INTRODUCTION
Thrombosis, i.e., formation of pathological intravascular blood clots is the most common cause for obstructive cardiovascular diseases leading to ischemic damage to the involved blood vessels and tissue ischemia (1). Pathologically altered vasculature (e.g., in inflammation sites) is predisposed for thrombosis, in part due to suppression of natural anti-thrombotic mechanisms in endothelium lining vascular lumen (2). Activation of the coagulation cascade generates thrombin that cleaves fibrinogen, producing a fibrin meshwork that along with the activation of platelets forms large intravascular aggregates (2). In the venous vasculature, clots are red and consist predominantly of fibrin and entrapped red blood cells (RBC), whereas in the arterial vasculature clots formed at high shear stress are mostly populated by platelets (2).
Approaches to pharmacologically manage thrombosis include prevention and therapy (Figure 1). Prevention is attained by prophylactic use of anticoagulants and platelet inhibitors (3). Anticoagulants with a delayed onset and relatively prolonged effect are used for long-term prevention (e.g., Warfarin), whereas thrombin inhibitors heparin and hirudin act within minutes and can be used for an immediate short-term thromboprophylaxis (4). A second approach is emergency therapy of thrombosis which employs intravascular injection of plasminogen activators (PA) (5). These middle-size proteases (50–60kD) include tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) that generate plasmin, which cleave fibrin clots and help to restore perfusion. Table 1 introduces current anti-thrombotic agents.
Figure 1. Insufficiencies of current anti-thrombotic agents.
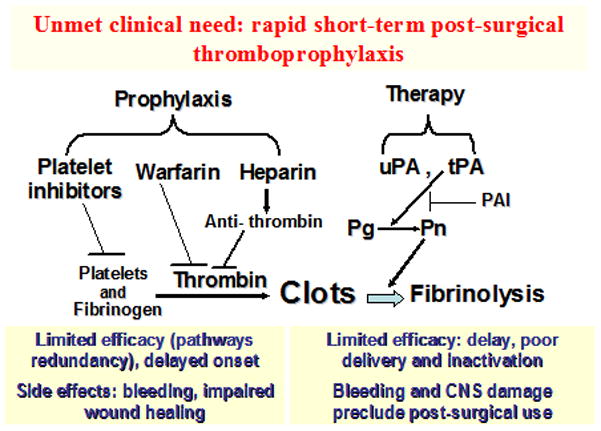
This schema illustrates current anti-thrombotic agents in use for either prophylaxis or treatment, and their limitations. Platelet inhibitors, warfarin and heparin are common prophylactic agents, these agents work to inhibit clot formation. However, these agents have limited efficacy, and severe adverse effects such as bleeding and impaired wound healing. In therapy, uPA is effective by directly inhibiting plasminogen, while tPA activates the conversion of plasminogen (Pg) to plasmin (Pn), which results in clot breakdown. Although, both of these agents have significant side effects, limited efficacy, such as, poor delivery, and bleeding complications. Abbreviations found within the figure- PAI, Plasminogen activator inhibitor; Pg, Plasminogen; Pn, Plasmin; tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator.
Table 1. Current anti-thrombotic agents.
This table describes the current anti-thrombotic agents that are commonly/currently used in patients. Included are descriptions of how the respective drugs are delivered, their half-life, when it is used, how it works and any adverse effects.
| Drug | Time/Onset of Action | Description |
|---|---|---|
| Warfarin |
|
|
| Heparin |
|
|
| tPA |
|
|
| SK |
|
|
| UK |
|
|
Abbreviations found within the table-IV, intravenous; SK, streptokinase; SubQ, Subcutaneous; tPA, tissue plasminogen activator; UK, Urokinase; uPA, urokinase plasminogen activator; uPA-T, urokinase plasminogen activator-thrombin.
Alas, both approaches provide rather limited efficacy of action and are liable in serious side effects, first of all, bleeding. Agents that act expeditiously (e.g., fibrinolytics) are rapidly cleared from the bloodstream. Several strategies to prolong the half-life in the circulation have been devised including structural modification and mutation of a protein drug to remove clearance recognition sites and replace labile amino acids, conjugation with PEG and application of drug delivery systems such as liposomes and biodegradable particles (6–9). However, despite these efforts, delivery of anti-thrombotic drugs to the site of their preferential effect, i.e., vascular sites predisposed to thrombosis or nascent intravascular clots, remain grossly suboptimal. A major fraction of the injected drug is an expensive and dangerous waste. In particular, impermeability of occlusive clots restricts therapeutic fibrinolysis by PA. Within minutes after infusion, mega-doses of fibrinolytics (e.g., 100mg of t-PA) are needed to overcome its inefficiency and achieve fibrinolysis locally, excess drug diffuses into pre-existing hemostatic mural clots predisposing to bleeding and into tissues such as the brain (Figure 1).
