Abstract
Although the roles of the SREBP1 and SREBP2 transcription factors in regulating fatty acid and cholesterol synthesis and uptake have been known for some time, it was recently discovered that two related microRNAs, miR-33a and miR-33b, are embedded in these genes. Studies indicate that miR-33a and miR-33b act with their host genes, Srebp2 and Srebp1, respectively, to reciprocally regulate cholesterol homeostasis and fatty acid metabolism in a negative feedback loop. miR-33 has been shown to post-transcriptionally repress key genes involved in cellular cholesterol export and HDL metabolism (Abca1, Abcg1, Npc1), fatty acid oxidation (Crot, Cpt1a, Hadhb, Ampk), and glucose metabolism (Sirt6, Irs2). Delivery of inhibitors of miR-33 in vitro and in vivo relieves repression of these genes resulting in up-regulation of the associated metabolic pathways. In mouse models, miR-33 antagonism has proven has proven to be an effective strategy for increasing plasma HDL cholesterol and fatty acid oxidation, and protecting from atherosclerosis. These exciting findings have opened up promising new avenues for the development of therapeutics to treat dyslipidemia and other metabolic disorders.
Unraveling the role of microRNAs (miRNAs) in the regulation of gene pathways is an exciting new frontier in many different areas of biological research. This class of short (22 nt) non-coding RNAs post-transcriptionally represses gene expression through binding to complementary target sites in the 3′untranslated regions (3′UTRs) of messenger RNA (mRNA)1-4. Since their discovery in Caenorhabditis elegans5, 6, our understanding of miRNA processing and action has increased tremendously through the work of many investigators in this field. MicroRNAs are encoded in intergenic or intronic regions of the genome of metazoan animals, plants and viruses, and are processed from primary transcripts through the sequential actions of Drosha and Dicer enzymes1, 3, 4. Mature miRNAs are then incorporated into the RNA-induced silencing complex (RISC) in the cytoplasm and bind to partially complementary target sites in mRNAs, thereby inhibiting their expression through mRNA destabilization, repression of translation or a combination of both processes1-4. Bioinformatic predictions and experimental approaches indicate that a single microRNA may simultaneously target more than 100 mRNAs that function in the same or related pathways, thus providing a mechanism of “fine-tuning” entire gene networks involved in a physiological process or biological pathway.
SREBP-miR-33a/b
The elegance of this mechanism of post-transcriptional gene control is exemplified by the recent identification of microRNA-33a and b (miR-33a/b) as intronic microRNAs located within the sterol response element binding protein (SREBP) genes Srebp2 and Srebp17-9. These loci code for the membrane bound transcription factors SREBP1 and SREBP2 that activate the synthesis of fatty acid and the synthesis and uptake of cholesterol10-12. Coincident with the transcription of Srebp1 and Srebp2, the embedded miR-33a and miR-33b are transcribed and these negative regulators act to repress a number genes involved in fatty acid oxidation and cholesterol export7-9, 13-15. This cleverly designed negative feedback loop helps to boost intracellular cholesterol and fatty acid levels by simultaneously balancing transcriptional induction and post-transcriptional repression of lipid metabolism genes.
miR-33 regulates cholesterol metabolism
The presence of miR-33a in the intron of Srebp2 is remarkably conserved in many species, including large and small mammals, chickens and frogs, suggesting a critical function7-9. By contrast, there is a gap in the evolutionary conservation of miR-33b, which is present in the Srebp1 gene of mammals, with the exception of rodents. Interestingly, miR-33 and Srebp gene are conserved in some cholesterol auxotroph animals, where the regulation of SREBP protein and its transcriptional targets are related to fatty acid and phospholipid metabolism. This observation suggests that the function of ancestral miR-33 may have been more related to miR-33b than miR-33a. The two isoforms of miR-33 differ by only 2 nucleotides in their mature sequences, but are identical in their seed sequence, which dictates target recognition. Thus, miR-33a and b are likely to repress a similar subset of target genes. However the metabolic conditions that regulate their induction are quite different. In a series of parallel studies, it was reported that miR-33a is co-regulated with its host gene Srebp2 under low sterol conditions7-9. SREBP2 activates the transcription of genes involved in cholesterol homeostasis, such as 3-hydroxy-3-methylglutaryl coenzyme A (HMGCR) reductase, which catalyzes a rate-limiting step in cholesterol biosynthesis, and the low-density lipoprotein receptor (LDLr), which imports cholesterol from the blood10, 12, 16. Like SREBP2, miR-33a is widely expressed and is induced 2-3 fold under low sterol conditions suggesting that these two regulatory elements are co-transcribed9. Similar studies have recently been performed demonstrating that conditions that induce SREBP1 transcription, such as insulin and liver X receptor (LXR) activation, also induce expression of miR-33b13. Notably, the amplitude of SREBP1, and thus miR-33b, induction can be quite large compared to SREBP2 under these conditions, and thus in humans, this isoform may be more abundant.
