Abstract
Background
The National Kidney Foundation has recommended that the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation replace the Modification of Diet in Renal Disease (MDRD) Study equation. Before implementing this change in the Kidney Early Evaluation Program (KEEP), we compared characteristics of reclassified individuals and mortality risk predictions using the new equation.
Methods
Of 123,704 eligible KEEP participants, 116,321 with data available for this analysis were included. Glomerular filtration rate (GFR) was estimated using the MDRD Study (eGFRMDRD) and CKD-EPI (eGFRCKD-EPI) equations with creatinine level calibrated to standardized methods. Participants were characterized by eGFR category: >120, 90-119, 60-89, 45-59, 30-44, and <30 mL/min/1.73 m2. Clinical characteristics ascertained included age, race, sex, diabetes, hypertension, coronary artery disease, congestive heart failure, cerebrovascular disease, peripheral vascular disease, and anemia. Mortality was determined over a median of 3.7 years of follow-up.
Results
The prevalence of eGFRCKD-EPI <60 mL/min/1.73 m2 was 14.3% compared with 16.8% using eGFRMDRD. Using eGFRCKD-EPI, 20,355 participants (17.5%) were reclassified to higher eGFR categories, and 3,107 (2.7%), to lower categories. Participants reclassified upward were younger and less likely to have chronic conditions, with a lower risk of mortality. A total of 3,601 deaths (3.1%) were reported. Compared with participants classified to eGFR of 45-59 mL/min/1.73 m2 using both equations, those with eGFRCKD-EPI of 60-89 mL/min/1.73 m2 had a lower mortality incidence rate (6.4 [95% CI, 5.1-7.7] vs 18.5 [95% CI, 17.1-19.9]). Results were similar for all eGFR categories. Net reclassification improvement was 0.159 (P < 0.001).
Conclusions
The CKD-EPI equation reclassifies people at lower risk of CKD and death into higher eGFR categories, suggesting more accurate categorization. The CKD-EPI equation will be used to report eGFR in KEEP.
Keywords: Chronic kidney disease, glomerular filtration rate estimation, mortality, risk factors
Glomerular filtration rate (GFR) is the best overall index of kidney function. Decreased GFR is associated with increased risk of complications related to kidney disease, including uremic manifestations of kidney disease, acute kidney injury, kidney failure, and cardiovascular disease. GFR also is important for making many clinical decisions, including listing for kidney transplant, medication dose adjustment, and avoidance of toxic medications. GFR most often is assessed using estimating equations derived from serum levels of endogenous filtration markers, the most common being creatinine.
The most commonly used estimating equation is the Modification of Diet in Renal Disease (MDRD) Study equation, developed from 1,628 people with chronic kidney disease (CKD) with a mean measured GFR of 40 mL/min/1.73 m2.1 It has been shown to be valid in similar populations, but to underestimate measured GFR at the higher range,2 around 60 mL/ min/1.73 m2, leading to misclassification to a lower category and thus overdiagnosis of CKD. The CKD Epidemiology Collaboration (CKD-EPI) equation was developed in 8,254 people and validated in a separate data set of 3,896.3 Both the development and validation data sets included people with and without kidney disease and a wide range of GFRs, with mean measured GFR of 68 mL/min/1.73 m2. The CKD-EPI equation has been shown to be a better estimate of measured GFR than the MDRD Study equation, particularly at higher levels.
Approximately 80% of clinical laboratories currently report estimated GFR (eGFR) when serum creatinine is measured.4 Most laboratories now use the MDRD Study equation.5 The National Kidney Foundation (NKF) has recommended that the CKD-EPI equation replace the MDRD Study equation in calculating eGFRs reported by clinical laboratories and in clinical practice, analogous to a software upgrade.3,6 The basis for this recommendation is that the new equation provides a more accurate estimate of GFR, especially at higher levels, and results in a decreased false-positive rate for the identification of CKD. In addition, because the CKD-EPI equation uses the same 4 variables as the MDRD Study equation, its use does not require that additional variables be collected by clinical laboratories.
The Kidney Early Evaluation Program (KEEP) is a free community-based health screening program that targets populations 18 years and older at high risk of kidney disease, defined as a history of diabetes or hypertension or first-order relative with diabetes, hypertension, or kidney disease.7 The goal of KEEP is to screen for CKD in people at high risk of it. Thus, GFR estimates that are accurate in the higher range are particularly important for detecting incipient CKD in this population. As part of the NKF strategy to implement the CKD-EPI equation, a decision was made to use it to report eGFR in the KEEP population. Before implementing this change in KEEP, we sought to evaluate the impact of the new equation in the KEEP data set. In this study, we compare the 2 equations regarding the characteristics of patients identified with CKD and patients who died in this large cohort of people at high risk of CKD. We hypothesized that people with CKD classified using the CKD-EPI equation would be more likely to have risk factors for CKD and a higher risk of mortality.