In theory, targeted delivery of anti-thrombotic agents, either to pathological vessels or into nascent clots may help solve the problem (10). Cardiovascular disease and thrombosis are typically located to discrete vascular regions, affording opportunity for targeted anti-thrombotic pharmacotherapy. This review will highlight the studies on affinity targeting anti-thrombotic therapeutics to fibrin, platelets, red blood cells (RBC) and endothelium.
Vascular targeted delivery of anti-thrombotic agents: drug targeting to fibrin and platelets
Since the fibrin molecule exposes epitope determinants absent on fibrinogen, it has been viewed as a molecular target for delivery of thrombolytic agents selectively to the thrombi (5,11). Diverse anti-thrombotic agents including hirudin and plasminogen activators have been conjugated with fibrin-specific antibodies and their fragments. For example, in rabbit and baboon models of jugular vein thrombolysis, an anti-fibrin/tPA conjugate proved to be more potent vs. tPA (12–13). Conjugation of a single chain urokinase type plasminogen activator (scuPA) to anti-fibrin has also resulted in marked increase in potency vs scuPA (14–15). Anti-fibrin coated nanoparticles conjugated with streptokinase (SK) have also been shown to be more effective in clot lysis vs. free SK in vitro (16).
However, a conundrum in targeting fibrinolytics to fibrin (or other specific clot components) is that the target does not exist before the thrombosis, whereas these drugs do not circulate for a prolonged time. This, and side effects of systemically active fibrinolytics preclude prophylactic use. On the other hand, after thrombosis majority of the formed clot mass becomes inaccessible to drugs. In fact, somewhat paradoxically, the higher fibrin affinity drugs have, the more impeded their penetration into the clot, due to retention on the upstream surface layer that works like an affinity column (17).
Platelets have also been used as a target for thromboprophylaxis and thrombolytic therapy. Potential limitations of this strategy include a relatively short life-time of platelets, especially carrying targeted drugs. However, this approach seems especially attractive for interventions in arterial clots. For this purpose, investigators used antibodies and antibody fragments to platelet membrane glycoproteins, such as fibrinogen receptor GPIIb/IIIa (18–19). The conformational changes induced in the GPIIb/IIIa complex by thrombin or collagen markedly enhance fibrinogen affinity (18–19). Ligands that recognize activated GPIIb/IIIa can be used for targeting drugs to platelet-rich clots (with the permeability caveat similar to that of fibrin targeting), whereas ligands binding to either resting or activated targets can be used for loading drugs on platelets prior to thrombosis. In addition, blocking GPIIb/IIIa inhibits platelets, providing the basis for anti-thrombotic effect of Reo-Pro and other anti-platelet agents. For example, urokinase conjugated with GPIIb/IIIa antibody has been shown to bind platelets and platelet-rich thrombi and dissolve arterial thrombi (20). It is likely that some activity of the conjugate could be attributed to blocking of the fibrinogen receptor GPIIb/IIa. Antibodies against other platelet glycoproteins such as GPIIIa and GPIIb, have also been used for the targeted delivery of plasminogen activators (21). In general, targeting anti-thrombotic agents to platelets seems an interesting avenue, worth further development and testing.
Targeting to red blood cells (RBC)
RBCs provide a natural carrier vascular drug delivery (22), in particular, for intervention in venous thrombosis (23). Hemodynamic factors propel RBCs towards the blood mainstream, restricting contact with vascular walls and hemostatic mural clots, reducing side effects. RBCs do not penetrate pre-existing hemostatic clots and restrict effect of the drug in blood. RBCs circulate for several weeks, providing a sufficient prophylactic window. Recent animal studies showed that biocompatible coupling to RBC converts plasminogen activators from problematic therapeutic agents into effective and safe agents for intravascular thromboprophylaxis, thereby shifting the paradigm of the fibrinolysis (23).
In prototype studies, utilizing homologous RBC carrying chemically coupled tPA, prophylactic infusion of RBC/tPA complexes into rats and mice delivered tPA into the interior of intravascular venous and arterial nascent clots, lysing within and during clot extension in settings where even a 10-fold higher dose of soluble tPA was ineffective (23) (Table 2 and Figure 2A). Coupling tPA to RBC prolonged tPA circulation by orders of magnitude without side effects including bleeding, RBC damage or activation of complement (24–25). In addition, RBC glycocalyx protected tPA from plasma inhibitors (26). Both direct coupling of tPA to RBCs and coupling of urokinase to RBCs carrying conjugated urokinase receptor yielded remarkable improvements in the benefit/risk ratio, reducing side effects and enhancing bioavailability (23, 27).