Recent studies identified a role for miR-33a/b in the repression of genes involved in cholesterol export, including the adenosine triphosphate binding cassette (ABC) transporters, ABCA1 and ABCG1, and the endolysosomal transport protein, NPC19. Of these targets, the most prominent is ABCA1, a cholesterol transporter that promotes the movement of excess free cholesterol out of the cell17, 18. In the liver, ABCA1 is essential for efflux of cholesterol to apolipoprotein AI (apoAI), the building block of high-density lipoprotein (HDL)17, 18. In peripheral cells, ABCA1 effluxes excess cholesterol to these nascent HDL particles for delivery to the liver for excretion to the bile and feces17-20. This process, termed reverse cholesterol transport, plays an essential role in both cellular and whole body cholesterol homeostasis.
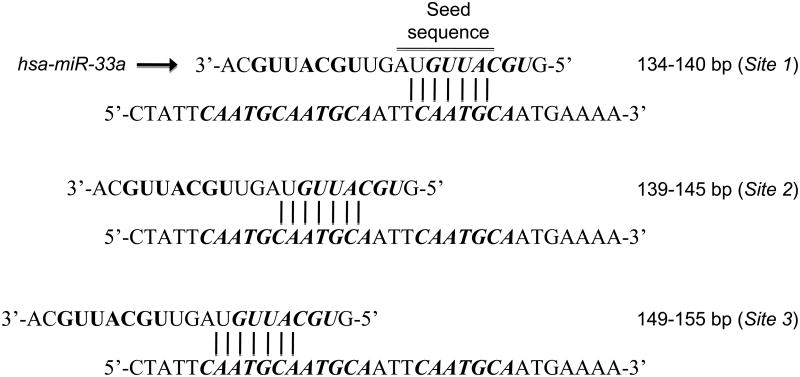
The 3′UTR of mouse and human Abca1 contain three binding sites for miR-33a/b (Figure 1) and transfection of miR-33 mimics strongly represses ABCA1 mRNA and protein in a variety of cell types7-9. Functionally, miR-33 overexpression in hepatocytes and macrophages results in decreased cellular cholesterol efflux to apoAI7-9. Furthermore, inhibition of endogenous miR-33 in both these cell types promotes an increase in the expression of ABCA1 protein and a concomitant increase in cholesterol efflux to apoA1, indicating a physiologically relevant role for this microRNA in regulating ABCA17-9. In mouse cells, miR-33 was also shown to target a second member of the ABC transporter family, ABCG19. ABCG1 also mobilizes excess free cholesterol from the cell, but uses more mature HDL particles as its acceptor17, 18. This pathway appears to be most active in peripheral cells, particularly macrophages in atherosclerotic lesions, where ABCA1 and ABCG1 are thought to act in sequence to lipidate nascent and then mature HDL to generate larger α-HDL particles destined for clearance by the liver17, 18. Notably, miR-33 repression of ABCG1 is not conserved in human cells due to the loss of miR-33 binding sites in the 3′UTR of human Abcg1. However, an additional component of this pathway, Neimann Pick C1 (NPC1), is targeted in human cells. NPC1 is a lysosomal protein that facilitates the transport of cholesterol to other parts of the cell in need and acts in concert with ABCA1 to promote cholesterol efflux to apoA19. The 3′UTR of human Npc1 contains 2 miR-33 binding sites, resulting in the repression of NPC1 protein in hepatocytes and macrophages9. Thus, miR-33 targets multiple genes in the pathway regulating efflux of cholesterol to HDL.
Figure 1.
