Abstract
Local natural resources are central to rural livelihoods across much of the developing world. Such “natural capital” represents one of several types of assets available to households as they craft livelihood strategies. In order to explore the potential for environmental scarcity and change to contribute to perpetuation of the HIV/AIDS pandemic, we examine the association between declining natural capital and engaging in risky sexual behaviors, as potentially another livelihood strategy. Such association has been demonstrated in Kenya and Tanzania, through the fish-for-sex trade. To explore the possibility of this connection within rural Haitian livelihoods we use Demographic and Health Survey data, with a focus on rural women, combined with vegetation measures generated from satellite imagery. We find that lack of condom use in recent sexual encounters is associated with local environmental scarcity – controlling for respondent age, education, religion and knowledge of AIDS preventive measures. The results suggest that explicit consideration of the environmental dimensions of HIV/AIDS may be of relevance in scholarship examining factors shaping the pandemic.
Keywords: deforestation, environment, Haiti, HIV/AIDS, rural livelihoods, natural resources, population, remote sensing
Demographic scholarship is increasingly exploring the ways in which environmental context shapes demographic processes and patterns. Consider, for example, land scarcity and tenure constraints as factors shaping recent Kenyan fertility decline (Shreffler and DoDoo 2009). In addition, evidence of an association between environmental change and increased risk of HIV/AIDS infection has begun to surface in sub-Saharan Africa (Gordon 2005; Merten and Haller 2007; Mojola 2010; Torrel et al. 2007). Such work suggests that research into core demographic processes may be incomplete (or worse, incorrect) unless it considers interactions among individual behavior, socioeconomic and political structural forces, and the local environment. Neglecting environmental factors may yield incomplete understanding especially in regions where households are highly dependent on nearby natural resources.
In many of these rural resource-dependent regions, both individual well-being and large-scale demographic dynamics are also dramatically impacted by HIV/AIDS. Today, an estimated 33.4 million individuals are living with HIV. In 2008, over 2.7 million became newly infected and there were over 2 million AIDS-related deaths (UNAIDS 2009). Still, scholarship examining the AIDS-environment association has been hampered by a shortage of HIV/AIDS data at the individual and household level (Barnett and Whiteside 2002). Even so, recent work does suggest that AIDS-impacted households rely more than others on natural resources to maintain food security (see, e.g., Hunter, Twine, and Patterson 2007; Kaschula 2008); and AIDS mortality also shapes households’ future access to land resources (Frank and Unruh 2008).
In this study we add to explorations of the environmental dimensions of the AIDS pandemic through focus on risky sexual behavior in Haiti as related to proximate environmental degradation. Higher risk sexual behaviors are believed to fuel the HIV/AIDS pandemic (e.g., Dunkle et al. 2004) and risky sexual behavior has been documented as an “alternative livelihood strategy” in the face of environmental pressures (e.g., Bene and Merten 2009; Mojola 2010). Haiti is a logical setting to explore these interconnections given the nation’s relatively high HIV prevalence and large-scale, sustained environmental degradation.
For this paper, we rely on the Demographic and Health Survey definition of higher risk sex as sex with a noncohabiting, nonmarital partner within the last 12 months (TACAIDS 2008). We further focus on women who report engaging in sex without condoms, controlling for knowledge of condom use as a preventive measure against AIDS.
Making use of DHS data from Haiti (2000) combined with satellite imagery reflecting shifts in vegetation availability across time (1990–2000), we examine associations between risky sexual behavior and proximate environmental change for rural women in Haiti -- an impoverished island nation with the oldest generalized HIV/AIDS epidemic outside of Sub-Saharan Africa (Gilbert et al. 2007).1 Satellite imagery allows broad spatial and temporal coverage of vegetation as a way to gauge natural resource availability and change. Within the analyses, we use a Rural Livelihoods Framework to carefully consider the broader suite of capital assets available to people making livelihood decisions.
In the remainder of this paper, we provide a general overview of the HIV/AIDS pandemic in Haiti, followed by a short review of existing literature on how HIV/AIDS affects rural livelihoods. We then outline this project’s theoretical framework and present our hypotheses. A review of the Haiti Demographic and Health Survey and remotely sensed data follows, and we conclude with presentation and discussion of results and implications.
Background
HIV/AIDS and Risky Sexual Behavior in Haiti
The vast majority of studies on the socio-environmental aspects of the AIDS pandemic have focused on sub-Saharan Africa. We have chosen to focus this work on Haiti because it has the highest rates of HIV/AIDS in the western hemisphere, combined with intense poverty and environmental degradation. In such a setting, consideration of the intersections of these socio-environmental processes is of paramount importance.
According to the most recent UNAIDS data, an estimated 230,000 individuals in the Caribbean were living with HIV in 2007, while an estimated 20,000 were newly infected. In addition, some 14,000 died of AIDS (UNAIDS 2008). Within the Caribbean region, 85% of the HIV/AIDS positive population lives on the island of Hispaniola, comprising Haiti and the Dominican Republic (Hempstone et al. 2004). Indeed, Haiti is the most heavily HIV/AIDS-affected nation in the region, with an estimated 94,000 adults living with HIV (UNAIDS 2008). In the year before the collection of the data used here, adult HIV/AIDS prevalence in Haiti exceeded 5 percent -- the only country outside Sub-Saharan Africa with a prevalence of this magnitude.
Yet recent years have seen improvements in Haiti’s AIDS prevalence. Current estimates put HIV prevalence at 2.2% -- slightly higher for women age 15–49 (2.3%) and lower for men in that age group (2.0%) (MEASURE DHS 2005–2006). Even so, the current prevalence represents the same rate estimated six years before (UNAIDS 2008) and, given this consistency, the nation has been characterized as “in the throes of a generalized HIV epidemic” (Pan American Health Organization (PAHO) 2007: 420).
In Haiti, HIV is spread primarily through heterosexual intercourse, paid or otherwise, in addition to mother-to-child transmission (Deschamps et al. 1996; UNAIDS 2008). The recent prevalence declines have been attributed to HIV/AIDS education campaigns and greater access to medical treatment (UNAIDS 2008). More women are using condoms although numbers still remain low -- 18% in 2006, more than double the 8% in 1995 (MEASURE DHS 2005–2006). Young people are also increasingly aware of means of preventing HIV infection, with 8 in 10 young Haitians understanding the importance of abstinence, having only one faithful partner, and/or using condoms (MEASURE DHS 2005–2006).
Factors that have facilitated the emergence of Haiti’s generalized HIV/AIDS epidemic include poverty, sociopolitical instability, stigmatization, social norms that permit multiple concurrent partners, limited health care, and poor governmental response (Hempstone et al. 2004). Thus HIV/AIDS represents more than a biological condition; rather it is rooted in broader socioeconomic and political contexts (Barnett and Whiteside 2002; Farmer 1992, 1999; Hunter 2007). Farmer, who has written extensively about the HIV/AIDS epidemic in Haiti, argues that poverty and inequality, both of which are tied to larger political economic conditions, have greatly accelerated the spread and devastation of HIV/AIDS (e.g., Farmer 1999). A more than century-long oppressive and extractive relationship between Haiti and international powers, most importantly the United States, has resulted in what Farmer terms structural violence, which in turn predisposes “the human body to pathogenic vulnerability by shaping the risk of infection” (Castro and Farmer 2005:55). We need to recognize that “the international community shares...the blame for the crisis in Haiti” (Faubert 2006).
Consideration of both the spatial gender and dimensions of the Haiti AIDS epidemic are important for the work presented here. With regard to spatial dimensions, urban/rural distinctions in the pandemic have recently surfaced; although general improvements in prevalence have been seen, UNAIDS (2008) reports that significant levels of high-risk behavior have been documented in Haiti’s rural areas and among young people (Cayemittes et al. 2006; Gaillard et al. 2006). Some evidence suggests that education and prevention messages aren’t as effectively reaching rural regions. A project focusing on commercial sex workers in the neighboring Dominican Republic suggests that although sex workers are increasingly likely to protect themselves (and their clients) against HIV infection, these positive behavioral changes are more common in the main urban and tourist centers (Kerrigan et al. 2006). Both trends leave rural individuals, the focus of our study, most at risk.