METHODS
Study Participants
We included 123,704 eligible KEEP participants, August 2000 through December 31, 2009, from 48 NKF affiliates and 2,634 screening programs in 50 states and the District of Columbia. We excluded participants with missing CKD data, leaving a study population of 116,321.
GFR Estimation
GFR was estimated using the 4-variable MDRD Study equation8 (eGFRMDRD) and the CKD-EPI equation3 (eGFRCKD-EPI):
where SCr is serum creatinine, κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum of SCr/κ or 1, and max indicates the maximum of SCr/κ or 1. Participants of race other than African American were considered as not African American for calculation of eGFR. Serum creatinine values were calibrated to standardized serum creatinine levels at the Cleveland Clinic Research Laboratory.5,9 GFR was categorized as >120, 90-119, 60-89, 45-59, 30-44, and <30 mL/min/1.73 m2. The categories are based on NKF Kidney Disease Outcomes Quality Initiative (KDOQI) CKD stages, but modified to allow for a category >120 mL/min/1.73 m2 because of are recognized J-shaped relationship between eGFR using the MDRD Study equation and risk, splitting the category 30-59 mL/min/1.73 m2 into 2 categories, as recently suggested,10,11 and combining CKD stages 4 and 5 given the small number of people in these categories.
Definitions of CKD Risk Factors and Comorbid Conditions
Diabetes, hypertension, and older age are the primary risk factors for CKD identified in KEEP. Diabetes was defined as history of diabetes (self-report or retinopathy) or use of medications to treat diabetes. Hypertension was defined as history of hypertension (self-report) or use of medications to treat hypertension.12 Coronary artery disease, congestive heart failure, stroke, and peripheral vascular disease were ascertained using self-report. Hemoglobin was measured for all participants, and anemia was defined using the World Health Organization definition; hemoglobin <13 g/dL for men and <12 g/dL for women.13
Ascertainment of Mortality
KEEP obtains informed consent from individual KEEP participants to use Social Security Number, first name, last name, and birth date in potential linkages for future research studies. All-cause mortality data in this study were ascertained by linking the KEEP study cohort to the first-quarter 2010 Social Security Administration Death Master File. All KEEP study participants were followed up through December 31, 2009, a median of 3.7 years of follow-up.
Statistical Analysis
Classification into eGFR categories was determined using both the MDRD Study and CKD-EPI equations for the overall study population and by CKD risk factors. Most analyses were descriptive, and χ2 tests were used to compare CKD prevalence by risk factors by CKD status. Clinical characteristics of KEEP participants from 2000-2009 by eGFR categories according to the CKD-EPI equation are reported using frequencies and percentages. Prevalence of disease is reported by eGFR categories according to both the MDRD Study and CKD-EPI equations. Mortality expressed as deaths per 1,000 patient-years and confidence intervals (CIs) was calculated using eGFR categories. In calculating mortality, KEEP participants were followed up from the screening date to December 31, 2009, and censored at date of death. The standard error for CIs was calculated as the square root of number of deaths divided by total follow-up time in each category and expressed per 1,000 patient-years. To determine changes in participant characteristics and mortality within eGFR categories from one equation to the other, clinical characteristics and mortality were evaluated according to eGFR classification using each equation. For mortal-ity, the net reclassification index was calculated14 as the sum of the proportion of participants reclassified downward to a lower eGFR category for people who died and the proportion of participants reclassified upward to a higher eGFR category for people who did not die minus the sum of the proportion of participants reclassified upward for people who died and the proportion of participants reclassified downward for people who did not die.
RESULTS
The median value for eGFRCKD-EPI was higher than for eGFRMDRD (85.5 [interquartile range, 31] vs 79.2 [interquartile range, 43] mL/min/1.73 m2). Participants in lower eGFR categories determined using eGFRCKD-EPI were more likely to be older, male, and white and have higher blood pressure than participants in higher categories (Table 1). They also were more likely to be anemic and have chronic conditions, such as diabetes, hypertension, coronary artery disease, congestive heart failure, and vascular disease. Similar results were observed for eGFRMDRD (Table S1, provided as online supplementary material).
Table 1.