Evolutionary conserved sequences in the 3′UTR of ABCA1 is partially complementary to miR-33. Annealing of miR-33 to some sequences are shown
miR-33 regulates fatty acid metabolism
miR-33a is found within the same intron of Srebp2 from many animal species, including large and small mammals. Interestingly, the fruit fly Drosophila melanogaster also has a highly conserved mature form of miR-33a, but these organisms do not synthesize sterols. This observation pointed to broader roles for miR-33 and lead to the identification of additional targets of miR-33a/b. Of note, several genes involved in fatty acid metabolism including Crot, Cpt1a, Hadhb and Ampk contain predicted binding sites for miR-33a/b13, 14. Overexpression of miR-33a/b reduces fatty acid oxidation and leads the accumulation of triglycerides in human hepatic cells and in the fat body of miR-33 transgenic flies13, 14. Of particular interest is the inhibitory effect of miR-33 on AMPK protein expression13. The role of AMPK in regulating cellular energy places this enzyme at a central point in maintaining energy homeostasis21. AMPK promotes hepatic fatty acid β-oxidation and inhibits cholesterol and triglyceride (TAG) synthesis. In this way, miR-33 inhibition of AMPK also increases cellular cholesterol and TAG content. Altogether, these data implicate miR-33a/b as central regulators of multiple aspects of lipid metabolism by limiting cholesterol efflux and fatty acid degradation as SREBP boosts their production.
miR-33 regulates glucose metabolism and insulin signaling
miR-33 has also been implicated in regulating insulin signaling via targeting of insulin receptor substrate 2 (IRS2), an essential signaling molecule that mediates the effects of insulin13. miR-33a/b over-expression reduces IRS2 levels and inhibits the activation of downstream messenger cascades, including AKT13. Moreover, miR-33a/b also target FSR-2 which has been suggested to participate in insulin signaling by recruiting Src-homology-phosphatase 2 (SHP2) and to function as a docking molecule similar to IRS213. In addition with IRS2 and FRS2, miR-33 also regulates the expression of other genes involved in glucose metabolism such as sirtuin-6 (SIRT6)13. Interestingly, hepatic-specific disruption of SIRT6 in mice results in fatty liver formation because of enhanced glucolysis and triglyceride synthesis22 which correlates with the increased triglyceride content observed in human hepatic cell lines transfected with miR-33.
Other functions of miR-33
A recent report has suggested a role for miR-33 in regulating stem cell self-renewal via down regulation of p5323. p53 has two putative miR-33 binding sites in the 3′UTR region and miR-33 transfection represses p53 expression and p53-mediated apoptosis23. This study suggests that miR-33 may promote the repopulation capacity of hematopoietic stem cell (HSC). Interestingly, SREBP-1 and cellular cholesterol content have also been shown to regulate cell cycle progression24-27. Thus, miR-33a/b might cooperate with their host genes in regulating cell proliferation and cell cycle progression. Indeed, it has been recently reported that miR-33 over-expression reduces cell proliferation by direct targeting the serine/threonine-protein kinase Pim-128.
Pre-clinical studies with miR-33 inhibitors
The physiological relevance of miR-33 targeting of cellular cholesterol efflux has been demonstrated by short-term overexpression or silencing of miR-33 in mice using strategies such as viral delivery of miR-33 mimics or hairpin inhibitors7, 9 or parenteral administration of modified anti-sense oligonucleotides8, 29. In vivo overexpression of miR-33 reduced expression of ABCA1 in the liver and decreased plasma HDL levels by 25%9. Conversely, various methods of miR-33 inhibition increased hepatic ABCA1 expression, resulting in up to 40% increases of plasma HDL cholesterol7-9. The results of these miR-33 antagonism studies were recently confirmed by the generation of a miR-33 knock-out mouse15. Targeted deletion of miR-33a from the intron of SREBP2 generated mice that were viable and fertile and showed no disruption of SREBP-2 function15. These miR-33 deficient mice had circulating HDL cholesterol levels that were 25-40% higher than wild type C57BL/6 mice15. Notably, whereas no differences in male and female mice were observed in studies using pharmacologic inhibitors of miR-33, female miR-33 knock-out mice showed larger increases in plasma HDL than their male counterparts15. The molecular mechanisms of this difference are currently being investigated.