As to gender, some reports argue there is increasing feminization of the pandemic although others report less substantial gender distinctions. Female prevalence is higher in urban areas (2.7% for urban women; 2.0% for rural women), while the opposite is true for men (1.8% for urban men; 2.1% for rural men) (MEASURE DHS 2005–2006). As to trends, although there is evidence of decline in prevalence among pregnant women in urban areas, this is not the case among those in rural regions (Gaillard et al. 2006). Gender inequality, oppressive and entrenched female impoverishment, economic dependency, and low levels of education have all been linked to greater female susceptibility to HIV in Haiti (Farmer 1992, 1999; Smith Fawzi et al. 2005). Men are better informed with regard to AIDS prevention, with 90 percent knowing that having only one faithful partner and using a condom are ways to avoid transmission; eighty-one percent of women are so informed (MEASURE DHS 2005–2006). Further, Haitian women have little influence concerning condom use or other safe sexual behaviors (Hempstone et al. 2004). Elsewhere, gender-based economic disparities have also been linked to increased risk of HIV (Dunkle et al. 2004; TACAIDS 2008), as have poverty, low educational attainment (Tladi 2006), hunger, and malnutrition (Oyefara 2006).
It is important to distinguish here among commercial sex work (which is legal in Haiti), “transactional” sex arrangements, “survival sex,” and risky sexual behavior, broadly defined. The term “prostitution,” thoroughly implicating western conceptualizations of sexuality and exchange, is not necessarily an appropriate lens through which to view sexual relations in nonwestern settings (Hunter 2002). In some social settings, transactional sex is viewed as normative and can be a means of achieving a particular desired standard of living, not simply securing basic needs. Leclerc-Madlala (2004) contends that not all women who engage in transactional sex self-identify as engaging in commercial sex – especially when this livelihood strategy is coupled with other means of generating income and/or is used to achieve a particular level of consumption. Such sex-based exchange may be seen as an alternative livelihood strategy – one with important implications for the HIV/AIDS pandemic. DHS data, however, do not indicate motives for the sexual behavior reported; thus, we focus more broadly upon risky sexual behavior, defined as sex without a condom or sex outside the woman’s union.
On risky sexual behaviors, data from Haiti reveals that HIV risk is very much related to number of sexual partners. For example, prevalence rates are 8.0% among women who have had 5–9 partners whereas only 1.3% for those with a single partner (MEASURE DHS 2005–2006). Further, early sexual debut is common among Haitians, although delayed sexual initiation reduces the risk of contracting HIV. A nationally representative study in 10,000 households (2005–2006) revealed that more than 75% of Haitian men between the ages of 20–24, and over half of Haitian women between those same ages, had had first intercourse before age 18 (MEASURE DHS 2005–2006).
Overall, although recent trends in risky sexual behavior in Haiti are indicating some improvement, risky behavior remains pervasive as indicated by overall low levels of condom use. In addition, although HIV prevalence has declined in recent years, the epidemic remains generalized. Finally, these factors, combined with chronic impoverishment and environmental degradation, reviewed next, come together to justify our consideration of environmental scarcity, risky sexual behavior and rural livelihoods within this particularly vulnerable national setting.
Rural Livelihoods
The “Rural Livelihoods” framework has been successfully used to explore health behaviors (Rugalema 2000), food security (Bank 2005), and household diversification strategies (Yaro 2006). Central to the framework is the understanding that the relative availability of various “capital assets” shapes the livelihood options of rural households in developing countries. Capital assets include human capital (e.g., education), financial capital (e.g., income), physical capital (e.g., automobiles), social capital (e.g., kin networks), and natural capital (e.g., wild foods from communal lands). Of course, the assets’ relative availability is shaped by individual and household actions as well as broader socioeconomic-political structures and processes.
Household livelihood strategies may include the use of human capital (e.g., migration in search of employment [Collinson et al. 2006]) or natural capital (e.g., making reed-based craft products for market sale [Pereira, Shackleton, and Shackleton 2006]). Some livelihood strategies yield beneficial outcomes (such as food security) that improve household resilience and thus are self-reinforcing. Other livelihood strategies may have long-term negative impacts on resilience. For example, risky transactional sex may increase vulnerability to HIV infection, which in turn decreases human capital through illness, death, and the household’s loss of experience-driven techniques for using natural capital; depletes financial capital and often causes liquidation of physical capital resources to secure medical treatment; and is likely to impair social capital because of the disease’s stigma.
With regard to the socio-economic context of Haitian livelihoods, Haiti is the poorest country in the western hemisphere; in 2007 it ranked 149th out of 182 nations in the UNDP Human Development Index (UNDP 2008). Current life expectancy is 59 years for men and 63 years for women, with a per capita GDP of $1,070 USD (WHO 2008). Unemployment rates are presently around 70%, and only 1 in 50 Haitians has regular employment (Farmer et al. 2001). It is estimated that 70% of the population lives in chronic poverty, while 56% lives on less than $1 a day (World Food Program 2008). Only 40% of the population has access to basic health care (Cayemittes et al. 2001). The World Health Organization indicates that the 1990s, the decade examined in this paper, witnessed a worsening of poverty in Haiti, with rates of inflation hovering around 15% and food prices increasing by 10.4% (WHO 2008). Furthermore, Haiti experienced a decline in GDP growth, attributable to negative growth in the agricultural sector. This is particularly critical since agriculture remains the mainstay of the Haitian people’s livelihoods (Dolisca et al. 2008). Sixty-seven percent of the population relies primarily on agriculture for income, and daily food insecurity affects 40% of Haitian households (World Food Program 2008).
Within the midst of this environmental dependence, estimates suggest that only 1.5% of Haiti’s original forests remain (Roc 2008). Demographic pressures and rising food demand heightened forest clearing across Haiti both for food subsistence crops such as cassava, potatoes and cabbage, and to establish cash crops such as cotton and sugar (Dolsica et al. 2008). In addition, rural farmers have historically been unable to engage technological advances due to lack of rural infrastructure, including extension programs and credit facilities (Dolsica et al. 2008). This has resulted in further extensification and deforestation as additional land is required for production. A consistent demand for charcoal also puts pressure on Haitian landscapes, while nearly 15,000 hectares of cultivable land are lost annually to soil erosion (Verner 2008). In all, livelihood options in rural Haiti are few.
As noted by historians, however, land degradation is not solely the product of recent activity: “deforestation in Haiti in a long-established and … continuous process,” (Lewis and Coffey 1985:159). Scholars contend that at the time of Columbus’s arrival in 1492, approximately 80% of the island of Hispaniola, which comprises Haiti and the Dominican Republic, was heavily vegetated. Yet by the end of the nineteenth century, Haiti’s tropical rainforests had been “systematically destroyed … in order to operate the brickworks, kilns and tanneries, wood was used that was not replanted” (Michel 2005:70–71 quoted in Roc 2008:2). Deforestation pressures continued with American occupation in the early twentieth century (Lewis and Coffey 1985) and scholars now contend that land and livelihood pressures caused by environmental degradation, chiefly deforestation and soil erosion, are the largest threats to rural Haitians today (Parsons 2001 and Smucker et al. 2002).
On livelihood options, formal sector jobs are few and 90% of formal economy jobs in Haiti are located in Port-au-Prince (Verner 2008). Nonfarm sectors appear more vibrant in more populated areas – lack of human capital and infrastructure constrain opportunities in rural regions (Verner 2008). With regard to gender, a substantial gender-earnings gap exists in rural Haiti, with female workers earning less than their male peers (Verner 2008). This reflects, in part, the female educational disadvantage; twenty percent of women have never attended school (MEASURE DHS 2005–2006).
Land degradation in Haiti also has important implications for individual illness and broader demographic trends. As Donohoe (2003:573) aptly stated, “the greatest effects on the health of individuals and populations result from environmental degradation and social injustice.” In 1925, Haiti had 60% original forest cover, but since that time, more than 98% of the countryside has been deforested, currently leaving less than 3% of the natural forest intact (Haggerty 1989). Clearly this is problematic in a country where 71% of all energy consumed is derived from wood or charcoal (WHO 2008). Deforestation also increases soil erosion, and often results in declining water availability, additional negative impacts for the 70+% of Haiti’s population that relies on subsistence agriculture. As Myers (2002) argues, the large-scale degradation of environmental resources in Haiti has created intense livelihood pressures, since agriculture is fundamentally resource based. Indeed, Haitians have few alternatives to resource-based livelihoods, and the resource base itself is in tatters.