Clinical Characteristics of KEEP Participants, 2000-2009, by eGFR Categories Defined Using the CKD-EPI Equation
| CKD-EPI eGFR (mL/min/1.73 m2) |
|||||||
|---|---|---|---|---|---|---|---|
| No. of Participants |
≥120 | 90-119 | 60-89 | 45-59 | 30-44 | <30 | |
| No. (row percent) | 116,321 | 6,827 (5.9) | 42,469 (36.5) | 50,412 (43.3) | 11,261 (9.7) | 4,234 (3.6) | 1,118 (1.0) |
| Age | |||||||
| 18-30 y | 8,263 | 3,214 (47.1) | 3,897 (9.2) | 1,077 (2.1) | 47 (0.4) | 15 (0.4) | 13 (1.2) |
| 31-45 y | 23,165 | 2,656 (38.9) | 13,660 (32.2) | 6,343 (12.6) | 390 (3.5) | 63 (1.5) | 53 (4.7) |
| 46-60 y | 40,633 | 935 (13.7) | 17,945 (42.2) | 18,751 (37.2) | 2,308 (20.5) | 522 (12.3) | 172 (15.4) |
| 61-75 y | 33,156 | 20 (0.3) | 6,678 (15.7) | 18,920 (37.5) | 5,282 (46.9) | 1,837 (43.4) | 419 (37.5) |
| >75 y | 11,104 | 2 (0.0) | 289 (0.7) | 5,321 (10.6) | 3,234 (28.7) | 1,797 (42.4) | 461 (41.2) |
| Sex | |||||||
| Men | 37,160 | 1,446 (21.2) | 13,125 (30.9) | 17,290 (34.3) | 3,592 (31.9) | 1,283 (30.3) | 424 (37.9) |
| Women | 79,161 | 5,381 (78.8) | 29,344 (69.1) | 33,122 (65.7) | 7,669 (68.1) | 2,951 (69.7) | 694 (62.1) |
| Race | |||||||
| White | 57,355 | 1,323 (19.4) | 17,677 (41.6) | 27,813 (55.2) | 7,130 (63.3) | 2,770 (65.4) | 642 (57.4) |
| African American | 38,521 | 4,080 (59.8) | 14,886 (35.1) | 15,378 (30.5) | 2,878 (25.6) | 999 (23.6) | 300 (26.8) |
| Other | 20,445 | 1,424 (20.9) | 9,906 (23.3) | 7,221 (14.3) | 1,253 (11.1) | 465 (11.0) | 176 (15.7) |
| Self-reported conditionsa | |||||||
| Diabetes | 34,283 | 1,151 (17.0) | 11,017 (26.1) | 15,187 (30.3) | 4,341 (38.7) | 2,015 (47.7) | 572 (51.4) |
| Hypertension | 65,340 | 1,937 (28.6) | 19,187 (45.6) | 30,699 (61.3) | 8,773 (78.4) | 3,719 (88.3) | 1,025 (92.1) |
| Coronary artery disease | 10,104 | 155 (2.3) | 2,075 (4.9) | 4,766 (9.5) | 1,855 (16.5) | 972 (23.0) | 281 (25.1) |
| Congestive heart failure | 2455 | 60 (1.6) | 571 (2.3) | 1,088 (3.5) | 396 (5.8) | 245 (9.6) | 95 (14.9) |
| Cerebrovascular disease | 5,643 | 125 (1.9) | 1,294 (3.2) | 2,655 (5.6) | 903 (8.5) | 500 (12.6) | 166 (15.7) |
| Peripheral vascular disease | 4,644 | 185 (2.7) | 1,389 (3.3) | 2,005 (4.0) | 629 (5.6) | 318 (7.5) | 118 (10.6) |
| WHO anemiab | 13,839 | 1,245 (18.6) | 4,153 (9.9) | 4,620 (9.3) | 1,820 (16.4) | 1,350 (32.4) | 651 (59.5) |
Note: Values are number (column percent) unless otherwise indicated. Conversion factor for eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667.
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; KEEP, Kidney Early Evaluation Program; WHO, World Health Organization.
Diabetes includes self-reported diabetes and using medication; hypertension includes self-reported hypertension and using medication; coronary artery disease includes history of heart attack, coronary artery bypass graft, angioplasty, and coronary artery disease; congestive heart failure data available starting 2005; cerebrovascular disease is defined as stroke; peripheral vascular disease includes peripheral vascular disease for 2000-2004 and limb amputation for 2005-2009.
Defined as hemoglobin level <13 g/dL for men and <12 g/dL for women.
Using eGFRCKD-EPI, the overall prevalence of eGFR <60 mL/min/1.73 m2 was 14.3% compared with 16.8% using eGFRMDRD. Using eGFRCKD-EPI, 23,462 participants (20.1%) were reclassified to a different eGFR category; 20,355 (17.5%) were reclassified to higher, and 3,107 (2.7%), to lower categories. Of 14,075 participants with eGFRMDRD of 45-59 mL/min/1.73 m2, 3,438 (24.4%) were reclassified to 60-89 mL/min/1.73 m2 and would not have been defined as having CKD in the absence of a concomitant marker of kidney damage (ie, albuminuria).