Table 2. RBC and EC targeted anti-thrombotic interventions.
This table describes anti-thrombotic agents that have been targeted to different vascular targets. Included is the location of their targeting, influence on fibrinolysis and any other notable findings.
| Drug | Target | Description |
|---|---|---|
| tPA | RBC |
|
| uPA-T | RBC |
|
| tPA | ICAM-1 |
|
| uPA | PECAM-1 |
|
| uPA-T | PECAM-1 |
|
Abbreviations found within the table- ICAM-1, Intercellular adhesion molecule, 1; PECAM-1, Platelet endothelial cell adhesion molecule-1; RBC, red blood cell; tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator; uPA-T, urokinase plasminogen activator-thrombin.
Figure 2. Strategies for coupling therapeutic agents to RBC and ECs.
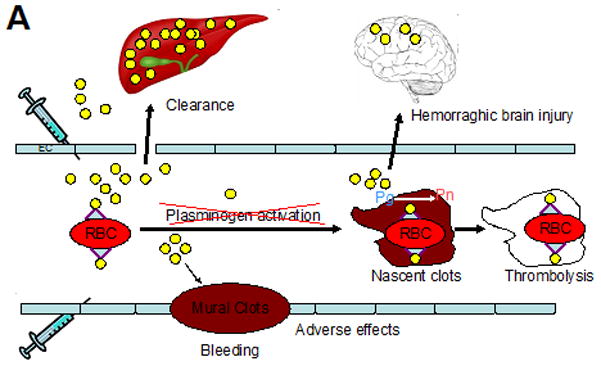
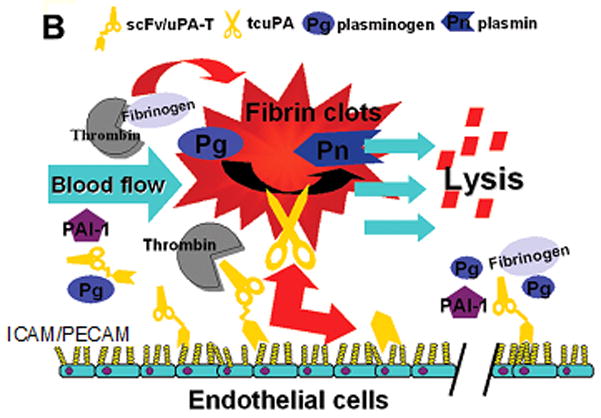
(A) Plasminogen activators (yellow dots) are relatively ineffective, in part due to rapid uptake by liver, and unsafe due to bleeding (indiscriminate lysis of hemostatic mural clots), vascular side effects (e.g. activation of receptors on endothelial cells, EC) and injurious effects of tPA diffusing into the CNS. Coupling to RBC will dramatically prolong the longevity of the scFv/tPA variant. RBC will restrain scFv/tPA binding to cellular receptors, and restrict its access into mural hemostatic clots and the CNS. Propulsion of RBC towards the mainstream will further offset interactions of the pro-drug with hemostatic clots and vascular walls. RBC-bound scFv/tPA will have virtually unlimited access to the interior of nascent pathological thrombi and thereby will dissolve pathological intravascular clots and prevent vascular occlusion. (B) As with RBC/tPA, ICAM or PECAM targeting enables one to target more locally in this case the pulmonary vasculature versus more systemically in the case of RBCs. By diversifying targeting, we can improve specificity and limit adverse effects. Abbreviations found within the figure-ICAM-1, Intercellular adhesion molecule, 1; PAI-1, Plasminogen activator inhibitor-1; PECAM-1, Platelet endothelial cell adhesion molecule-1; Pg, Plasminogen; Pn, Plasmin; RBC, red blood cell; tPA, tissue plasminogen activator; uPA-T, urokinase plasminogen activator-thrombin.