We contend that in combination, environmental degradation and the lack of alternatives to resource-based livelihoods may further disenfranchise women and push them toward dangerous alternative livelihood strategies, including risky sex. This is of direct relevance to HIV prevalence, since “HIV affects those households and areas that have the smallest degree of flexibility in respect to agriculture and other possibilities of making a living” (Hammarskjold 2003:11).
This association has been empirically supported in work addressing the migration of Haitian women to sugarcane plantations in the neighboring Dominican Republic (Brewer et al. 1998). In the Dominican Republic, social disruption fuelled by poverty and migration led to increased transactional sex for food and accommodation, particularly among the poorest female immigrants from Haiti. This project examines the potential for a similar association to play out in rural Haiti itself.
HIV/AIDS and Rural Livelihoods
That HIV/AIDS is affecting the viability of rural livelihoods across the world has been widely established in the academic literature (e.g., Edstrom and Samuels 2007; Hunter et al. 2008; Kaschula 2008; Murphy 2008; Murphy, Harvey, and Silvestre 2005). Loss of household labor quality and quantity decreases household income generation potential and, therefore, available financial capital. Lessened human capital also affects natural resource collection strategies (Hunter, Twine, and Johnson forthcoming). Land uses shift, and access to land may be altogether lost because of inheritance customs (Frank and Unruh 2008). Dependency ratios increase and experience-based knowledge is lost as prime age adults succumb to AIDS (Edstrom and Samuels 2007).
In particular, HIV/AIDS decreases food security. Kaschula (2008) documents higher levels of food insecurity among AIDS-impacted households as well as greater dietary dependence on wild foods. Hunter et al. (2007) also note important substitution effects, wherein rural households rely on their environmental surroundings more heavily once affected by HIV/AIDS. Noticeable shifts in farming strategies include greater use of kitchen gardens for both sustenance and income generation (Murphy 2008). While this is widely perceived as a form of coping, Rugalema (2000) argues that we might more productively label it a “struggling strategy.” Additionally, Mitka (2000 Additionally, Mitka (2001) and Tumushabe (2004) argue that HIV impairs rural livelihoods by compromising networks of social reciprocity, which had previously provided another coping mechanism for suffering households.
These impacts perpetuate a vicious poverty-AIDS cycle (Loewenson and Whiteside 2001; Masanjala 2007). De Waal and Whiteside (2003) argue that with this “new variant famine” we are witnessing the emergence of a new, permanently disenfranchised group, the AIDS-poor (de Waal and Whiteside 2003). The effects of HIV/AIDS upon the rural poor and food security are so significant that “the consequences of the epidemic affect all spheres of life. The human capital loss has a serious social and economic impact in all sectors, and at community and individual levels” (TACAIDS 2008).
As an example of the AIDS-livelihoods connection within Haiti, Farmer (1992) recounts a tale of rural Haitians forced from their agricultural land by hydroelectric dam development. The Haitians moved to safer ground, but could not continue farming for lack of arable land in the resettlement area. Lacking the sustainable rural livelihood strategies that had supported their families for generations, the relocated population became highly susceptible to HIV, which proceeded to decimate the community. More generally Farmer’s work demonstrates that, even though prevalence is higher in urban areas, it is important to examine the HIV/AIDS-poverty-livelihoods nexus within the context of rural Haiti.
Farmer’s example reveals another important point. Most of the AIDS-livelihoods literature is notably directional – existing studies focus almost exclusively on how HIV/AIDS undermines rural livelihoods through capital impacts, thereby increasing vulnerability to further shocks/stresses. But a reciprocal relationship is also possible. The lessening of rural livelihood options may predispose certain individuals to engage in risky sexual behavior as a livelihood strategy. The result may be HIV and further harm to livelihood – susceptibility yielding further susceptibility. Indeed, Loevinsohn and Gillespie (2003) go so far as to contend that HIV is endogenous to livelihood and agricultural systems – that such systems shape both the susceptibility to HIV and its consequences. We grant that HIV/AIDS cannot be simply described as a disease of the poor. The pandemic is also rooted more broadly in political, socioeconomic, and cultural contexts (Barnett and Whiteside 2002; Farmer 1999, 1992; Hunter 2007). Still, it remains true that …
…. poverty and inequality can drive those on the margin of destitution into risky livelihood and coping strategies that raise their likelihood of contracting HIV. (Masanjala 2007:1032)
Barnett and Whiteside (2002) contend that scholars must look not only at the effects of HIV/AIDS – the focus of most previous work on HIV/AIDS and rural livelihoods--but also at its social and economic causes. In other words, in what ways might livelihood strategies shape vulnerability to HIV infection? In this paper, we use changes in land use and coverage as a proxy for environmental deterioration and lessened access to natural capital and resource-based livelihood strategies, in order to examine how environmental degradation may be associated with HIV exposure and infection.
Specifically, we hypothesize that rural Haitian women in areas of declining natural capital, and those most intensely impoverished, will be relatively more likely to engage in risky sexual behavior net of the effects of other forms of capital assets, and net of HIV knowledge. The following sections present the methodological approach used in this study, the results, and a discussion.
Data & Methods
The 2000 Demographic and Health Survey (DHS) is a standardized survey addressing a wide breadth of sociodemographic information and health topics. Within those DHS settings where HIV-related data were collected, data are available on knowledge regarding HIV and prevention, knowledge source, and sexual history. A women’s module is also included addressing female autonomy, decision-making authority, freedom of movement, asset possession and control, and access to money. The 2000 Haiti DHS was administered to 10,159 fecund women, ages 15–49, in 9,595 households.
Sociodemographic Data
Dependent Variables
Our dependent variables measure risky sexual behavior in two ways: (1) number of partners, other than husband or long-term partner, in last 12 months, and (2) used a condom during last intercourse As we noted above, the DHS does not include data reflecting reason for sex outside of union. In addition, we chose not to focus upon the relationship of sexual partner to respondents (with focus on commercial sex work) since in the Haitian setting, not all those who engage in transactional sex would self-identify as engaging in commercial sex.
Independent Variables
Our two independent variables of primary interest reflect impoverishment and natural resource availability and change.
Poverty
Since poverty combined with natural resource scarcity forms the basis of our examination, we include a measure of socioeconomic status. As in other less economically developed settings, we make use of an asset index representing the sum of specific household possessions that reflect household economic well-being (e.g., Schellenberg et al. 2003). In Haiti, these include household electrification, and ownership of a radio, television, bicycle, refrigerator, car/truck, and/or motorcycle/scooter. We explore the importance of individual assets, while also making use of the overall index.
Natural Resource Availability and Change
Our measure of change in proximate natural resources available for rural livelihood strategies is the normalized difference vegetation index (NDVI), calculated from satellite remote sensing imagery obtained from the Landsat GeoCover Database (Global Land Cover Facility 2006). NDVI is a relative measure of biomass and is often used to show impacts of environmental change on vegetation health and abundance (Roerink et al. 2003; Wang, Rich, and Price 2003; Zhou et al. 2003). Vegetation indices such as NDVI exploit vegetation’s reflectance of near-infrared light and absorption of red. In healthy plant tissue, chlorophyll absorbs red light and mesophyll tissues scatter near-infrared light; the NDVI is the difference between the values in the red and near-infrared bands divided by the sum of these same values (Tucker 1979; Jensen, 2007). NDVI values approach +1 where there is actively growing green vegetation; senescent or dead vegetation values approach −1. Where there is no vegetation, NDVI values are nearly 0. These relationships make NDVI a particularly good measure of the indicators of environmental scarcity we expect to see in Haiti: bare ground and deforestation.
We analyzed images from the Geocover database that covered all the DHS respondent households at two timesteps, ca. 1990 and ca. 2000; nine tiled images were required at each timestep to cover essentially all of Haiti. The GeoCover database is a publicly available collection of selected Landsat images produced under a National Aeronautics and Space Administration (NASA) program to provide geodetically accurate, multitemporal, global imagery, with minimal cloud cover, to facilitate studies of land use and land cover change (Global Land Cover Facility 2006; Tucker et al. 2004). Pixel resolution of the Landsat data is 30 meters, which is adequate to show variation in land cover around our study households. To compare vegetation greenness across years, we needed to use images acquired as closely as possible to the same part of the growing season, minimizing annual changes in plant growth and solar illumination angle (Coppin et al. 2004). Haiti has distinct wet and dry seasons, so Geocover’s standard for minimal cloud cover meant that we could select images that were all acquired either during August through October, or in January. Where mismatches between months occurred from one decadal timestep to another, we tested inclusion of the month of acquisition as an independent variable within our regression models, finding little sensitivity in the results.