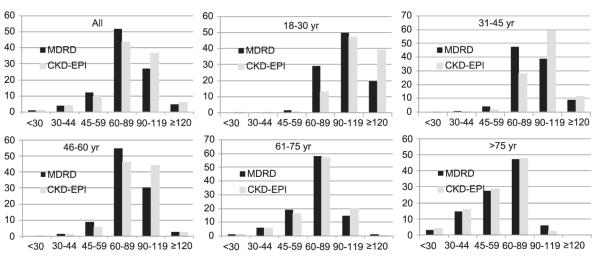
Overall, participants reclassified to higher eGFR categories were more likely to be younger, female, and African American than participants not reclassified (Table S2, provided as online supplementary material). Figure 1 shows changes in distributions of eGFR categories overall and by age. Participants who were reclassified upward also were less likely to have chronic conditions (Table 2). For example, compared with participants classified as eGFR of 45-59 mL/min/ 1.73 m2 using both equations, those reclassified as eGFRCKD-EPI of 60-89 mL/min/1.73 m2 were less likely to have diabetes (38.7% vs 29.3%), hypertension (78.3% vs 60.3%), coronary artery disease (16.5% vs 8.0%), congestive heart failure (5.7% vs 4.4%), cerebrovascular disease (8.4% vs 5.1%), peripheral vascular disease (5.5% vs 5.3%), and anemia (15.7% vs 8.1%). In contrast, participants reclassified as eGFRCKD-EPI of 30-44 mL/min/1.73 m2 were more likely to have diabetes (38.7% vs 43.2%), hypertension (78.3% vs 87.4%), coronary artery disease (16.5% vs 16.8%), congestive heart failure (5.7% vs 6.2%), cerebrovascular disease (8.4% vs 12.9%), peripheral vascular disease (5.5% vs 7.9%), and anemia (15.7% vs 35.8%). The pattern was similar for all comorbid conditions for other eGFR categories, including >120 mL/min/1.73 m2. The pattern for anemia was similar for eGFR category of 60-89 mL/min/1.73 m2, but for the category 90-119 mL/min/1.73 m2, the prevalence of anemia increased for participants reclassified using eGFR to >120 mL/min/1.73 m2 CKD-EPI (16.7% vs 10.5%).
Figure 1.
Distribution of estimated glomerular filtration rate categories determined using the Modification of Diet in Renal Disease (MDRD) Study and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations by age category.
Table 2.
Characteristics of KEEP Participants by eGFR Categories Defined Using the MDRD Study and CKD-EPI Equations
| CKD-EPI eGFR (mL/min/1.73 m2) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDRD Study eGFR (mL/min/ 1.73 m2) |
No. of Participants (N = 116,321) |
≥120 (n = 6,827) |
90-119 (n = 42,469) |
60-89 (n = 50,412) |
45-59 (n = 11,261) |
30-44 (n = 4,234) |
<30 (n = 1,118) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| ≥120 | ||||||||||||||
| No. | 5,392 | 3,897 | 1,489 | 6 | ||||||||||
| DMa | 1,329 | 24.9 | 752 | 19.5 | 574 | 39.0 | 3 | 50.0 | ||||||
| HTNa | 1,926 | 36.1 | 1,124 | 29.1 | 798 | 54.2 | 4 | 66.7 | ||||||
| CAD | 175 | 3.3 | 89 | 2.3 | 86 | 5.8 | 0 | 0.0 | ||||||
| CHFa | 63 | 2.2 | 35 | 1.7 | 28 | 3.5 | 0 | 0.0 | ||||||
| CVDa | 155 | 3.0 | 89 | 2.4 | 66 | 4.7 | 0 | 0.0 | ||||||
| PVD | 199 | 3.7 | 120 | 3.1 | 79 | 5.3 | 0 | 0.0 | ||||||
| Anemiab | 944 | 17.8 | 766 | 19.9 | 178 | 12.2 | 0 | 0.0 | ||||||
| 90-119 | ||||||||||||||
| No. | 31,080 | 2,930 | 27,464 | 686 | ||||||||||
| DMa | 8,118 | 26.3 | 399 | 13.7 | 7,455 | 27.3 | 264 | 38.8 | ||||||
| HTNa | 14,078 | 45.7 | 813 | 27.8 | 12,787 | 47.0 | 478 | 69.9 | ||||||
| CAD | 1,593 | 5.1 | 66 | 2.3 | 1,403 | 5.1 | 124 | 18.1 | ||||||
| CHFa | 418 | 2.3 | 25 | 1.5 | 370 | 2.3 | 23 | 4.9 | ||||||
| CVDa | 950 | 3.2 | 36 | 1.3 | 856 | 3.3 | 58 | 9.0 | ||||||
| PVD | 1,055 | 3.4 | 65 | 2.2 | 968 | 3.5 | 22 | 3.2 | ||||||
| Anemiab | 3,392 | 11.1 | 479 | 16.7 | 2,844 | 10.5 | 69 | 10.2 | ||||||
| 60-89 | ||||||||||||||
| No. | 60,303 | 13,516 | 46,282 | 505 | ||||||||||
| DMa | 17,093 | 28.5 | 2,988 | 22.2 | 13,919 | 30.2 | 186 | 37.0 | ||||||