Furthermore, RBC/tPA has provided effective and safe thromboprophylaxis in more challenging settings such as cerebral embolism, thereby preventing deleterious consequences of brain ischemia. In mice, RBC/tPA dissolved subsequently formed occlusive cerebrovascular thrombi, leading to rapid and stable reperfusion, marked alleviation of ischemic brain injury and improved survival, whereas free tPA failed to provide reperfusion and in fact enhanced mortality (28). In a pig model of cerebral ischemia/hypoxia, RBC/tPA alleviated, whereas tPA aggravated vascular abnormalities (29). A similar outcome has been observed in a pig model of cerebral thrombosis: RBC/tPA restored perfusion and alleviated cerebral abnormalities, whereas tPA aggravated pro-inflammatory and vasoconstrictor side effects (30). Of note, RBC/tPA injected in rats shortly prior or after traumatic brain injury caused no brain hemorrhage, in contrast with free tPA (31).
These animal studies utilizing infusion of RBC carrying conjugated tPA revealed superiority of RBC/tPA thromboprophylaxis vs free tPA use. Subsequent recent studies further translated this promising prototype closer to the potential clinical applications, by advancing the methodology for coupling drugs to RBC, avoiding the need of having to couple tPA to isolated RBC’s ex vivo followed by transfusion. In the prototype study, tPA conjugated with antibody to RBC determinant CR1 showed binding to circulating RBC after IV injection, leading to prolonged circulation and thromboprophylaxis in a mouse model of thrombosis (32).
Next, in order to avoid technical and regulatory challenges associated with chemical conjugation of antibodies, this molecular format has been replaced by single-chain Fv fragments (scFv, comprising variable domains of heavy chain VH and light chain VL). A recombinant fusion protein combining a single chain antigen-binding fragment of a monoclonal antibody against mouse glycophorin A was fused with truncated urokinase providing scFv-uPA (33). Urokinase is an attractive drug for vascular targeting. It exists as an inactive single-chain zymogen of 411 amino acid residues (single-chain uPA, scuPA) that is converted by plasmin cleavage into fully active two-chain uPA (tcuPA) (33). The scuPA consists of three domains: the N-terminal domain homologous to the epidermal growth factor, the kringle domain, and the C-terminal catalytic domain. Urokinase binding to a cellular receptor via the “growth factor-like domain” (GFD) activates vascular cells (34), but deletion of the GFD provides a low-molecular-weight form of scuPA (MW 32-kD) that has enzymatic features similar to those of full-length scuPA. When injected in mice, low molecular weight scuPA fused with anti-GPA scFv (scFv-uPA) safely bound to RBC, which markedly prolonged its intravascular circulation and fibrinolytic activity compared with its non-targeted uPA counterpart, and resulted in prevention of thrombotic occlusion caused by vascular injury (33).
The scuPA zymogen (pro-urokinase) can be activated by trace amounts of plasmin over time and formed tcuPA may cause adverse effects, which would limit prophylactic use. Since tcuPA is rapidly inactivated by plasminogen activator inhibitor (PAI-1), the duration and effectiveness of prophylaxis would also be limited. Further, thrombin inactivates uPA by cleaving Arg156-Phe157, negating its effect at sites of active thrombosis. These problems might be solved by deleting Phe157 and Lys158, which yields a plasmin-resistant mutant activated by thrombin (uPA-T) (35). This pro-enzyme will not be activated by plasmin in vivo (thus avoiding systemic effects and premature PAI-1 inactivation), while thrombin will activate it locally at sites of nascent thrombosis within seconds of clotting. In order to further minimize premature activation and localize it to the sites of active ongoing thrombosis, uPA portion of the anti-GPA scFv-uPA fusion has been mutated to replace natural plasmin-sensitive activation site by a thrombin-sensitive one (36). This scFv-uPA-T has been shown to bind specifically to RBC’s without altering their biocompatibility, while causing thrombin-induced fibrinolysis even 20 hours after intravenous injection (36). These results provide proof-of-principle for the development of a recombinant PA variant that binds to circulating RBC and provides thromboprophylaxis in the settings associated with high risk of acute severe intravascular thrombosis.
Targeting to endothelium
Endothelial cells line the luminal surface of blood vessels and control vascular tone, blood fluidity and extravasation of blood components. In particular, the endothelium plays a central role in thrombosis and represents a key target for pharmacological anti-thrombotic interventions. The main goal of endothelial targeting anti-thrombotic drugs is for prophylactic purpose, to boost anticoagulant or thrombolytic potency in the vascular areas predisposed to thrombosis. Several endothelial determinants have been employed for this function (10). For example, tPA chemically conjugated with anti-ACE retained fibrinolytic and antigen-binding activities and exhibited sustained preferential accumulation in rat pulmonary vasculature (37). Other endothelial determinants have also been explored for this goal; both tPA and uPA chemically conjugated with antibodies against antigens enriched in the pulmonary endothelium accumulated in the pulmonary vasculature (37–38).