Our ca. 1990 and ca. 2000 GeoCover images were acquired, respectively, by Landsat’s Thematic Mapper and Enhanced Thematic Mapper satellite sensors. For the GeoCover database, NASA orthorectified the ca. 1990 images and then geometrically registered the ca. 2000 images to them, ensuring positional accuracy and elimination of distortion due to varying topography within each image and among images covering the same location at both timesteps. A further key preprocessing step involves radiometric and atmospheric normalization to minimize spatial and temporal variation within and among images due to light absorption and scattering by water vapor and aerosols, differences in sun illumination, and sensor calibration inconsistencies (Lu et al. 2004, Coppin et al. 2004; Song et al. 2001). Although the NDVI index reduces some of these effects, we conducted further atmospheric correction, using ENVI software to implement the Dark Object Subtraction method (Song et al. 2001; 233–234). Also using ENVI, we calculated NDVI for all our images and created masks to eliminate cloud and sea pixels from the analysis.
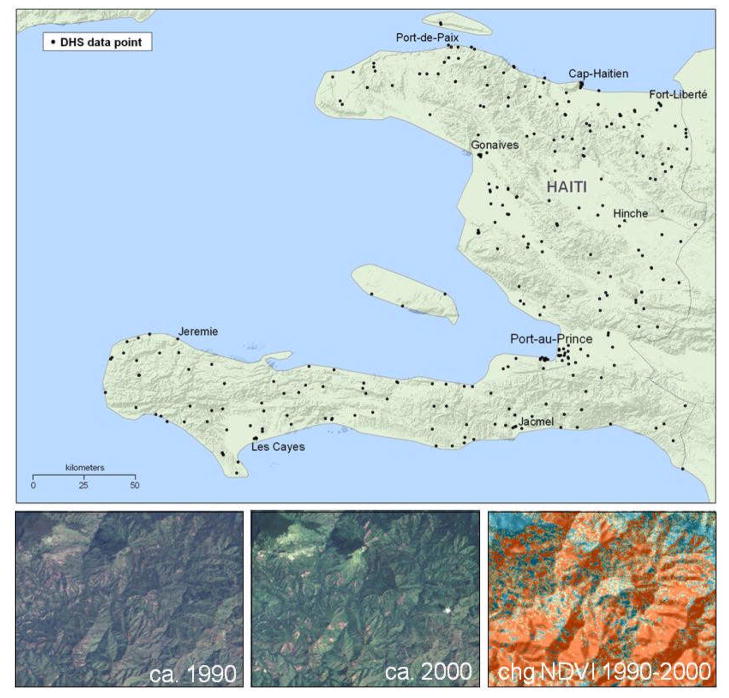
The DHS data do not provide geographic location of individual households; instead, local clusters of households are linked to the centroid of the small populated area in which they occur. For each of these 317 sample points, in ArcGIS 9.1 we located its geographic coordinates and created buffer zones in order to calculate mean NDVI at a range of distances surrounding each point. We generated a variety of buffers, circling each sample point at intervals from 500 meters to 15 kilometers. Within these buffers, we calculated three vegetation measures: (1) mean NDVI, 2000, (2) change in mean NDVI, 1990–2000, and (3) percentage change in mean NDVI, 1990–2000 (see Figure 1).2 After preliminary analyses and focused review of natural resource harvesting literature, we chose to use a 5-kilometer buffer (2.31 miles) within the subsequent analyses. The choice represents a trade-off; this distance is sufficiently small to reflect natural resource conditions relatively proximate to each study household, while also being sufficiently large to reflect broader regional environmental conditions and change.
Figure 1.
DHS “cluster” data points, illustrative satellige imagery ca. 1990 and ca. 2000, and change in NDVI 1990–2000.
Control Variables
Since risky sexual behavior may be influenced by many factors other than poverty and natural resource availability and change, we control for a variety of characteristics that have been shown to shape sexual behavior: awareness that condoms reduce the risk of AIDS transmission during sex; union status; age; education; and religion (Catholic).3
Methods
Following a descriptive overview of the DHS sample, we link our dependent variables reflecting risky sexual behavior to measures of proximate natural resource availability and change through simple bivariate correlations. We next engage logistic regression models to incorporate control variables and further isolate the potential role of diminished livelihood options resulting from natural resource decline and poverty within decision-making about risky sexual behavior.
Results
On the dependent variables, fewer than 5% of women reported condom use during their last intercourse. This low rate of condom use has been linked to religious beliefs, high levels of illiteracy, the economic value tied to children in agricultural settings, and the “plurality and looseness of unions” (Maynard-Tucker 1996:1379–1380). Although the majority of respondents (~79%) reported not having had sex outside their marriage or long-term partnership within the past year, approximately 22% had done so.
Poverty status and natural resource availability and change represent our primary predictor variables. The descriptive table clearly reveals the impoverishment of rural Haitians. Although the asset index ranges from 0 to 7, the mean value for SES is just 0.875, suggesting that, on average, rural households have fewer than one of the assets measured (household electrification, or ownership of a radio, television, bicycle, refrigerator, car/truck, and/or motorcycle/scooter).
Natural resource availability and change vary dramatically across respondent households. The mean NDVI varies from a minimum of 0.00 to a maximum of 0.83, with 0.56 the mean. The trend of deforestation is clear: mean change in NDVI, 1990–2000, ranges from a minimum of −0.23 to a maximum of only 0.1, with a mean of −0.06 suggesting loss on average. There is substantial variation in percentage change, with an average 9.41% vegetation loss during the decade, although some gain was also experienced (maximum 17.88%). The maximum loss was approximately 40%, suggesting that some households (N=8) lost nearly half their proximate vegetation coverage between 1990 and 2000.
On control variables, the data present a picture wherein nearly everyone is aware of HIV/AIDS, yet this does not translate into modified behavior or accurate knowledge. Specifically, although 97% of respondents were aware of the existence of HIV/AIDS (not shown), nearly one-third (32%) responded that they were unaware of any means of AIDS prevention. Further, more than half of the respondents either indicated that HIV/AIDS could be transmitted by supernatural means and sorcery or did not know that this means of transmission was not possible (not shown). Considered from a cultural perspective, this is understandable since Haiti is characterized by multiple belief systems, some of which focus on what we term the occult or voodoo. In the end, we chose to include one variable reflecting AIDS knowledge; our “know how to avoid AIDS” indicator is coded so that 0 represents lack of knowledge or incorrect knowledge (those expressing a belief that condoms do not protect against AIDS are coded zero, 51%). Those coded 1 for “know how to avoid AIDS” noted that condoms are a means of protection.
On other control variables, our sample is, on average, 25 years of age with only 3 years of education. Over half are married or living with a partner (53%), and nearly half are Catholic (49%).
Bivariate Associations
Table 2 presents bivariate relationships. Results reveal some statistically significant associations among risky sexual behaviors, poverty, and natural resource availability and change.
Table 2.
Bivariate Associations Among Risky Sexual Behavior, Poverty, and Natural Resource Availability and Change, Haiti Demographic and Health Survey, Rural Respondents, 2000
| Used a condom during last sex? Significant associations in bold |
Number of partners, other than husband or cohabiting partner, For respondents in union; Significant associations in bold |
|||||
|---|---|---|---|---|---|---|
| Primary Predictor Variables | Mean | Sig | N | Primary Predictor Variables | Mean | Sig |
| by SES1 | by SES1 | |||||
| yes | 2.19 | 0.00 | 1012 | 0 | 1.85 | 0.44 |
| no | 0.79 | 1+ | 1.77 | |||
| by NDVI, 20002 | by NDVI, 20002 | |||||
| yes | 55.18% | 0.18 | 1012 | 0 | 0.58 | 0.42 |
| no | 57.60% | 1+ | 0.59 | |||
| by change NDVI, 1990–20002 | by change NDVI, 1990–20002 | |||||
| yes | −0.06 | 0.57 | 1012 | 0 | −0.06 | 0.78 |
| no | −0.06 | 1+ | −0.06 | |||
| by % change NDVI, 1990–20002 | by % change NDVI, 1990–20002 | |||||
| yes | −9.67% | 0.83 | 1012 | 0 | −9.82% | 0.8 |
| no | −10.04% | 1+ | −9.57% | |||
| Control Variables | Control Variables | |||||
| by awareness of ways to avoid AIDS | by awareness of ways to avoid AIDS | |||||
| aware - no condom | 92.68% | 0.00 | 1013 | aware - extra-union partners | 21.23% | 0.32 |
| not aware - no condom | 98.13% | not aware - extra-union partners | 18.55% | |||
| Age (in years) | Age (in years) | |||||
| yes | 25.85 | 0.03 | 1002 | yes | 30.56 | 0.00 |
| no | 28.59 | no | 25.42 | |||
| Education (in years) | Education (in years) | |||||
| yes | 3.79 | 0.00 | 1012 | yes | 2.94 | 0.47 |
| no | 2.82 | no | 2.83 | |||
| With partner (married or cohabiting) | Catholic | |||||
| with partner - no condom | 96.27% | Catholic - extra-union partners | 22.05% | |||
| no partner - no condom | 89.74% | 0.00 | 1013 | Not Catholic - extra-union partners | 17.77% | 0.11 |
| Catholic | ||||||
| Catholic - no condom | 94.64% | |||||
| Not Catholic -- no condom | 95.93% | 0.33 | 1013 | |||
asset index: higher value = more assets, lower level of poverty
NDVI = “greenness” measure calculated from satellite imagery.