Plasma HDL cholesterol levels bear a strong inverse correlation with cardiovascular disease risk, and thus the finding that HDL levels can be modulated by manipulating miR-33 has generated considerable interest in its therapeutic potential. In mouse models of atherosclerosis, overexpression of apoA1 to increase HDL has been shown to hinder plaque progression30-33, and to promote regression34-36. Furthermore, direct infusion of HDL in apolipoprotein E (apoE) deficient mice, cholesterol-fed rabbits37, or human subjects38 with established atherosclerosis, reduces plaque size. To test whether miR-33 inhibition might have a similar impact, Ldlr−/− mice were treated with an oligonucleotide inhibitor of miR-33 for 4 weeks following establishment of atherosclerotic plaques by western diet feeding for 14 weeks. Notably, in this mouse model of atherosclerosis, miR-33 inhibition increased HDL by 35% as previously seen in wild type mice, and this was associated with a 35% reduction in both plaque size and lipid content29. Using an in vivo assay to measure the efficiency of reverse cholesterol transport, it was shown that the HDL generated by miR-33 inhibition was functional and increased the transport of cellular radiolabelled cholesterol to the plasma, liver and feces29. The atheroprotective effects of HDL have been largely attributed to its function in reverse cholesterol transport and in line with this, atherosclerotic lesions in anti-miR33 treated mice showed increased markers of plaque stability, including reduced macrophage accumulation and inflammatory gene expression, as well as an increase in collagen content29. Notably, in addition to raising ABCA1 in the liver, anti-miR33 oligonucleotides were also detected within macrophages of the atherosclerotic plaque. Isolation of these macrophages by laser capture microdissection showed an increase in ABCA1 expression of anti-miR-33 treated mice, as well as a decrease in inflammatory gene expression. Thus, oligonucleotide inhibitors of miR-33 may promote the reverse cholesterol transport pathway in two ways: by directly increasing HDL biogenesis in the liver and by increasing cellular cholesterol efflux from plaque macrophages.
While the preclinical studies of miR-33 inhibition in mice are encouraging, extrapolation of these findings to humans is complicated by the fact that mice lack miR-33b. This difference between mice and humans may be particularly relevant under conditions in which the transcription of Srebp1 is highly up-regulated, such as hyperinsulinemia11, which would lead to profound increases in miR-33b expression. Not only would such a condition lead to greater downregulation of cellular cholesterol efflux and plasma HDL levels, but the increased miR-33b/Srebp1 transcription in insulin-resistant states would be predicted to promote hypertriglyceridemia by inhibiting fatty acid oxidation and promoting fatty acid synthesis. Thus, inhibitors of miR-33a/b may relieve repression of both of these metabolic pathways resulting in reduced plasma triglycerides as well as increased plasma HDL. However, a comprehensive understanding of the effects of inhibiting both miR-33a and miR-33b awaits translational studies in animal models containing both isoforms of miR-33, such as a non-human primate model.
Future aspects
The therapeutic manipulation of miRNA-regulated pathways is emerging as a promising avenue for the treatment of dyslipidemia and other metabolic disorders. Given the role of miR-33a/b in repressing cholesterol efflux, fatty acid oxidation and insulin signaling (Figure 2), pharmacologic targeting of miR-33a/b may be a promising strategy to treat metabolic syndrome. Cardinal features of this syndrome include dyslipidemia, characterized by an increase in plasma triglycerides and a decrease in plasma high-density lipoproteins (HDL), as well as obesity and insulin resistance39. The metabolic syndrome is a growing public health concern worldwide, with complex and interrelated risk factors for both cardiovascular disease (CVD) and diabetes. Despite widespread use of statins to lower levels of low density lipoproteins (LDL) and apolipoprotein B-containing lipoproteins, considerable residual CVD risk persists in this patient population. Major goals in the pursuit of novel therapies to target this residual risk have focused on raising levels of HDL to exploit its atheroprotective functions, lowering triglycerides and improving insulin signaling. Whether miR-33 could be such a panacea awaits future studies.
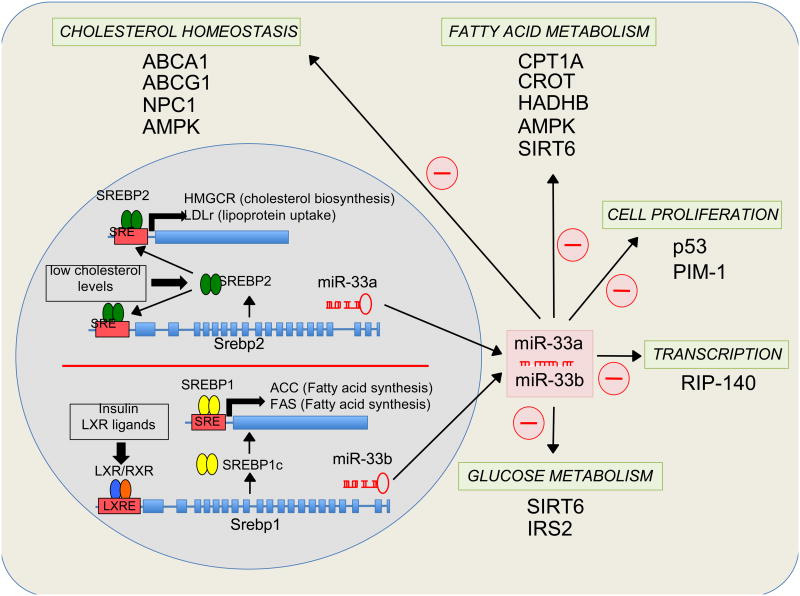
Figure 2.