In particular, Platelet-Endothelial Adhesion Molecule-1 (PECAM-1, CD31) and Inter-Cellular Adhesion Molecule-1 (ICAM-1, CD54), transmembrane glycoproteins constitutively expressed on endothelial cells represent attractive targets for anchoring anti-thrombotic agents in the vasculature predisposed to thrombosis or embolism (Table 2). The pulmonary vasculature contains ~30% of the endothelial surface in the body and receives the entire cardiac output and, as a result, agents with an endothelial affinity accumulate in the lungs after intravenous (IV) injection. This vascular bed is an important target for treatment of acute lung injury, oxidative stress, thrombosis and inflammation, among other conditions (39). Agents conjugated with anti-ICAM and anti-PECAM infused IV accumulate in the lungs (40–41), whereas local infusion in a conduit artery enriches the binding in the downstream vascular areas (e.g., cardiac, cerebral and mesentery vasculature) (42–43). PECAM and ICAM are involved in mechanisms of cellular recognition, adhesion and trans-endothelial migration of leukocytes. Thus blocking these molecules may inhibit leukocyte trafficking, a bonus in treatment of inflammation and thrombosis. Endothelial cells do not internalize anti-ICAM and anti-PECAM or their monovalent fragments such as scFv (44–45), which provides the optimal strategic position of anti-thrombotic drugs anchored to these molecules in the vascular lumen.
PECAM is stably expressed on the endothelium at the level of a million copies per cell predominantly localized in inter-endothelial borders, whereas endothelial cells in the vasculature express ICAM-1 at a surface density of 2×104–2×105 surface copies per cell and this level doubles upon pro-inflammatory challenge (46). Diverse agents conjugated to anti-PECAM and ICAM accumulate and display their functional activity in the endothelium as soon as 10 min after IV injection in mice, rats and pigs (10). After IV injection in rats, pulmonary uptake of anti-ICAM/tPA conjugate is two orders of magnitude higher than that of control IgG/tPA, which resulted in enhanced fibrinolysis of subsequent pulmonary emboli (47).
Chemical conjugation of proteins with antibodies yields multivalent conjugates that may cause endocytosis undesirable in the context of sustained retention of fibrinolytics on the endothelial lumen (44). In contrast, recombinant fusion of uPA with scFv antibody fragments yields monovalent, homogeneous and relatively small drugs (50–70kD, hence low immunogenicity and lack of Fc-fragment mediated side effects). As a proof of principle for prophylactic thrombolysis by endothelium-targeted thrombolytic fusions, using a linker of three Gly4Ser repeats, an anti-PECAM scFv was fused with low-molecular weight uPA described above in the context of anti-RBC fusions. After IV injection, the protein composed of an anti-PECAM scFv fused with lmw-scuPA (anti-PECAM scFv/uPA) preferentially accumulated in the lungs of wild-type but not PECAM deficient mice, persisted in the lungs for at least 3 hours and remained on the endothelial surface. Compared with non-targeted uPA, scFv/uPA augmented local lysis of pulmonary emboli in a mouse pulmonary thrombotic model (41). Further, scFv/uPA accumulated in the cerebral vasculature after intra-arterial and IV injection, dissolved cerebral clots and improved blood reperfusion without hemorrhagic complications, thereby mitigating post-thrombotic brain edema in a mouse model of cerebral embolism (48).
Replacing the native plasmin activation site in the uPA moiety of scFv/uPA with a thrombin activation site provided thrombin-activated anti-PECAM scFv/uPA-T (49). This construct was also found to contain an intrinsic thrombin-sensitive cleavage site in the anti-PECAM scFv moiety, providing a built-in mechanism for local drug release. The scFv/uPA-T is latent and resists the PA inhibitor PAI-1 until activated by thrombin. After IV injection in mice, scFv/uPA-T did not consume plasma fibrinogen, in contrast with scFv/uPA that has this liability. However, scFv/uPA-T bound to the endothelium and accumulated in the vascularized organs, particularly the lungs. In a mouse model of thrombin-induced pulmonary thrombosis, scFv/uPA-T provided more potent and durable thromboprophylaxis than both plasmin-sensitive scFv/uPA and lmw-scuPA. Further, injection of mice with scFv/uPA-T prior to unilateral lung ischemia/reperfusion attenuated pulmonary fibrin deposition, to a significantly greater extent than plasmin-sensitive scFv/uPA, and restored arterial oxygen tension, while PAI-1 susceptible plasmin-activated scFv/uPA did not improve blood oxygenation (49). Therefore, vascular targeting (attained by fusion with scFv) of a pro-drug locally activated by the key pathological mediator, thrombin, provides the maximal degree of spatiotemporal precision and efficacy of the pharmacotherapy of occlusive vascular disease.