| HTNa | 34,174 | 57.1 | 5,602 | 41.8 | 28,156 | 61.3 | 416 | 83.0 | ||||||
| CAD | 5,042 | 8.4 | 586 | 4.3 | 4,366 | 9.4 | 90 | 17.8 | ||||||
| CHFa | 1,170 | 3.2 | 173 | 2.1 | 982 | 3.5 | 15 | 5.6 | ||||||
| CVDa | 2,858 | 5.0 | 372 | 2.9 | 2,430 | 5.5 | 56 | 11.8 | ||||||
| PVD | 2,182 | 3.6 | 342 | 2.5 | 1,802 | 3.9 | 38 | 7.5 | ||||||
| Anemiab | 5,539 | 9.3 | 1,131 | 8.5 | 4,275 | 9.4 | 133 | 26.6 | ||||||
| 45-59 | ||||||||||||||
| No. | 14,075 | 3,438 | 10,333 | 304 | ||||||||||
| DMa | 5,110 | 36.5 | 1,001 | 29.3 | 3,978 | 38.7 | 131 | 43.2 | ||||||
| HTNa | 10,367 | 74.1 | 2,061 | 60.3 | 8,042 | 78.3 | 264 | 87.4 | ||||||
| CAD | 2,030 | 14.4 | 276 | 8.0 | 1,703 | 16.5 | 51 | 16.8 | ||||||
| CHFa | 454 | 5.4 | 83 | 4.4 | 360 | 5.7 | 11 | 6.2 | ||||||
| CVDa | 1,018 | 7.7 | 167 | 5.1 | 814 | 8.4 | 37 | 12.9 | ||||||
| PVD | 776 | 5.5 | 181 | 5.3 | 571 | 5.5 | 24 | 7.9 | ||||||
| Anemiab | 1,984 | 14.3 | 276 | 8.1 | 1,602 | 15.7 | 106 | 35.8 | ||||||
| 30-44 | ||||||||||||||
| No. | 4,422 | 423 | 3,882 | 117 | ||||||||||
| DMa | 2,093 | 47.5 | 177 | 42.6 | 1,862 | 48.1 | 54 | 46.2 | ||||||
| HTNa | 3,836 | 87.2 | 315 | 75.2 | 3,415 | 88.5 | 106 | 90.6 | ||||||
| CAD | 1,009 | 22.8 | 62 | 14.7 | 914 | 23.5 | 33 | 28.2 | ||||||
| CHFa | 263 | 9.8 | 21 | 8.3 | 231 | 9.8 | 11 | 15.7 | ||||||
| CVDa | 506 | 12.3 | 33 | 8.3 | 457 | 12.6 | 16 | 14.8 | ||||||
| PVD | 316 | 7.2 | 20 | 4.7 | 289 | 7.4 | 7 | 6.0 | ||||||
| Anemiab | 1,370 | 31.5 | 85 | 20.5 | 1,222 | 32.0 | 63 | 54.3 | ||||||
| <30 | ||||||||||||||
| No. | 1,049 | 48 | 1,001 | |||||||||||
| DMa | 540 | 51.7 | 22 | 45.8 | 518 | 520 | ||||||||
| HTNa | 959 | 91.9 | 40 | 83.3 | 919 | 92.3 | ||||||||
| CAD | 255 | 24.3 | 7 | 14.6 | 248 | 24.8 | ||||||||
| CHFa | 87 | 14.6 | 3 | 10.0 | 84 | 14.8 | ||||||||
| CVDa | 156 | 15.6 | 6 | 13.0 | 150 | 15.7 | ||||||||
| PVD | 116 | 11.1 | 5 | 10.4 | 111 | 11.1 | ||||||||
| Anemiab | 610 | 59.5 | 22 | 45.8 | 588 | 60.1 | ||||||||
Note: All numbers are total; values are missing in each category. Conversion factor for eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667.
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, cerebrovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; KEEP, Kidney Early Evaluation Program; MDRD, Modification of Diet in Renal Disease; PVD, peripheral vascular disease.
DM includes self-reported DM and using medication; HTN includes self-reported HTN and using medication; CAD includes history of heart attack, coronary artery bypass graft, angioplasty, and CAD; CHF data available starting 2005; CVD is defined as stroke; PVD includes PVD for 2000-2004 and limb amputation for 2005-2009.
Defined as hemoglobin level <13 g/dL for men and <12 g/dL for women.
Participants reclassified to higher eGFR categories had the lowest incidence rate for mortality compared with those reclassified to lower categories or not reclassified (3.1 [95% CI, 2.7-5.3] vs 23.2 [95% CI, 20.3-26.6] vs 8.96 [95% CI, 8.6-11.1]). Table 3 lists incidence rates for mortality for each eGFR category for both equations. The incidence rate for all-cause mortality for participants classified as eGFR of 45-59 mL/min/1.73 m2 using both equations was 18.5 (95% CI, 17.1-19.9). The mortality incidence rate was lower at 6.4 (95% CI, 5.1-7.7) for participants with eGFRCKD-EPI of 60-89 mL/min/1.73 m2 and higher at 47.6 (95% CI, 34.2-60.9) for participants with eGFRCKD-EPI of 30-44 mL/min/1.73 m2.