Women who did not use a condom during their last sexual encounter had, on average, far lower SES (0.79) than those who did (2.19). No significiant bivariate associations were exhibited between condom use and proximate natural resources.
As to engaging in sex with individuals other than husbands/cohabiting partners, very few significant associations were revealed; among control variables only age varies across respondents in relation to extra-union sexual encounters. Women with an extra-union sexual encounter within the past year tended to be older (~30 years) than those without such experience (~25 years, p<0.00). No significiant bivariate associations were exhibited between extra-union partners and either SES or natural resource availability or change.
Of course, given that the condition of proximate natural resources is often intimately tied to household well-being, it is logical to assume that correlation will exist between SES and proximate resource availability and change. Indeed, our measure of assets is correlated at −0.20 (p<0.00) with mean NDVI within a 5-km buffer (not shown). Further, the percentage change in NDVI, 1990–2000, is also significantly correlated with SES, with greater percentage change associated with lower asset scores (−0.04, p<0.01). As a result, we include an interaction term reflecting this association within the multivariate logistic regression models.
As to associations between control variables, awareness of ways to avoid AIDS is associated with greater condom use (p<0.00). Even so, about 93% of respondents who were aware of condoms’ preventive uses still did not use a condom at last sex. Women not using condoms tended to be older (approximately 29 years vs. 26 years, p<0.03) and also less educated (approximately 3 vs. 4 years of schooling, p<0.00). Women with extra-union partners tended to be older (approximately 26 vs. 24 years, p<0.00) and Catholic (21% had extra-union partners, p<0.01). In an effort to examine impacts of multicollinearity, we ran models in a variety of forms, and concluded that inclusion of all variables did not substantially alter conclusions on our key predictors.
Multivariate Models
Our logistic regression models estimate the log odds that a woman engaged in risky sexual behavior (i.e., no condom, or at least one extra-union partner for those in unions) as a function of poverty and proximate natural resource availability and change, net of control variables including age, education, partnership status, religion, and awareness of ways to avoid AIDS. Some factors significantly predict risky sex.
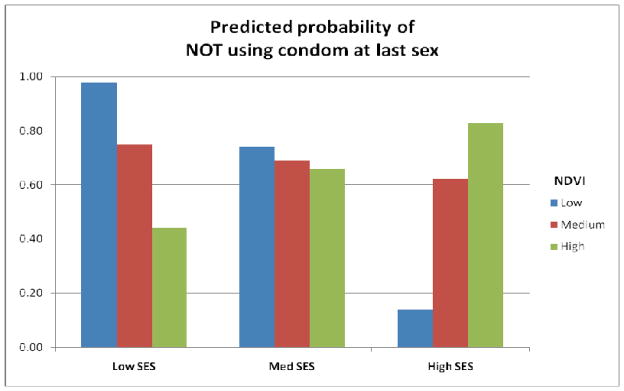
With regard to lack of condom use, women in areas with greater vegetation coverage were far less likely to engage in such risky sex than women in less vegetated areas (b = −9.56), who may have been more vulnerable because they lacked proximate natural capital. Women in households with more assets (i.e., higher SES) also exhibited lesser propensity to engage in sex without a condom (b = −2.87). Yet the interaction between these variables also exhibits statistical significance, with a positive estimate. The coefficient of 4.57 suggests that at higher levels of SES and vegetation coverage, the association changes. In this way, the data suggest that the negative effect of SES and lack of vegetation exists only for those most vulnerable, as is evidenced in the predicted probabilities displayed in Figure 2. In this graph, assets range from 0 to 3 (coded as 1 to 4 for SES), and NDVI ranges from 0.00 to 0.83. As the graph shows, women in particularly low SES households exhibit the highest probability of engaging in risky sexual behavior when local vegetation coverage is especially sparse. Yet the opposite is true for women with higher socioeconomic standing – suggesting that it may be those most impoverished who are particularly vulnerable to the negative impacts of low levels of proximate natural capital.
Figure 2.
Interestingly, the measures of vegetation change are not importantly associated with lack of condom use. However, the estimates suggest the importance of several controls. In particular, more educated women are more likely to regularly use condoms, as are women with husbands or live-in partners. As one would expect, a lack of awareness of how to avoid AIDS also predicts less condom use.
On our other dependent variable, extra-union sexual partners among women in unions, we find no association with either SES or proximate vegetation coverage/change. Most important in this case are two control variables. In particular, older women are less likely to have extra-union encounters, and women who are Catholics exhibit slightly higher probability of engaging in such encounters.
Discussion
We examined the possibility that livelihood vulnerability due to environmental degradation in rural Haiti may drive women to engage in risky sexual behaviors, as has been documented in the fish-for-sex trade in Kenya and Tanzania. Acknowledging cultural distinctions across these study settings, like many rural regions of Africa, the livelihoods of many rural Haitians centrally integrate the “natural capital” available through local environmental resources. In these socio-environmental spaces, environmental change has immediate and direct impacts on the health and well-being of millions of households (Koziell and Saunders 2001). We model the association between sexual behaviors among women of reproductive age in Haiti and measures of poverty and proximate resource availability and change. Our data are drawn from the Demographic and Health Survey, 2000, with a focus on rural women, combined with vegetation measures generated from satellite imagery. In all, the work has contributed in two ways: (1) its focus on Haiti, a setting outside sub-Saharan Africa but also impacted by HIV/AIDS, and (2) its focus on livelihood decline as a potential driver of HIV/AIDS vulnerability through risky sexual behaviors.
Our results suggest that impoverished women in particularly deforested settings are most likely to have engaged in sex without condoms at their last sexual encounter – net of age, education, union status, religion, or knowledge about ways to avoid AIDS. On further exploration, we find that 172 DHS respondents were in both the most impoverished category and the lowest quartile of vegetation cover in 2000. The respondents geographically overrepresented Haiti’s northwestern and Arbonite departments, the two least vegetated of Haiti’s rural regions. Importantly, women of higher socioeconomic status do not exhibit the same vulnerability to lack of condom use even within similarly deforested settings. In this way, the results suggest that sustainable livelihood strategies, particularly those that do not put the individual’s health in tremendous peril, are not equally available.
Understanding the dynamics of condom use in Haiti is important partly because the HIV/AIDS pandemic is impairing rural livelihoods there, as in so many other developing regions. By eroding social networks and social capital (Pronyk et al. 2008, Gaede et al. 2006) and undermining subsistence strategies and food security (e.g., Kaschula 2008, Murphy 2008, Hunter et al. 2007), the pandemic is undermining livelihoods already vulnerable. The DHS data confirm that rural Haitians possess very few livelihood assets and, therefore, few livelihood options. Indeed, as the aftermath of Hurricane Gustav and the tragic earthquake of January 2010 have recently demonstrated, the vulnerability of Haitians is extreme.