SUMMARY
Thrombolytic/fibrinolytic agents used for prevention and therapy of occlusive cardiovascular diseases including acute myocardial infarction, pulmonary embolism and thrombosis have limited efficacy and serious side effects. Conjugating these agents, for example thrombolytic plasminogen activators, with affinity carriers that deliver cargoes to blood elements or to the lumen of the predisposed vasculature may improve thromboprophylaxis of nascent pathological clots. In particular, RBC and endothelial cell targeting which has been shown to result in a reduced risk of hemorrhage and toxicity associated with systemic drug administration. Using a modular recombinant format of scFv fragments directed to specific blood cell or endothelial determinants provides a modular approach to achieve this goal. Mutating plasminogen activators such as urokinase in a way that renders them activated selectively by thrombin further enhances specificity of the thromboprophylaxis. Results of the recent animal studies give us a strong reason to believe that with further work, these novel targeted therapeutics will offer safe and effective means for management of thrombosis.
References
- 1.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) New England J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 2.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1(7):1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 3.McGuire M, Dobesh PP. Therapeutic update on the prevention and treatment of venous thromboembolism. J Pharmacy Practice. 2004;17:289. [Google Scholar]
- 4.Ng HJ, Crowther MA. New Anti-thrombotic agents: Emphasis on Hemorrhagic Complications and Their Management. Seminars in Hematology. 2006;43(suppl 1):S77–S83. doi: 10.1053/j.seminhematol.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Lijnen HR, Collen D. Development of thrombolytic agents. Biotec Adv. 1993;11:115–130. doi: 10.1016/0734-9750(93)90411-f. [DOI] [PubMed] [Google Scholar]
- 6.Heeremans JL, Prevost R, Bekkers ME, Los P, Emeis JJ, Kluft C, Crommelin DJ. Thrombolytic treatment with tissue-type plasminogen activator (t-PA) containing liposomes in rabbits: a comparison with free t-PA. Thromb Haemost. 1995;73:488–494. [PubMed] [Google Scholar]
- 7.Leach JK, Patterson E, O’Rear EA. Distributed intraclot thrombolysis: mechanism of accelerated thrombolysis with encapsulated plasminogen activators. J Thromb Haemost. 2004;2:1548–1555. doi: 10.1111/j.1538-7836.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Kim JK, Park JS, Byun Y, Kim CK. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials. 2009;30(29):5751–6. doi: 10.1016/j.biomaterials.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Chung TW, Wang SS, Tsai WJ. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials. 2008;29:228–237. doi: 10.1016/j.biomaterials.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Carnemolla R, Shuvaev VV, Muzykantov VR. Targeting Antioxidant and Antithrombotic Biotherapeutics to Endothelium. Seminars in Thrombosis and Haemostasis. 2010;26:332–342. doi: 10.1055/s-0030-1253455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas SP, Vaidya B. Targeted delivery of thrombolytic agents: role of integrin receptors. Expert Opin Drug Deliv. 2009;6(5):499–508. doi: 10.1517/17425240902878002. [DOI] [PubMed] [Google Scholar]
- 12.Runge MS, Bode C, Matsueda GR, Haber E. Antibody-enhanced thrombolysis:targeting of tissue plasminogen activator in vivo. Proc Natl Acad Sci USA. 1987;84(21):7659–62. doi: 10.1073/pnas.84.21.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Runge MS, Harker LA, Bode C, Ruef J, Kelly AB, Marzee UM, Allen E, Caban R, Shaw SY, Haber E, Hanson SR. Enhanced thrombolytic and antithrombotic potency of a fibrin-targeted plasminogen activator in baboons. Circulation. 1996;94(6):1412–22. doi: 10.1161/01.cir.94.6.1412. [DOI] [PubMed] [Google Scholar]
- 14.Bode C, Runge MS, Schönermark S, Eberle T, Newell JB, Kübler W, Haber E. Conjugation of antifibrin Fab’ enhances fibrinolytic potency of single-chain urokinase plasminogen activator. Circulation. 1990;81(6):1974–80. doi: 10.1161/01.cir.81.6.1974. [DOI] [PubMed] [Google Scholar]