Table 3.
Cross-tabulation of Mortality Incidence Rates According to Classification to eGFR Categories by the MDRD Study and CKD-EPI Equations
| CKD-EPI eGFR (mL/min/1.73 m2) |
|||||||
|---|---|---|---|---|---|---|---|
| MDRD Study eGFR (mL/min/1.73 m2) |
Total | ≥120 | 90-119 | 60-89 | 45-59 | 30-44 | <30 |
| ≥120 | |||||||
| No. | 5,392 | 3,897 | 1,489 | 6 | 0 | 0 | 0 |
| Death | 81 | 32 | 49 | 0 | |||
| Death/1,000 patient-years | 3.6 | 1.9 | 8.2 | ||||
| 95% CI | 2.6-4.6 | 1.2-2.6 | 5.9-10.5 | ||||
| 90-119 | |||||||
| No. | 31,080 | 2,930 | 27,464 | 686 | 0 | 0 | 0 |
| Death | 470 | 15 | 400 | 55 | |||
| Death/1,000 patient-years | 3.9 | 1.3 | 3.8 | 25.6 | |||
| 95% CI | 1.3-6.5 | 0.7-1.9 | 3.4-4.2 | 18.8-32.4 | |||
| 60-89 | |||||||
| No. | 60,303 | 0 | 13,516 | 46,282 | 505 | 0 | 0 |
| Death | 1,517 | 119 | 1,320 | 78 | |||
| Death/1,000 patient-years | 6.9 | 2.4 | 7.8 | 42.4 | |||
| 95% CI | 3.5-10.3 | 2.0-2.8 | 7.4-8.2 | 33.0-51.8 | |||
| 45-59 | |||||||
| No. | 14,075 | 0 | 0 | 3,438 | 10,333 | 304 | 0 |
| Death | 823 | 88 | 686 | 49 | |||
| Death/1,000 patient-years | 15.9 | 6.4 | 18.5 | 47.6 | |||
| 95% CI | 10.6-21.3 | 5.1-7.7 | 17.1-19.9 | 34.2-60.9 | |||
| 30-44 | |||||||
| No. | 4,422 | 0 | 0 | 0 | 423 | 3,882 | 117 |
| Death | 488 | 15 | 441 | 32 | |||
| Death/1,000 patient-years | 32.3 | 9.8 | 33.3 | 90.9 | |||
| 95% CI | 19.1-45.5 | 4.8-14.8 | 30.2-36.4 | 59.4-122.4 | |||
| <30 | |||||||
| No. | 1,049 | 0 | 0 | 0 | 0 | 48 | 1,001 |
| Death | 222 | 5 | 217 | ||||
| Death/1,000 patient-years | 62.7 | 30.1 | 64.3 | ||||
| 95% CI | 45.2-80.2 | 3.7-56.5 | 55.8-72.8 | ||||
| Total | |||||||
| No. | 116,321 | 6,827 | 42,469 | 50,412 | 11,261 | 4,234 | 1,118 |
| Death | 3,601 | 47 | 568 | 1,463 | 779 | 495 | 249 |
| Death/1,000 patient-years | 8.3 | 1.6 | 3.5 | 7.9 | 19.3 | 34.3 | 66.8 |
| 95% CI | 1.7-14.9 | 1.0-2.2 | 2.5-4.5 | 5.8-10.0 | 14.0-24.6 | 20.0-48.6 | 46.8-86.8 |
Note: Values expressed as crude incidence rate per 1,000 person-years (95% CI). Conversion factor for eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667.
Abbreviations: CI, confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
The net reclassification index for improvement in risk of mortality was calculated (Table 4). Of 3,601 participants who died, 242 (6.7%) were incorrectly reclassified to a higher GFR category using eGFRCKD-EPI. In contrast, of 112,720 participants who did not die, 20,113 (17.8%) were correctly reclassified to a higher eGFR category, for an overall net reclassification index of 0.159 (P < 0.001). The index varied by subgroup; for all subgroups except age, net reclassification index values ranged from 0.101-0.188 (P for all < 0.001). The net reclassification index was −0.010 (P = 0.05) for participants younger than 45 years, 0.049 (P = −0.003) for those aged 45-60 years, and 0.078 (P < 0.001) for those older than 60 years.
Table 4.