Pathways by which activated miR-33 may contribute to regulating cholesterol, fatty acid and glucose metabolism, cell proliferation and transcription. Transcriptional activation of SREBP-2 under low intracellular cholesterol levels and SREBP-1 upon insulin-LXR stimulation leads the co-transcription of miR-33a and miR-33b respectively. miR-33a/b target several genes involved in the regulation of cholesterol efflux and trafficking, fatty acid oxidation, cell proliferation and cell cycle progression, and glucose metabolism.
Acknowledgments
Source of funding: C.F.-H. is supported by grants from the National Institute of Health (RO1HL106063 and RO1HL107953) and American Heart Association (SDG-0835585D). K.J.M. supported by grants from the National Institute of Health (R01AG020255 and R01HL108182).
Footnotes
Disclosures: None
References
- 1.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 6.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 7.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 11.Horton JD. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem Soc Trans. 2002;30:1091–1095. doi: 10.1042/bst0301091. [DOI] [PubMed] [Google Scholar]
- 12.Osborne TF. Sterol regulatory element-binding proteins (SREBPs): key regulators of nutritional homeostasis and insulin action. J Biol Chem. 2000;275:32379–32382. doi: 10.1074/jbc.R000017200. [DOI] [PubMed] [Google Scholar]
- 13.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, Esplugues E, Fisher EA, Penalva LO, Moore KJ, Suarez Y, Lai EC, Fernandez-Hernando C. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, Macdougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–61. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 18.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Oram JF. Tangier disease and ABCA1. Biochim Biophys Acta. 2000;1529:321–330. doi: 10.1016/s1388-1981(00)00157-8. [DOI] [PubMed] [Google Scholar]
- 20.Oram JF, Yokoyama S. Apolipoprotein-mediated removal of cellular cholesterol and phospholipids. J Lipid Res. 1996;37:2473–2491. [PubMed] [Google Scholar]
- 21.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HS, Xiao C, Wang RH, Lahusen T, Xu X, Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, Gao B, Deng CX. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 2010;12:224–236. doi: 10.1016/j.cmet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera-Merchan A, Cerrato C, Luengo G, Dominguez O, Piris MA, Serrano M, Gonzalez S. miR-33-mediated downregulation of p53 controls hematopoietic stem cell self-renewal. Cell Cycle. 2010;9:3277–3285. doi: 10.4161/cc.9.16.12598. [DOI] [PubMed] [Google Scholar]
- 24.Bengoechea-Alonso MT, Ericsson J. Cdk1/cyclin B-mediated phosphorylation stabilizes SREBP1 during mitosis. Cell Cycle. 2006;5:1708–1718. doi: 10.4161/cc.5.15.3131. [DOI] [PubMed] [Google Scholar]
- 25.Bengoechea-Alonso MT, Punga T, Ericsson J. Hyperphosphorylation regulates the activity of SREBP1 during mitosis. Proc Natl Acad Sci U S A. 2005;102:11681–11686. doi: 10.1073/pnas.0501494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez C, Lobo Md Mdel V, Gomez-Coronado D, Lasuncion MA. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp Cell Res. 2004;300:109–120. doi: 10.1016/j.yexcr.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Botas J, Suarez Y, Ferruelo AJ, Gomez-Coronado D, Lasuncion MA. Cholesterol starvation decreases p34(cdc2) kinase activity and arrests the cell cycle at G2. FASEB J. 1999;13:1359–1370. doi: 10.1096/fasebj.13.11.1359. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71:5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- 29.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhury RP, Rong JX, Trogan E, Elmalem VI, Dansky HM, Breslow JL, Witztum JL, Fallon JT, Fisher EA. High-density lipoproteins retard the progression of atherosclerosis and favorably remodel lesions without suppressing indices of inflammation or oxidation. Arterioscler Thromb Vasc Biol. 2004;24:1904–1909. doi: 10.1161/01.ATV.0000142808.34602.25. [DOI] [PubMed] [Google Scholar]
- 31.Paszty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 34.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 36.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 37.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 39.Betteridge DJ. Lipid control in patients with diabetes mellitus. Nat Rev Cardiol. 8:278–290. doi: 10.1038/nrcardio.2011.23. [DOI] [PubMed] [Google Scholar]