- 15.Peter K, Graeber J, Kipriyanov S, Zewe-Welschof M, Runge MS, Kübler W, Little M, Bode C. Construction and functional evaluation of a single-chain antibody fusion protein with fibrin targeting and thrombin inhibition after activation by factor Xa. Circulation. 2000;101(10):1158–64. doi: 10.1161/01.cir.101.10.1158. [DOI] [PubMed] [Google Scholar]
- 16.Marsh JN, Senpan A, Hu G, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Fibrin-targeted perfluorocarbon nanoparticles for targeted thrombolysis. Nanomedicine (London) 2007;2(4):533–43. doi: 10.2217/17435889.2.4.533. [DOI] [PubMed] [Google Scholar]
- 17.Sakharov DV, Rijken DC. Superficial accumulation of plasminogen during clot lysis. Circulation. 1995;92(7):1883–90. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- 18.Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–86. doi: 10.1160/TH07-11-0685. [DOI] [PubMed] [Google Scholar]
- 19.Topol EJ, Mark DB, Lincoff AM, Cohen E, Burton J, Kleiman N, Talley D, Sapp S, Booth J, Cabot CF, Anderson KM, Califf RM. Outcomes at 1 year and economic implications of platelet glycoprotein IIb/IIIa blockade in patients undergoing coronary stenting:results from multicentre randomized trial:EPISTENT Investigators. Evaluation of Platelet IIb/IIIa for stenting. Lancet. 1999;354(9195):2019–24. doi: 10.1016/s0140-6736(99)10018-7. [DOI] [PubMed] [Google Scholar]
- 20.Bode C, Meinhardt G, Runge MS, Freitag M, Nordt T, Arens M, Newell JB, Kubler W, Haber E. Platelet-targeted fibrinolysis enhances clot lysis and inhibits platelet aggregation. Circulation. 1991;84(2):805–13. doi: 10.1161/01.cir.84.2.805. [DOI] [PubMed] [Google Scholar]
- 21.DeMeyer SF, Vanhoorelbeke K, Broos K, Salles, Deckmyn H. Antiplatelet drugs. Br J Haemotol. 2008;142:515–528. doi: 10.1111/j.1365-2141.2008.07233.x. [DOI] [PubMed] [Google Scholar]
- 22.Rossi L, Serafini S, Pierigé F, Antonelli A, Cerasi A, Fraternale A, Chiarantini L, Magnani M. Erythrocyte-based drug delivery. Expert Opin Drug Deliv. 2005;2(2):311–22. doi: 10.1517/17425247.2.2.311. [DOI] [PubMed] [Google Scholar]
- 23.Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylatic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21(8):891–6. doi: 10.1038/nbt846. [DOI] [PubMed] [Google Scholar]
- 24.Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther. 2005;312(3):1106–13. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 25.Ganguly K, Goel MS, Krasik T, Bdeir K, Diamond SL, Cines DB, Muzykantov VR, Murciano JC. Fibrin affinity of erythrocyte-coupled tissue type plasminogen activators endures hemodynamic forces and enhances fibrinolysis in vivo. J Pharmacol Exp Ther. 2006;316(3):1130–6. doi: 10.1124/jpet.105.093450. [DOI] [PubMed] [Google Scholar]
- 26.Ganguly K, Murciano JC, Westrick R, Leferovich J, Cines DB, Muzykantov VR. The glycocalyx protects erythrocyte-bound tissue-type plasminogen activators from enzymatic inhibition. J Pharmacol Exp Ther. 2007;321(1):158–64. doi: 10.1124/jpet.106.114405. [DOI] [PubMed] [Google Scholar]
- 27.Murciano JC, Higazi AA, Cines DB, Muzykantov VR. Soluble urokinase receptor conjugated to red blood cells binds latent pro-urokinase and alters its functional profile. J Control Release. 2009;139(3):190–6. doi: 10.1016/j.jconrel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danielyan K, Ganguly K, Ding BS, Atochin D, Zaitsev S, Murciano JC, Huang PL, Kasner SE, Cines DB, Muzykantov VR. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118(14):1442–9. doi: 10.1161/CIRCULATIONAHA.107.750257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29(8):1463–74. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstead WM, Ganguly K, Riley J, Kiessling JW, Cines DB, Higazi AA, Zaitsev S, Muzykantov VR. Red blood cell-coupled tissue plasminogen activator prevents impairment of cerebral vasodilatory responses through inhibition of c-Jun-N-terminal kinase and potentiation of p38 mitogen-activated protein kinase after cerebral photothrombosis in the newborn pig. Pediatr Crit Care Med. 2010 Oct 28; doi: 10.1097/PCC.0b013e3181fe40a7. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein SC, Ganguly K, Belfield CM, Xu X, Swanson EW, Chen XH, Browne KD, Johnson VE, Smith DH, LeBold DG, Cines DB, Muzykantov VR. Erythrocyte-bound tissue plasminogen activator is neuroprotective in experimental traumatic brain injury. J Neurotrauma. 