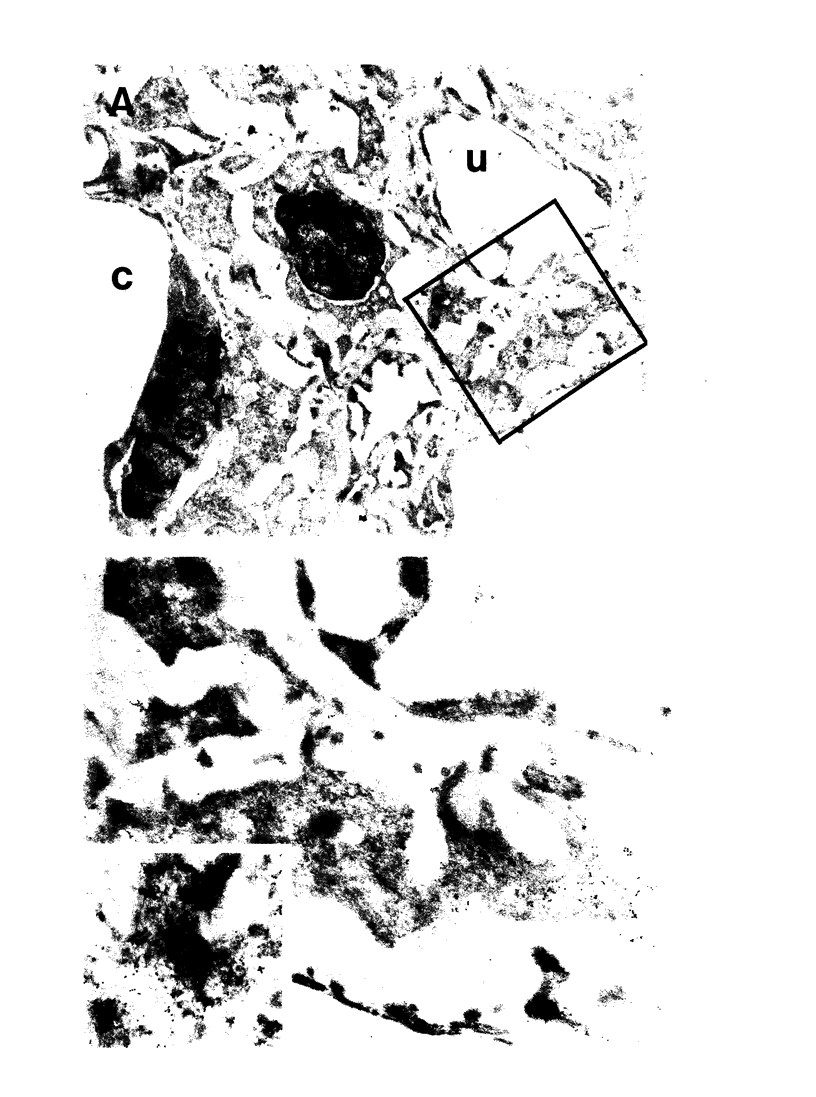
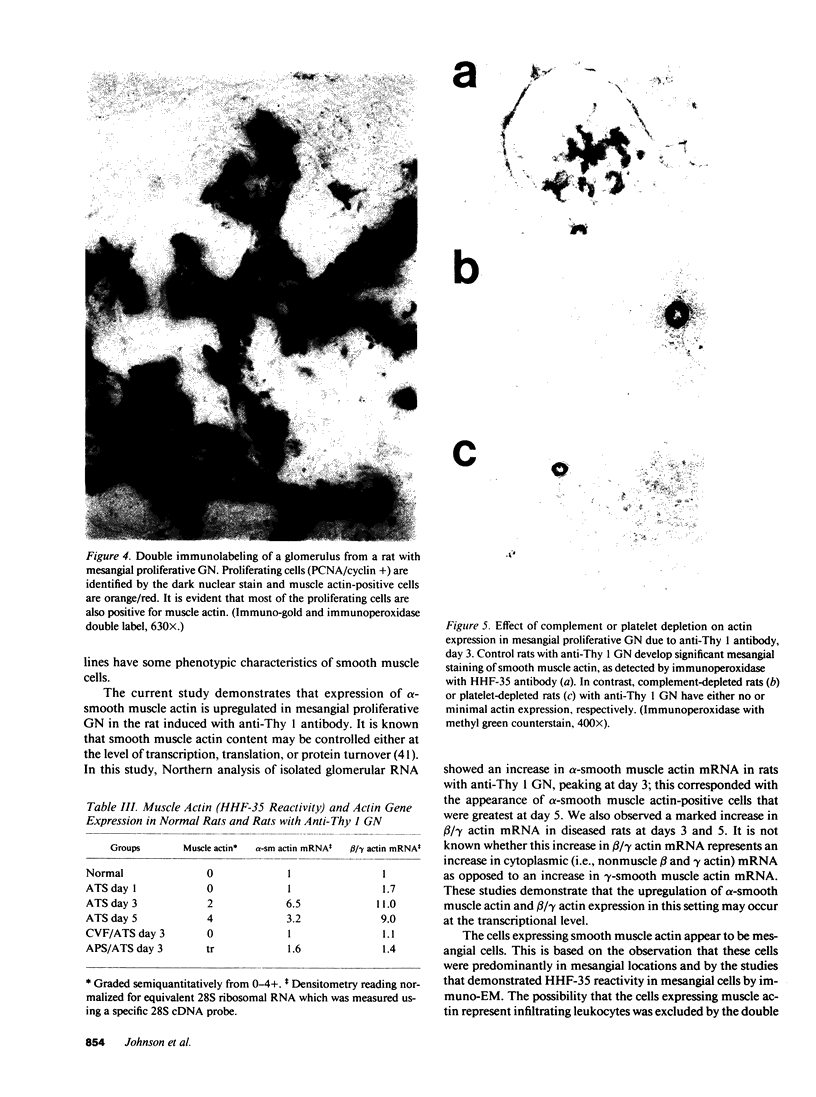
Mesangial cell proliferation is common in glomerulonephritis but it is unclear if proliferation is associated with any in vivo alteration in phenotype. We investigated whether mesangial of mesangial proliferative nephritis induced with antibody to the Thy-1 antigen present on mesangial cells. At day 3 glomeruli displayed de novo immunostaining for alpha-smooth muscle actin in a mesangial pattern, correlating with the onset of proliferation, and persisting until day 14. An increase in desmin and vimentin in mesangial regions was also noted. Immunoelectron microscopy confirmed that the actin-positive cells were mesangial cells, and double immunolabeling demonstrated that the smooth muscle actin-positive cells were actively proliferating. Northern analysis of isolated glomerular RNA confirmed an increase in alpha and beta/gamma actin mRNA at days 3 and 5. Complement depletion or platelet depletion prevented or reduced proliferation, respectively; these maneuvers also prevented smooth muscle actin and actin gene expression. Studies of five other experimental models of nephritis confirmed that smooth muscle actin expression is a marker for mesangial cell injury. Thus, mesangial cell proliferation in glomerulonephritis in the rat is associated with a distinct phenotypic change in which mesangial cell assume smooth muscle cell characteristics.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Baker P. J., Johnson R. J., Ochi R. F., Pritzl P., Couser W. G. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J Clin Invest. 1986 Mar;77(3):762–767. doi: 10.1172/JCI112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann S., Kriz W., Kuhn C., Franke W. W. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77(3):365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- Barja F., Coughlin C., Belin D., Gabbiani G. Actin isoform synthesis and mRNA levels in quiescent and proliferating rat aortic smooth muscle cells in vivo and in vitro. Lab Invest. 1986 Aug;55(2):226–233. [PubMed] [Google Scholar]
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud L., Oudinet J. P., Bens M., Noe L., Peraldi M. N., Rondeau E., Etienne J., Ardaillou R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989 May;35(5):1111–1118. doi: 10.1038/ki.1989.98. [DOI] [PubMed] [Google Scholar]
- Becker C. G. Demonstration of actomyosin in mesangial cells of the renal glomerulus. Am J Pathol. 1972 Jan;66(1):97–110. [PMC free article] [PubMed] [Google Scholar]
