Abstract
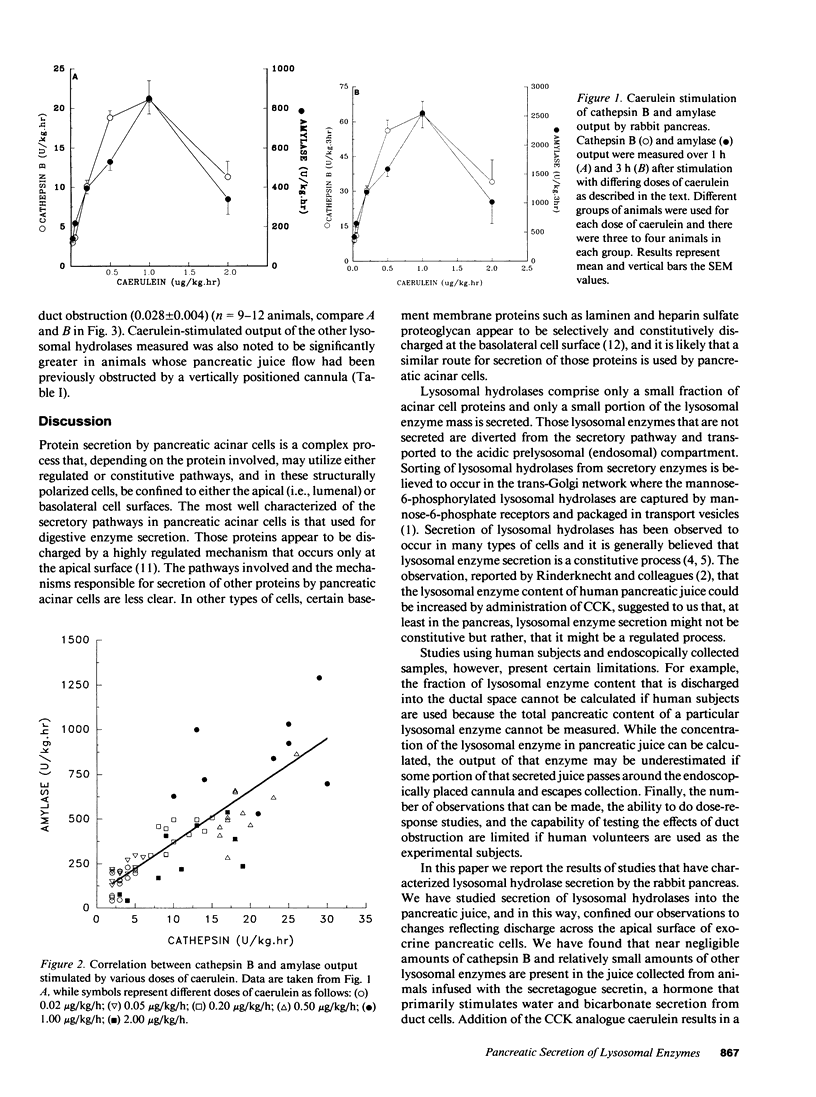
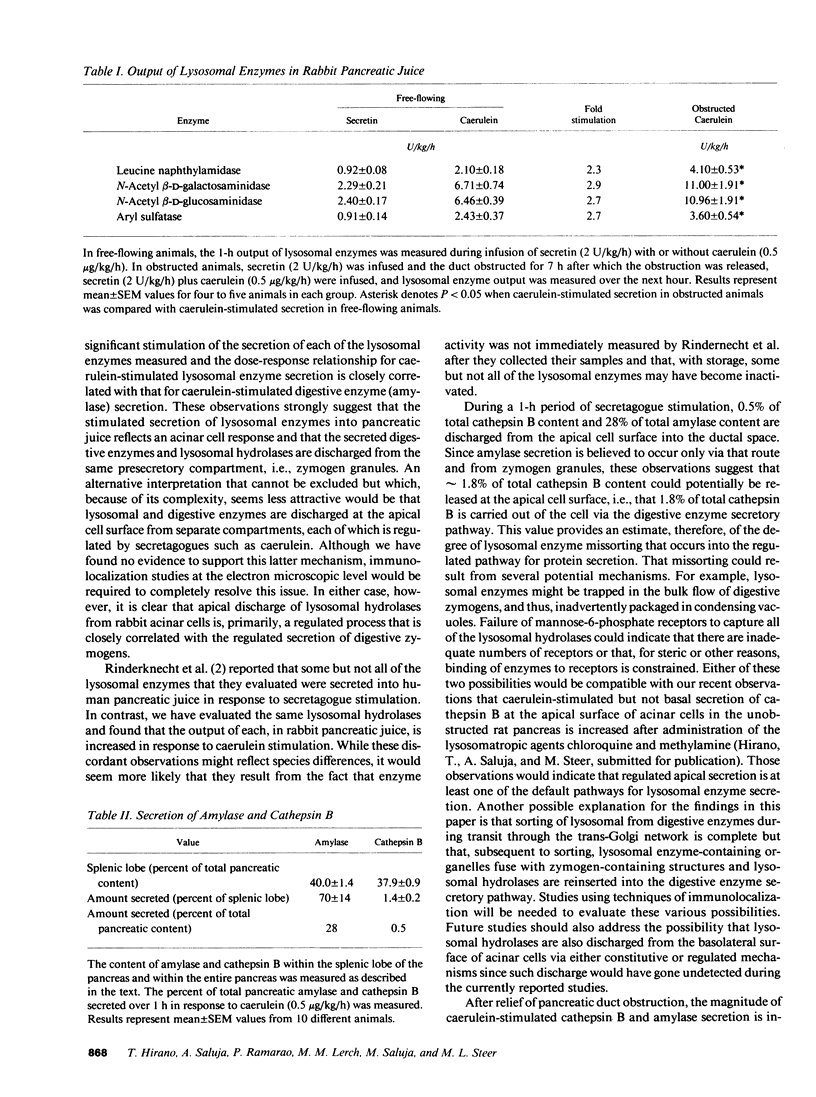
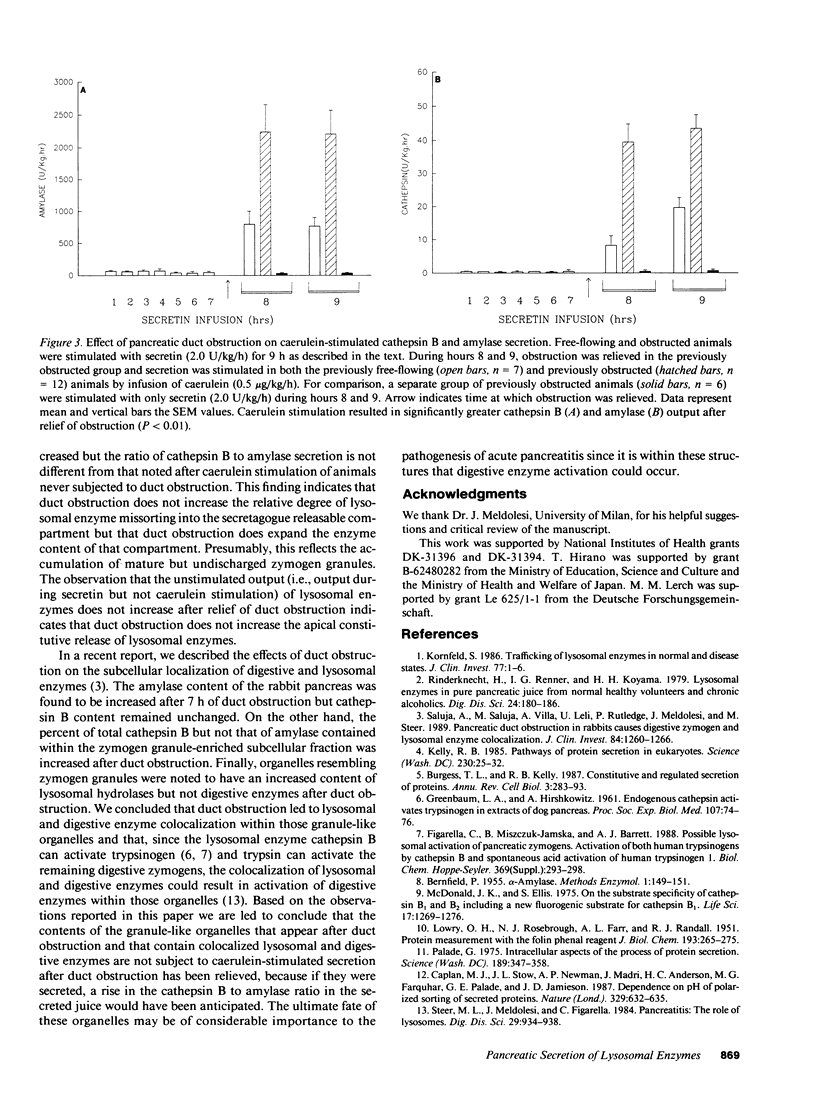
Lysosomal hydrolases such as cathepsin B are apically secreted from rabbit pancreatic acinar cells via a regulated as opposed to a constitutive pathway. Intravenous infusion of the cholecystokinin analogue caerulein results in highly correlated apical secretion of digestive and lysosomal enzymes, suggesting that they are discharged from the same presecretory compartment (zymogen granules). Lysosomal enzymes appear to enter that compartment as a result of missorting. After 7 h of duct obstruction is relieved, caerulein-stimulated apical secretion of cathepsin B and amylase is increased, but the ratio of cathepsin B to amylase secretion is not different than that following caerulein stimulation of animals never obstructed. These findings indicate that duct obstruction causes an increased amount of both lysosomal and digestive enzymes to accumulate within the secretagogue releasable compartment but that