Our motivating concern is the potential for the “environmental scarcity-risky sex linkage” to operate in areas where HIV prevalence is already high. Importantly, we found that natural resource availability and decline were associated with lack of condom use though not with extra-union sexual partnerships. In this respect, our results are suggestive although not definitive. Even so, they do point to the multi-faceted nature of livelihoods and the critical need for integrated development initiatives that bring health education and services, combined with environmental and conservation initiatives as well as diversified livelihood options to rural regions. In this way, improvements may be made within the variety of capitals necessary to craft sustainable livelihoods. Certainly, endangering one’s health (e.g. human capital) through risky sexual activity as a result of poverty (e.g. lack of financial capital) and lack of natural resource based livelihood options (e.g. natural capital scarcity) only shifts burdens resulting in unsustainable and unhealthy choices. Livelihood choices are integrated as should be livelihood interventions.
In addition, we recommend additional research more explicitly focused on transactional sexual partnerships in settings with high HIV prevalence. We also encourage researchers to consider the environmental context of these partnerships to better understand the processes fueling the pandemic, especially in regions where households are highly resource-dependent. It is our hope that the substantive results presented here will spur additional consideration of this linkage by researchers, policy makers, and practitioners in order to improve both social and environmental well-being in rural regions hard-hit by the HIV/AIDS pandemic.
Table 1.
Descriptive Profiles of Incorporated Variables
Haiti Demographic and Health Survey, Rural Respondents, 2000
| Dependent Variables | Yes | No | Don’t Know | N |
|---|---|---|---|---|
| Used a condom during last sex? | 4.52% | 95.48% | 0.25% | 1013 |
| # partners, other than husband, in last 12 months (for those in unions) | 896 | |||
| 0 | 77.80% | |||
| 1+ | 22.20% | |||
| Primary Predictor Variables | Mean | Min | Max | |
| Assets | 0.875 | 0 | 7 | 1707 |
| Within 5k buffer, NDVI=vegetation coverage | ||||
| Mean NDVI, 2000 | 0.56 | 0.00 | 0.83 | 1707 |
| Change Mean NDVI, 1990–2000 | −0.06 | −0.23 | 0.10 | 1707 |
| Percentage Change Mean NDVI, 1990–2000 | −9.41 | −39.91 | 17.88 | 1707 |
| Control Variables | ||||
| Aware of ways to avoid AIDS? | 50.91% | 49.09% | 1707 | |
| Age (in years) | 25.52 | 15 | 49 | 1697 |
| Education (in years) | 2.98 | 0 | 7 | 1707 |
| With partner (married or cohabiting) | 52.55% | 1707 | ||
| Catholic | 48.68% | 1707 | ||
Sampling weights used
Table 3.
Logistic Regression Estimates Predicting Associations Among Risky Sexual Behavior, Poverty, and Natural Resource Availability and Change, Haiti Demographic and Health Survey, Rural Respondents, 2000
| Primary Predictor Variables | Did NOT use condom during last sex | Sexual partner(s) other than husband/partner in past 12 months | ||||
|---|---|---|---|---|---|---|
| Within 5k buffer, NDVI=vegetation coverage | ||||||
| Mean NDVI, 2000 | −9.56 ** | 1.86 | ||||
| Change Mean NDVI, 1990–2000 | −6.46 | 1.16 | ||||
| Percentage Change Mean NDVI, 1990–2000 | −0.04 | 0.01 | ||||
| Assets | −2.87 *** | −0.42 ** | −0.38 * | 0.40 | −0.05 | −0.06 |
| Assets * Vegetation Measure | 4.57 ** | 1.87 | 0.01 | −0.78 | −0.43 | 0.00 |
| Control Variables | ||||||
| Age (in years) | −0.05 | 0.04 | 0.04 | −0.10 *** | −0.10 *** | −0.10 *** |
| Education (in years) | −0.19 * | −0.19 * | −0.20 * | −0.01 | −0.01 | −0.01 |
| With partner (married or cohabiting) | 0.75 * | 0.72 | 0.72 * | |||
| Catholic | −0.34 | −0.33 | −0.34 | 0.45 ** | 0.46 ** | 0.46 ** |
| Aware of ways to avoid AIDS? | −1.05 ** | −0.96 * | −1.01 * | 0.26 | −0.05 | −0.06 |
| Constant | 9.27 *** | 3.81 *** | 3.79 *** | −0.01 | 1.12 ** | 1.13 ** |
| Pseudo R2 | 0.21 | 0.18 | 0.19 | 0.08 | 0.08 | 0.08 |
| N | 1003 | 1003 | 1003 | 897 | 897 | 897 |
Footnotes
While there is more recent DHS survey data for Haiti, from 2005–2006, we chose to use data from 2000 because we were able to obtain high quality satellite imagery data for 1990 and 2000. A useful follow-on project would examine this association mid-decade.
Correlation also exists between the various environmental measures. As would be anticipated, higher levels of proximate vegetation at baseline (1990) are associated with greater change across the period 1990–2000 (0.63, p<0.00) as well as greater percentage change (0.69, p<0.00).
Religiosity has been linked to lower risk levels for HIV in several studies including Zaleski and Schiaffino (2000), Elifson, Klein and Sterk (2003) and Lehrere at al (2006).
Contributor Information
Lori M. Hunter, Department of Sociology, Institute of Behavioral Science, CU Population Center, Population, Environment and Society Programs, University of Colorado at Boulder
John Reid-Hresko, Department of Sociology, University of Colorado at Boulder.
Tom Dickinson, Institute of Behavioral Science, Computing and Research Services, Department of Geography, University of Colorado at Boulder.
References
- Bank L. On family farms and commodity groups: Rural livelihoods, households and development policy in the Eastern Cape. Social Dynamics. 2005;31(1):157–181. [Google Scholar]
- Barnett T, Whiteside A. AIDS in the twenty-first century: Disease and globalization. New York: Palgrave Macmillan; 2002. [Google Scholar]
- Béné C, Merten S. Women and fish-for-sex: Transactional sex, HIV/AIDS and gender in African fisheries. World Development. 2009;36(5):875–899. [Google Scholar]
- Castro A, Farmer P. Understanding and addressing AIDS-related stigma: From anthropological theory to clinical practice in Haiti. American Journal of Public Health. 2005;95(1):53–59. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayemittes M, Placide MF, Barrère B, Mariko S, Sévère B. Enquête mortalité, morbidité et utilisation des services, Haïti 2000. Calverton, MD: Ministère de la Santé Publique et de la Population, Institut Haïtien de l’Enfance et ORC Macro; 2001. [Google Scholar]
- Collinson MA, Tollman SM, Kahn K, Clark SJ, Garenne M. Highly prevalent circular migration: households, mobility and economic status in rural South Africa. In: Tienda M, Findley SE, Tollman SM, Preston-Whyte E, editors. Africans on the Move: Migration in Comparative Perspective. Wits University Press; Johannesburg, South Africa: 2006. [Google Scholar]
- Coppin P, Jonckheere I, Nackaerts K, Muys B, Lambin E. Digital change detection methods in ecosystem monitoring: a review. International Journal of Remote Sensing. 2004;25(9):1565–1596. [Google Scholar]
- Deschamps M, Pape JW, Hafner A, Johnson W. Annals of Internal Medicine. 4. Vol. 125. 1996. Heterosexual transmission of HIV in Haiti; pp. 324–330. [DOI] [PubMed] [Google Scholar]
- De Waal A, Tumushabe J. HIV/AIDS and food security in Africa. [Accessed 10 Sept 2009];A Report for DFID, draft. 2003 Available via http://www.sarpn.org.za/documents/d0000235/P227_AIDS_Food_Security.pdf.