Net Reclassification Index
| CKD-EPI eGFR (mL/min/1.73 m2) |
|||||||
|---|---|---|---|---|---|---|---|
| MDRD Study eGFR (mL/min/1.73 m2) |
≥120 | 90-119 | 60-89 | 45-59 | 30-44 | <30 | Total No. |
| People Who Died (n = 3,601) | |||||||
| ≥120 | 32 | 49 | 0 | 81 | |||
| 90-119 | 15 | 400 | 55 | 470 | |||
| 60-89 | 119 | 1,320 | 78 | 1,517 | |||
| 45-59 | 88 | 686 | 49 | 823 | |||
| 30-44 | 15 | 441 | 32 | 488 | |||
| <30 | 5 | 217 | 222 | ||||
| People Who Did Not Die (n = 112,720) | |||||||
| ≥120 | 3,865 | 1,440 | 6 | 0 | 0 | 0 | 5,311 |
| 90-119 | 2915 | 27,064 | 631 | 0 | 0 | 0 | 30,610 |
| 60-89 | 0 | 13,397 | 44,962 | 427 | 0 | 0 | 58,786 |
| 45-59 | 0 | 0 | 3350 | 9,647 | 255 | 0 | 13,252 |
| 30-44 | 0 | 0 | 0 | 408 | 3,441 | 85 | 3934 |
| <30 | 0 | 0 | 0 | 0 | 43 | 784 | 827 |
Note: Net reclassification improvement was calculated as follows: clinically correct reclassification [proportion of participants reclassified upward who did not die: (200,113/112,720) + proportion of participants classified downward who died (263/3601)] − clinically incorrect reclassification [proportion of participants reclassified upward who died: (1,242/3,601) + proportion of participants classified downward who did not die (2,844/112,720)]. Conversion factor for eGFR in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667.
Abbreviations: CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease.
DISCUSSION
GFR is used in many clinical settings. In KEEP, it is used to identify people with CKD and assess CKD severity. In this study, we show that compared with the MDRD Study equation, use of the CKD-EPI equation resulted in a lower prevalence of eGFR <60 mL/min/1.73 m2 and more participants classified to higher eGFR categories. Participants who were reclassified to higher categories using eGFRCKD-EPI were less likely to have CKD risk factors or comorbid conditions and were at lower risk of death compared with those who were classified to similar categories using both equations or reclassified to lower categories.
The 2 primary changes in the formulation of the CKD-EPI equation are use of a spline for serum creatinine level, which enables better identification of the differing relationships between creatinine level and GFR throughout the range of measured GFRs, and use of a linear instead of a logarithmic term for age.3 The linear term for age leads to a steeper decrease in eGFR with age, such that people older than 70 years have a lower eGFRCKD-EPI than eGFRMDRD. These differences result in higher eGFRs for a given creatinine level compared with the MDRD Study equation for most people younger than ~75 years. The selective reclassification of people with CKD risk factors and comorbid conditions does not directly result from the formulation of the equation because these variables are not specifically included in the equation and likely reflects the association of age with these factors.
Recent studies in the general population compared the 2 equations with respect to CKD prevalence and mortality risk.3,15,16 The CKD-EPI equation leads to a lower estimated prevalence of CKD in the National Health and Nutrition Examination Survey (NHANES), 11.1% compared with 13.2% using the MDRD Study equation. In particular, people at lower risk of the development and progression of CKD, such as women, younger people, and whites, were more likely to be reclassified to higher GFR categories.3 Analyses from the AusDiab16 (Australian Diabetes, Obesity and Lifestyle) Study showed that people reclassified to higher eGFR categories had lower cardiovascular disease risk profiles and lower risk of the development of cardiovascular disease. A study of participants in the Atherosclerosis Risk in Communities (ARIC) Study showed that the CKD-EPI equation led to reclassification of ~45% of participants to higher GFR categories.15 For those reclassified, risk was lower for mortality, end-stage renal disease, coronary heart disease, and stroke in eGFR categories <120 mL/min/1.73 m2. Our results extend the findings to a population at high risk of the development and progression of CKD and show that in this population, the CKD-EPI equation better categorizes people by eGFR consistent with their pre-dicted risk of comorbid conditions commonly associated with CKD and of mortality.
In KEEP, people reclassified as eGFRCKD-EPI of 90-119 mL/min/1.73 m2 had higher rates of CKD risk factors and comorbid conditions and lower risk of death compared with people classified as >120 mL/ min/1.73 m2 using both equations. This is in contrast to studies that have shown that creatinine-based equations result in a J-shaped curve in the relationship between GFR and adverse outcomes,17,18 such that people classified as >120 mL/min/1.73 m2 have a higher risk of death than people classified as 90-119 mL/min/1.73 m2. Consistent with these observations, in theARIC analyses, people reclassified as eGFRCKD-EPI of 90-119 from >120 mL/min/1.73 m2 had a lower rate of adverse events.15 The likely explanation for the difference between the KEEP population and prior analyses relates to differences in characteristics of the populations. Possibly, people with eGFR >120 mL/ min/1.73 m2 who are at high risk of adverse outcomes are too frail to participate in detection programs.