duct obstruction does not increase the degree of lysosomal enzyme missorting into that compartment. Pancreatic duct obstruction causes lysosomal hydrolases to become colocalized with digestive enzymes in organelles that, in size and distribution, resemble zymogen granules but that are not subject to secretion in response to secretagogue stimulation. These organelles may be of importance in the development of pancreatitis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caplan M. J., Stow J. L., Newman A. P., Madri J., Anderson H. C., Farquhar M. G., Palade G. E., Jamieson J. D. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987 Oct 15;329(6140):632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- Figarella C., Miszczuk-Jamska B., Barrett A. J. Possible lysosomal activation of pancreatic zymogens. Activation of both human trypsinogens by cathepsin B and spontaneous acid. Activation of human trypsinogen 1. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):293–298. [PubMed] [Google Scholar]
- GREENBAUM L. M., HIRSHKOWITZ A. Endogenous cathepsin activation of trypsinogen in extracts of dog pancreas. Proc Soc Exp Biol Med. 1961 May;107:74–76. doi: 10.3181/00379727-107-26539. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986 Jan;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McDonald J. K., Ellis S. On the substrate specificity of cathepsins B1 and B2 including a new fluorogenic substrate for cathepsin B1. Life Sci. 1975 Oct 15;17(8):1269–1276. doi: 10.1016/0024-3205(75)90137-x. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Renner I. G., Koyama H. H. Lysosomal enzymes in pure pancreatic juice from normal healthy volunteers and chronic alcoholics. Dig Dis Sci. 1979 Mar;24(3):180–186. doi: 10.1007/BF01308426. [DOI] [PubMed] [Google Scholar]
- Saluja A., Saluja M., Villa A., Leli U., Rutledge P., Meldolesi J., Steer M. Pancreatic duct obstruction in rabbits causes digestive zymogen and lysosomal enzyme colocalization. J Clin Invest. 1989 Oct;84(4):1260–1266. doi: 10.1172/JCI114293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]