- De Waal A, Whiteside A. New variant famine: AIDS and food crisis in Southern Africa. Lancet. 2003;362(9391):1234–1237. doi: 10.1016/S0140-6736(03)14548-5. [DOI] [PubMed] [Google Scholar]
- Dolisca F, McDaniel JM, Shannon DA, Jolly CM. Modeling farm households for estimating the efficiency of policy instruments on sustainable land use in Haiti. Land Use Policy. 2008;26:130–138. [Google Scholar]
- Donohoe M. Causes and health consequences of environmental degradation and social injustice. Social Science and Medicine. 2003;56:573–587. doi: 10.1016/s0277-9536(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Dunkle K, Jewkes R, Brown H, Gray G, McIntryre J, Harlow S. Transactional sex among women in Soweto, South Africa: Prevalence, risk factors, and associations with HIV infection. Social Science and Medicine. 2004;59(8):1581–1592. doi: 10.1016/j.socscimed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Edstrom J, Samuels F. A report for DFID, draft. 2007. HIV, nutrition, food and livelihoods in Sub-Saharan Africa: Evidence, debates and reflections for guidance. [Google Scholar]
- Elifson K, Klein H, Sterk C. Religiosity and HIV Risk Behavior Involvement Among “At Risk” Women. Journal of Religion and Health. 2003;42(1):47–66. [Google Scholar]
- Farmer PE. Infections and inequalities: The modern plagues. Berkeley: University of California Press; 1999. [Google Scholar]
- Farmer PE. Ethnography, social analysis, and the prevention of sexually transmitted infections among poor women in Haiti. In: Inhorn M, Brown P, editors. The anthropology of infectious disease. New York: Gordon and Breach; 1997. pp. 413–438. [Google Scholar]
- Farmer PE. Culture, poverty, and the dynamics of HIV transmission in rural Haiti. In: Brummelhuis HT, Herdt G, editors. Culture and sexual risk: Anthropological perspectives on AIDS. New York: Gordon and Breach; 1995. pp. 3–28. [Google Scholar]
- Farmer PE. AIDS and accusation: Haiti and the geography of blame. Berkeley: University of California Press; 1992. [Google Scholar]
- Farmer PE, Léandre F, Mukherjee J, Sidonise Claude M, Nevil P, Smith-Fawzi M, Koenig S, Castro A, Becerra M, Sachs J, Attaran A, Yong Kim J. Community-based approaches to HIV treatment in resource-poor settings. Lancet. 2001;358:404–409. doi: 10.1016/s0140-6736(01)05550-7. [DOI] [PubMed] [Google Scholar]
- Frank E, Unruh J. Demarcating forest, containing disease: Land and HIV/AIDS in southern Zambia. Population and Environment. 2008;29(3):108–132. [Google Scholar]
- Faubert C. Human security: Evaluation of UNDP assistance to conflict-affected areas –case study Haiti. New York: Evaluation Office, United Nations Development Programme; 2006. [Google Scholar]
- Gaede BM, Majeke SJ, Modeste RRM, Naidoo JR, Titus MJ, Uys LR. Social support and health behavior in women living with HIV in Kwa-Zulu Natal. Sahara J: Journal of Social Aspects of HIV/AIDS. 2006;3(1):362–368. doi: 10.1080/17290376.2006.9724862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard EM, Boulos L-M, André Cayemittes MP, Eustache L, Van Onacker JD, Duval N, Louissaint E, Thimoté G. Understanding the reasons for decline of HIV prevalence in Haiti. Sexually Transmitted Infections. 2006 April;82(Suppl 1):i14–i20. doi: 10.1136/sti.2005.018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Rambaut A, Wlasiuk G, Spira T, Pitchenik A, Worobey M. The emergence of HIV/AIDS in the Americas and beyond. PNAS. 2007;104(47):18566–18570. doi: 10.1073/pnas.0705329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Land Cover Facility. Landsat GeoCover Database. University of Maryland; [accessed Nov 3, 2006]. http://glcfapp.umiacs.umd.edu/ [Google Scholar]
- Gordon A. HIV/AIDS in the fisheries sector in Africa. Cairo, Egypt: World Fish Center; 2005. [Google Scholar]
- Haggerty R. Haiti: A country study. Washington, DC: GPO for the Library of Congress; 1989. [Google Scholar]
- Haiti Information Bureau. Neoliberalism in Haiti: The case of rice. Haiti Info. 1995;3(24):41–48. [Google Scholar]
- Hammarskjold M. Environment Policy Division Report. Swedish International Development Agency; 2003. The environment, natural resources, and HIV/AIDS. Available via www.sida.se/publications. Cited 21 Jan 2010. [Google Scholar]
- Hempstone H, Diop-Sidibe N, Ahanda KS, Lauredent E, Heerey M. A Report for USAID. Washington DC: USAID; 2004. HIV/AIDS in Haiti: A literature review. [Google Scholar]
- Hunter LM, De Souza R, Twine W. The environmental dimensions of the HIV/AIDS pandemic: A call for scholarship and evidence-based intervention. Population and Environment. 2008;29(3):103–107. doi: 10.1007/s11111-008-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LM, Twine W, Johnson A. Adult mortality and natural resource use in rural South Africa: Evidence from the Agincourt Health and Demographic Surveillance Site. University of Colorado, Institute of Behavioral Science; 2007. Working Paper EB2005–0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LM, Twine W, Patterson L. ‘Locusts Are Now Our Beef’: Adult Mortality and Household Dietary Use of Local Environmental Resources. Scandinavian Journal of Public Health, Vol. 2007;25(Suppl 69):165–174. doi: 10.1080/14034950701356385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M. The changing political economy of sex in South Africa: The significance of unemployment and inequalities to the scale of the AIDS pandemic. Social Science and Medicine. 2007;64(3):689–700. doi: 10.1016/j.socscimed.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Hunter M. The materiality of everyday sex: Thinking beyond “prostitution”. African Studies. 2002;61(1):99–120. [Google Scholar]
- Jensen JR. Remote sensing of the environment : an earth resource perspective. 2. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. [Google Scholar]
- Kaschula SA. Wild foods and household food security responses to AIDS: Evidence from South Africa. Population and Environment. 2008;29(3):162–185. [Google Scholar]
- Kerrigan D, Moreno L, Rosario S, Gomez B, Jerez H, Barrington C, Weiss E, Sweat M. Environmental-structural interventions to reduce HIV/STI risk among female sex workers in the Dominican Republic. American Journal of Public Health. 2006 Jan;96(1):120–5. doi: 10.2105/AJPH.2004.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc-Madlala S. Transactional sex and the pursuit of modernity. Social Dynamics. 2004;29(2):1–21. [Google Scholar]
- Lehrer J, Shrier L, Gortmaker S, Buka S. Depressive Symptoms as a Longitudinal Predictor of Sexual Risk Behaviors Among US Middle and High School Students. Pediatrics. 2006;118(1):189–200. doi: 10.1542/peds.2005-1320. [DOI] [PubMed] [Google Scholar]
- Loewenson R, Whiteside A. United Nations Development Program Policy Paper. New York: UNDP; 2001. HIV/AIDS: Implications for poverty reduction. [Google Scholar]
- Loevinsohn M, Gillespie S. Food Consumption and Nutrition Division Discussion Paper 157. Washington, DC: IFPRI; 2003. HIV/AIDS, food security and rural livelihoods: Understanding and responding. [Google Scholar]
- Lu D, Mausel P, Brondizio E, Moran E. Change detection techniques. International Journal of Remote Sensing. 2004;25(12):2365–2407. [Google Scholar]
- Masanjala W. The poverty-HIV/AIDS nexus in Africa: A livelihood approach. Social Science and Medicine. 2007;64(5):1032–1041. doi: 10.1016/j.socscimed.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Maynard-Tucker G. Haiti: Unions, Fertility, and the quest for Survival. Social Science & Medicince. 1996;43(9):1379–1387. doi: 10.1016/0277-9536(96)00034-2. [DOI] [PubMed] [Google Scholar]
- MEASURE DHS. EMMUS-IV 2005–2006. Institut Haitien de l’Enfance (IHE); Petion-Ville, Haiti: 2005–2006. HIV/AIDS in Haiti: Key Findings of the Mortality, Morbidity, and Utilization of Services Survey. [Google Scholar]
- Merten S, Haller T. Culture, changing livelihoods, and HIV/AIDS discourse: Reframing the institutionalization of fish-for-sex exchange in the Zambian Kafue Flats. Culture, Health, & Sexuality. 2007;9(1):69–83. doi: 10.1080/13691050600965968. [DOI] [PubMed] [Google Scholar]