These findings have implications for KEEP, and similar implications would be expected for the general clinical population. Using the CKD-EPI equation, the prevalence of eGFR <60 mL/min/1.73 m2 decreased by 20%. A major criticism of the CKD paradigm and use of the MDRD Study equation is that the underestimate of measured GFR using the MDRD Study equation leads to false-positive diagnoses, with subsequent anxiety imposed on people and excessive testing with consequent cost to the health care system.19 These concerns are highly relevant for a detection program such as KEEP. KEEP sends letters to participants’ physicians to verify positive results; thus, eGFR <60 mL/min/1.73 m2 would result in further potentially unnecessary testing. In addition, the KEEP laboratory tests for abnormalities of mineral metabolism and other CKD complications only in people with eGFR <60 mL/min/1.73 m2. Thus, identification of fewer people will lead directly to cost savings for the program. Similar decisions and behaviors would occur for physicians caring for individual patients. The selective reclassification of high- versus low-risk groups suggests that GFR estimates using the CKD-EPI equation will enable better prognostication of patients’ clinical courses.
Despite these improvements, the CKD-EPI equation is still based on serum creatinine level, allowing only a small improvement in precision compared with the MDRD Study equation. Additional markers may be required to further improve the precision of GFR estimates. At present, for patients at the extremes of muscle mass and diet or for whom highly accurate values are required for clinical decision making, confirmatory tests using clearance of exogenous markers or measured creatinine clearance are necessary.20
The strength of this analysis is the large well-characterized cohort of people at risk of CKD. Limitations include use of only 1 serum creatinine measurement, preventing verification of CKD chronicity. However, KEEP bases its recommendations on 1 test, and this does not detract from the comparison of the equations. Second, comorbid conditions are defined using only self-report, leading to possible error in assignment of these conditions. Third, we were unable to evaluate complications of CKD other than anemia, such as hyperphosphatemia or hyperparathyroidism, because these were measured for only participants with eGFRMDRD <60 mL/min/1.73 m2 and therefore were not ascertained uniformly for all participants.
In conclusion, the CKD-EPI equation resulted in reclassification to higher eGFR categories; participants reclassified to higher categories were less likely to have CKD risk factors or comorbid conditions and had a lower rate of death. More accurate identification of CKD is a major goal of a detection program and KEEP therefore will begin reporting eGFR using the CKD-EPI equation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Shane Nygaard, BA, and Nan Booth, MSW, MPH, ELS, of the Chronic Disease Group for manuscript preparation and editing, respectively.
Support: This study was supported by grant K23-DK081017, Kidney Function and Aging, from the National Institute of Diabetes and Digestive and Kidney Diseases. The KEEP is a program of the NKF Inc and is supported by Amgen, Abbott, Siemens, Astellas, Fresenius Medical Care, Genzyme, LifeScan, Nephroceuticals, and Pfizer. Dr Stevens receives grant support from Gilead Inc.
Footnotes
Financial Disclosure: Dr Norris has consulted with Amgen, King Pharmaceuticals, and Abbott. Dr Whaley-Connell receives support from the Veteran’s Affairs Career Development Award-2.
The remaining authors declare that they have no relevant financial interests.
SUPPLEMENTARY MATERIALS Note: The supplementary material accompanying this article (doi:10.1053/j.ajkd.2010.11.007) is available at www.ajkd.org.
REFERENCES
- 1.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the Modification of Diet in Renal Disease Study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.College of American Pathologists [Accessed August 17, 2010];Current status of reporting eGFR. 2010 http://www.cap.org/apps/docs/committees/chemistry/current_status_reporting_egfr_09.pdf.
- 5.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Becker BN, Vassalotti JA. A software upgrade: CKD testing in 2010. Am J Kidney Dis. 2010;55:8–10. doi: 10.1053/j.ajkd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 7.US Renal Data System . USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2005. [Google Scholar]
- 8.Research Data Assistance Center [Accessed August 17, 2009];Medicare data file descriptions. 2009 http://www.resdac.umn.edu/Medicare/file_descriptions. asp.
- 9.Stevens LA, Stoycheff N. Standardization of serum creatinine and estimated GFR in the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;51(suppl 2):S77–S82. doi: 10.1053/j.ajkd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) [Accessed August 17, 2010];KDIGO controversies conference: definition, classification, and prognosis in CKD. 2009 http://www.kdigo.org.
- 11.Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation [Accessed August 17, 2009];KDOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease. 2006 http://www.kidney.org/professionals/KDOQI/guidelines_anemia/index.htm.
- 14.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648–659. doi: 10.1053/j.ajkd.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660–670. doi: 10.1053/j.ajkd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 18.Astor BC, Hallan SI, Miller ER, III, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 19.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53:915–920. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.