- Blank R. S., Thompson M. M., Owens G. K. Cell cycle versus density dependence of smooth muscle alpha actin expression in cultured rat aortic smooth muscle cells. J Cell Biol. 1988 Jul;107(1):299–306. doi: 10.1083/jcb.107.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell V. Focal mesangial proliferative glomerulonephritis in the rat caused by Habu snake venom: the role of platelets. Br J Exp Pathol. 1979 Apr;60(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Kocher O., Ropraz P., Chaponnier C., Gabbiani G. Arterial smooth muscle cells in vivo: relationship between actin isoform expression and mitogenesis and their modulation by heparin. J Cell Biol. 1988 Nov;107(5):1939–1945. doi: 10.1083/jcb.107.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Determination and differentiation of the nephron. Med Biol. 1981 Jun;59(3):139–160. [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R. A., Lynch K. R., Sturgill B. C., Elwood J. P., Chevalier R. L., Carey R. M., Peach M. J. Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol. 1989 Nov;257(5 Pt 2):F850–F858. doi: 10.1152/ajprenal.1989.257.5.F850. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Horii Y., Muraguchi A., Iwano M., Matsuda T., Hirayama T., Yamada H., Fujii Y., Dohi K., Ishikawa H., Ohmoto Y. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989 Dec 15;143(12):3949–3955. [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Pritzl P., Schulze M., Baker P., Pruchno C., Couser W. G. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J Clin Invest. 1988 Oct;82(4):1225–1235. doi: 10.1172/JCI113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Garcia R. L., Pritzl P., Alpers C. E. Platelets mediate glomerular cell proliferation in immune complex nephritis induced by anti-mesangial cell antibodies in the rat. Am J Pathol. 1990 Feb;136(2):369–374. [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Analysis of alpha-smooth-muscle actin mRNA expression in rat aortic smooth-muscle cells using a specific cDNA probe. Differentiation. 1987;34(3):201–209. doi: 10.1111/j.1432-0436.1987.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Expression of actin mRNAs in rat aortic smooth muscle cells during development, experimental intimal thickening, and culture. Differentiation. 1986;32(3):245–251. doi: 10.1111/j.1432-0436.1986.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I. Cell biology and biochemistry of the glomerular mesangium. Miner Electrolyte Metab. 