2009;26(9):1585–92. doi: 10.1089/neu.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaitsev S, Danielyan K, Murciano JC, Ganguly K, Krasik T, Taylor RP, Pincus S, Jones S, Cines DB, Muzykantov VR. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108(6):1895–902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaitsev S, Spitzer D, Murciano JC, Ding BS, Tliba S, Kowalska MA, Bdeir K, Kuo A, Stepanova V, Atkinson JP, Poncz M, Cines DB, Muzykantov VR. Targeting of a mutant plasminogen activator to circulating red blood cells for prophylactic fibrinolysis. J Pharmacol Exp Ther. 2010;332(3):1022–31. doi: 10.1124/jpet.109.159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barinka C, Parry G, Callahan J, Shaw DE, Kuo A, Bdeir K, Cines DB, Mazar A, Lubkowski J. Structural basis of interaction between urokinase-type plasminogen activator and its receptor. J Mol Biol. 2006;363(2):482–95. doi: 10.1016/j.jmb.2006.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang WP, Goldstein J, Procyk R, Matsueda GR, Shaw SY. Design and evaluation of a thrombin-activable plasminogen activator. Biochemistry. 1994;33(8):606–12. doi: 10.1021/bi00174a043. [DOI] [PubMed] [Google Scholar]
- 36.Zaitsev S, Spitzer D, Murciano JC, Ding BS, Tliba S, Kowalska MA, Marcos-Contreras OA, Kuo A, Stepanova V, Atkinson JP, Poncz M, Cines DB, Muzykantov VR. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood. 2010;115(25):5241–8. doi: 10.1182/blood-2010-01-261610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muzykantov VR, Barnathan ES, Atochina EN, Kuo A, Danilov SM, Fisher AB. Targeting to antibody-conjugated plasminogen activators to the pulmonary vasculature. J Pharmacol Exp Ther. 1996;279(2):1026–34. [PubMed] [Google Scholar]
- 38.Murciano JC, Harshaw DW, Ghitescu L, Danilov SM, Muzykantov VR. Vascular immunotargeting to endothelial surface in a specific macrodomain in alveolar capillaries. Am J Respir Crit Care Med. 2001;164(7):1295–1302. doi: 10.1164/ajrccm.164.7.2010076. [DOI] [PubMed] [Google Scholar]
- 39.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 40.Muro S, Dziubla T, Qiu W, Leferovich J, Cui X, Berk E, Muzykantov VR. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J Pharm Exp Ther. 2006;317(3):1161–9. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 41.Ding BS, Gottstein C, Grunow A, Kuo A, Ganguly K, Albelda SM, Cines DB, Muzykantov VR. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106(13):4191–8. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther. 2002;300(3):777–86. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 43.Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman EH, Muzykantov V, Muro S. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharm Exp Ther. 2008;325(2):400–8. doi: 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- 44.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci USA. 1999;96(5):2379–84. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116(Pt 8):1599–609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 46.Almenar-Queralt A, Duperray A, Miles LA, Felez J, Altieri DC. Apical topography and modulation of ICAM-1 expression on activated endothelium. Am J Pathol. 1995;147(5):1278–1288. [PMC free article] [PubMed] [Google Scholar]
- 47.Murciano JC, Muro S, Koniaris L, Christofidou-Solomidou M, Harshaw DW, Albelda SM, Granger DN, Cines DB, Muzykantov VR. ICAM-directed vascular immunotargeting of antithrombotic agents to the endothelial luminal surface. Blood. 2003;101(10):3977–84. doi: 10.1182/blood-2002-09-2853. [DOI] [PubMed] [Google Scholar]
- 48.Danielyan K, Ding BS, Gottstein C, Dines DB, Muzykantov VR. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates post-ischemic brain edema. J Pharmacol Exp Ther. 2007;321(3):947–52. doi: 10.1124/jpet.107.120535. [DOI] [PubMed] [Google Scholar]
- 49.Ding BS, Hong N, Murciano JC, Ganguly K, Gottstein C, Christofidou-Solomidou M, Albelda SM, Fisher AB, Cines DB, Muzykantov VR. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111(4):1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]