- Mojola S. Fishing in Dangerous Waters: Ecology, Gender and Economy in HIV Risk. Institute of Behavioral Science, CU Population Center; 2010. [Accessed January 2010]. Working Paper Series. POP2009-12. http://www.colorado.edu/ibs/pop/pubs/wp.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtika M. The AIDS epidemic in Malawi and its threat to household food security. Human Organization. 2001;60(2):178–188. [Google Scholar]
- Mtika M. Social and cultural relations in economic action: The embeddedness of food security in rural Malawi amidst the AIDS epidemic. Environment and Planning A. 2000;32:345–360. [Google Scholar]
- Murphy L. AIDS and kitchen gardens: Insights from a village in western Kenya. Population and Environment. 2008;29(3):133–161. [Google Scholar]
- Murphy L, Harvey P, Silvestre E. How do we know what we know about the impact of AIDS on food and livelihood insecurity: A review of empirical research from rural Sub Saharan Africa. Human Organization. 2005;64(3):265–275. [Google Scholar]
- Myers N. Environmental refugees: A growing phenomenon of the 21st century. Philospohical Transactions of the Royal Society B. 2002;357:609–613. doi: 10.1098/rstb.2001.0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyefara JL. Food insecurity, HIV/AIDS pandemic and sexual behavior of female commercial sex workers in Lagos Metropolis, Nigeria. Sahara J: Journal of Social Aspects of HIV/AIDS. 2007;4(2):626–635. doi: 10.1080/17290376.2007.9724884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan American Health Organization (PAHO) Countries. II. WHO; Washington DC: 2007. Health in the Americas, 2007. [Google Scholar]
- Pereira T, Shackleton CM, Shackleton S. Trade in reed-based craft products in rural villages in the Eastern Cape, South Africa. Development Southern Africa. 2006;23(4):477–495. [Google Scholar]
- Pimental D, Cooperstein S, Randell H, Filiberto D, Sorrentino S, Kaye B, Nicklin C, Yagi J, Brian J, O’Hern J, Habas A, Weinstein C. Ecology of increasing diseases: Population growth and environmental degradation. Human Ecology. 2007;35:653–668. doi: 10.1007/s10745-007-9128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D, Tort M, D’Anna L, Krawic A, Berger J, Rossman J, Mugo F, Doon N, Shriberg M, Howard ES, Lee S, Talbot J. Ecology of increasing disease: Population growth and environmental degradation. BioScience. 1998;48:817–826. [Google Scholar]
- Piot P, Bartos M, Ghys P, Walker N, Schwartlander B. The global impact of HIV/AIDS. Nature. 2001;410:968–973. doi: 10.1038/35073639. [DOI] [PubMed] [Google Scholar]
- Pronyk PM, Harpham T, Morison L, Hargreaves JR, Kim J, Phetla G, Watts C, Porter JD. Is social capital associated with HIV risk in rural South Africa? Social Science and Medicine. 2008;66(9):1999–2010. doi: 10.1016/j.socscimed.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Putnam E, Deriveua J, Zalduondo B, Genece E, Mercier P, Soliman C, Timyan J. USAID support for sexually transmitted infections and HIV/AIDS programming in Haiti. Washington, DC: The Synergy Project; 2001. [Google Scholar]
- Roc N. Report commissioned by Fundacin para las Relaciones Internacionales y el Di logo Exterior. Madrid, Spain: 2008. Haiti-Environment: from the “Pearl of the Antilles” to desolation. Available via http://www.chilehaiti.cl/docs/roc.pdf. Cited 1 Dec 2009. [Google Scholar]
- Rugalema G. Coping or struggling? A journey into the impact of HIV/AIDS in southern Africa. Review of African Political Economy. 2000;27:537–545. [Google Scholar]
- Samuels F, Spraos H. HIV and Emergencies: Haiti Country Case Study. Overseas Developing Institute; London, UK: 2008. [Google Scholar]
- Schellenberg JA, Victoria CG, Mushi A, de Savigny D, Schellenberg D, Mshinda H. Inequities among the very poor: health care for children in rural southern Tanzania. The Lancet. 2003;361:561–566. doi: 10.1016/S0140-6736(03)12515-9. [DOI] [PubMed] [Google Scholar]
- Shreffler K, Nii-Amoo Dodoo F. The role of intergenerational transfers, land, and education in fertility transition in rural Kenya: The case of Nyeri district. Population and Environment. 2009;30(3):75–92. [Google Scholar]
- Smith Fawzi MC, Lambert W, Singler JM, Tanagho Y, Leandre F, Nevil P, Bertrand D, Claude MS, Louissaint M, Jeannis L, Mukherjee JS, Goldie S, Salazar JJ, Farmer PE. Factors associated with forced sex among women accessing health services in rural Haiti: Implications for the prevention of HIV infection and other sexually transmitted diseases. Social Science and Medicine. 2005;60(4):679–689. doi: 10.1016/j.socscimed.2004.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Woodcock CE, Seto KC, Lenney MP, Macomber SA. Classification and change detection using Landsat TM data: When and how to correct atmospheric effects? Remote Sensing of Environment. 2001;75:230–244. [Google Scholar]
- Stillwaggon E. AIDS and the ecology of poverty. Oxford: Oxford University Press; 2006. [Google Scholar]
- Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and Macro International Inc. Tanzania HIV/AIDS and malaria indicator survey 2007–2008. Dar Es Salaam, Tanzania: TACAIDS, ZAC, NBS, OCGS, and Macro International Inc; 2008. [Google Scholar]
- Tladi LS. Poverty and HIV/AIDS in South Africa: An empirical contribution. Sahara J: Journal of Social Aspects of HIV/AIDS. 2006;3(1):369–381. doi: 10.1080/17290376.2006.9724863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torell E, Kalangahe B, Thaxton M, Issa A, Pieroth V, Fahmy O, Tobey J. Guidelines for mitigating the impact of HIV/AIDS on coastal biodiversity and natural resource management. Washington, DC: Population Reference Bureau; 2007. [Google Scholar]
- Tucker CJ. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sensing of Environment. 1979;8:127–150. [Google Scholar]
- Tucker CJ, Grant DM, Dykstra JD. NASA’s global orthorectified landsat data set. Photogrammetric Engineering and Remote Sensing. 2004;70(3):313–322. [Google Scholar]
- Tumushabe J. HIV/AIDS and rural livelihoods: What do we know? Impacts and mitigation strategies. Paper presented at CHGA Interactive on Rural Livelihoods and Food Security/Nutrition in the Context of HIV/AIDS in ESAR; Addis Ababa. 2004. [Google Scholar]
- UNAIDS. AIDS epidemic update, December 2009. Geneva: UNAIDS; 2009. Available via http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. Cited 11 Jan 2010. [Google Scholar]
- UNAIDS. Haiti: Country situation. 2008a July; Available via http://data.unaids.org/pub/FactSheet/2008/sa08_hai_en.pdf. Cited 11 Jan 2010.
- UNAIDS. Epidemiological fact sheet for HIV/AIDS Haiti 2008. Geneva: 2008b. Available via ( http://www.unaids.org/en/CountryResponses/Countries/haiti.asp). Cited 19 Dec 2008. [Google Scholar]
- UNAIDS. Report on the global HIV/AIDS epidemic 2008. Geneva: UNAIDS; 2008c. [Google Scholar]
- UNDP. Human Development Report 2009. Haiti: 2008. http://hdrstats.undp.org/en/countries/country_fact_sheets/cty_fs_HTI.html Cited 7 Feb 2010. [Google Scholar]
- UNDP. Human development index report. New York: United Nations Development Program; 2007. [Google Scholar]
- Verner Dorte. Policy Research Working Paper 4574, The World Bank, Social Development, Sustainable Development Division. The World Bank; New York, NY: 2008. Mar, Labor Markets in Rural and Urban Haiti: Based on the First Household Survey for Haiti. [Google Scholar]
- Wang J, Rich PM, Price KP. Temporal responses of NDVI to precipitation and temperature in the central Great Plains, USA. International Journal of Remote Sensing. 2003;24(11):2345–2364. [Google Scholar]
- World Food Program. Haiti overview. 2008 Available via http://www.wfp.org/country_brief/indexcountry.asp?country=332#Facts%20&%20Figures. Cited 23 Dec 2008.
- World Health Organization. World health statistics – Haiti. 2008 Available via http://www.who.int/countries/hti/en/. Cited 27 Jan 2010.
- Yaro JA. Is deagrarianisation real? A study of livelihood activities in rural northern Ghana. The Journal of Modern African Studies. 2006;44(1):125–156. [Google Scholar]
- Zaleski E, Schiaffino K. Religiosity and sexual risk-taking behavior during the transition to college. Journal of Adolescence. 2000;23(2):223–227. doi: 10.1006/jado.2000.0309. [DOI] [PubMed] [Google Scholar]
- Zhou L, Kaufmann RK, Tian Y, Myneni RB, Tucker CJ. Relation between interannual variations in satellite measures of northern forest greenness and climate between 1982 and 1999. Journal of Geophysical Resesarch. 2003;108(D1):3-2–3-11. [Google Scholar]