1988;14(2-3):167–175. [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- LATTA H., MAUNSBACH A. B. Relations of the centrolobular region of the glomerulus to the juxtaglomerular apparatus. J Ultrastruct Res. 1962 Jun;6:562–578. doi: 10.1016/s0022-5320(62)80010-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986 Aug;30(2):246–254. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- Martin J., Davies M., Thomas G., Lovett D. H. Human mesangial cells secrete a GBM-degrading neutral proteinase and a specific inhibitor. Kidney Int. 1989 Nov;36(5):790–801. doi: 10.1038/ki.1989.264. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Kalnoski M. H., Lessard J. L., Bulinski J. C. Immunolocalization of the gamma isoform of nonmuscle actin in cultured cells. J Cell Biol. 1986 May;102(5):1726–1737. doi: 10.1083/jcb.102.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Sariola H., Timpl R., von der Mark K., Mayne R., Fitch J. M., Linsenmayer T. F., Ekblom P. Dual origin of glomerular basement membrane. Dev Biol. 1984 Jan;101(1):86–96. doi: 10.1016/0012-1606(84)90119-2. [DOI] [PubMed] [Google Scholar]
- Scheinman J. I., Fish A. J., Matas A. J., Michael A. F. The immunohistopathology of glomerular antigens. II. The glomerular basement membrane, actomyosin, and fibroblast surface antigens in normal, diseased, and transplanted human kidneys. Am J Pathol. 1978 Jan;90(1):71–88. [PMC free article] [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987 Oct;1(4):272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Schulze M., Baker P. J., Perkinson D. T., Johnson R. J., Ochi R. F., Stahl R. A., Couser W. G. Increased urinary excretion of C5b-9 distinguishes passive Heymann nephritis in the rat. Kidney Int. 1989 Jan;35(1):60–68. doi: 10.1038/ki.1989.8. [DOI] [PubMed] [Google Scholar]
- Silver B. J., Jaffer F. E., Abboud H. E. Platelet-derived growth factor synthesis in mesangial cells: induction by multiple peptide mitogens. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1056–1060. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Gabbiani G., Babaï F., Seemayer T. A., Pizzolato G., Schürch W. Intermediate filament proteins and actin isoforms as markers for soft tissue tumor differentiation and origin. II. Rhabdomyosarcomas. Am J Pathol. 1988 Mar;130(3):515–531. [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Skalli O., Vandekerckhove J., Gabbiani G. Actin-isoform pattern as a marker of normal or pathological smooth-muscle and fibroblastic tissues. Differentiation. 1987;33(3):232–238. doi: 10.1111/j.1432-0436.1987.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Skalli O., Gabbiani G. Distribution of intermediate filament proteins in normal and diseased human glomeruli. Am J Pathol. 1986 Dec;125(3):465–475. [PMC free article] [PubMed] [Google Scholar]
- Trenchev P., Dorling J., Webb J., Holborow E. J. Localization of smooth muscle-like contractile proteins in kidney by immunoelectron microscopy. J Anat. 1976 Feb;121(Pt 1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., McNutt M. A., Ross R., Gown A. M. HHF35, a muscle actin-specific monoclonal antibody. II. Reactivity in normal, reactive, and neoplastic human tissues. Am J Pathol. 1987 May;127(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Ueyama H., Hamada H., Battula N., Kakunaga T. Structure of a human smooth muscle actin gene (aortic type) with a unique intron site. Mol Cell Biol. 1984 Jun;4(6):1073–1078. doi: 10.1128/mcb.4.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasmant D., Maurice M., Feldmann G. Cytoskeleton ultrastructure of podocytes and glomerular endothelial cells in man and in the rat. Anat Rec. 1984 Sep;210(1):17–24. doi: 10.1002/ar.1092100104. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Witt D. P., Brown D. J., Gordon J. A. Transformation-sensitive isoactin in passaged chick embryo fibroblasts transformed by Rous sarcoma virus. J Cell Biol. 1983 Jun;96(6):1766–1771. doi: 10.1083/jcb.96.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Wilson C. B. Complement dependence of antibody-induced mesangial cell injury in the rat. J Immunol. 1987 Jun 1;138(11):3758–3765. [PubMed] [Google Scholar]
- Yamamoto T., Wilson C. B. Quantitative and qualitative studies of antibody-induced mesangial cell damage in the rat. Kidney Int. 1987 Oct;32(4):514–525. doi: 10.1038/ki.1987.240. [DOI] [PubMed] [Google Scholar]
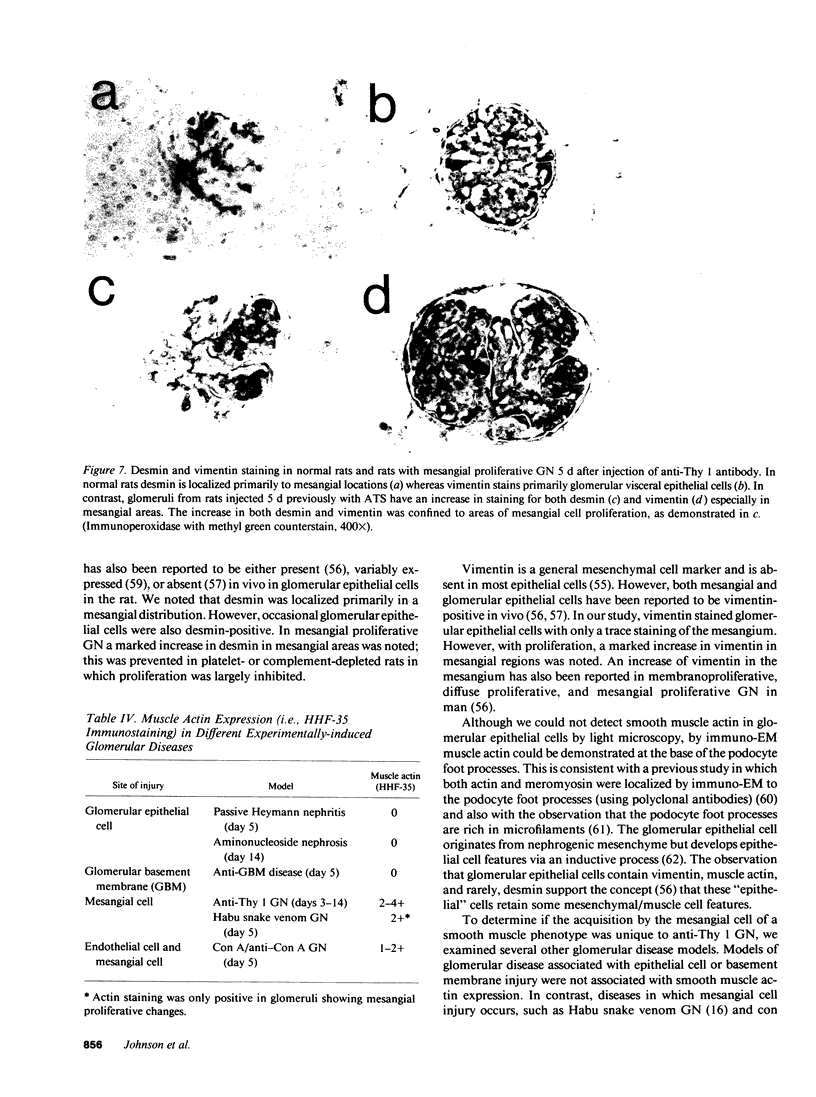
- Yaoita E., Kawasaki K., Yamamoto T., Kihara I. Variable expression of desmin in rat glomerular epithelial cells. Am J Pathol. 1990 Apr;136(4):899–908. [PMC free article] [PubMed] [Google Scholar]
- Yaoita E., Kazama T., Kawasaki K., Miyazaki S., Yamamoto T., Kihara I. In vitro characteristics of rat mesangial cells in comparison with aortic smooth muscle cells and dermal fibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(4):285–294. doi: 10.1007/BF02912106. [DOI] [PubMed] [Google Scholar]