Abstract
Fibroblasts are responsible for producing the majority of collagen and other extracellular matrix (ECM) proteins in tissues. In the injured tissue, transforming growth factor-β (TGF-β)-activated fibroblasts or differentiated myofibroblasts synthesize excessive ECM proteins and play a pivotal role in the pathogenesis of fibrosis in heart, kidney and other organs. Recent studies suggest that fibroblast-like cells, derived from endothelial cells by endothelial-to-mesenchymal transition (EndMT), contribute to the pathogenesis of cardiac fibrosis. The molecular basis of EndMT, however, is poorly understood. Here, we investigated the molecular basis of EndMT in mouse cardiac endothelial cells (MCECs) in response to TGF-β2. MCECs exposed to TGF-β2 underwent EndMT as evidenced by morphologic changes, lack of acetylated–low density lipoprotein (Ac-LDL) uptake, and the presence of alpha-smooth muscle actin (α-SMA) staining. Treatment with SB431542, a small molecule inhibitor of TGF-β-receptor I (TβRI) kinase, but not PD98059, a MEK inhibitor, completely blocked TGF-β2-induced EndMT. The transcript and protein levels of α-SMA, Snail and β-catenin as well as acetyltransferase p300 (ATp300) were elevated in EndMT derived fibroblast-like cells. Importantly, microRNA (miRNA) array data revealed that the expression levels of specific miRNAs, known to be dysregulated in different cardiovascular diseases, were altered during EndMT. The protein level of cellular p53, a bonafide target of miR-125b, was downregulated in EndMT-derived fibroblast-like cells. Here, we report for the first time, the differential expression of miRNAs during cardiac EndMT. These results collectively suggest that TβRI serine-threonine kinase-induced TGF-β signaling and microRNAs, the epigenetic regulator of gene expression at the posttranscriptional level, are involved in EndMT and promote profibrotic signaling in EndMT-derived fibroblast-like cells. Pharmacologic agents that restrict the progression of cardiac EndMT, a phenomenon that is found in adults only in the pathological conditions, in targeting specific miRNA may be helpful in preventing and treating cardiac fibrosis.
Keywords: Cardiac EndMT, TβRI kinase, microRNA, ATp300, Epigenetics, Fibrosis
1. Introduction
Cardiac fibrosis is a common end-stage pathologic manifestation of several cardiovascular diseases. While fibroblasts are the major source of extracellular matrix (ECM) proteins during tissue repair under normal physiologic conditions as well as during development of cardiac fibrosis under pathologic conditions, the origin of fibroblasts participating in cardiac fibrosis is not well understood. Originally, it was thought that in response to myocardial infarction, intracardiac resident fibroblasts derived from embryonic mesenchymal cells were the primary origin of myofibroblasts contributing to repair processes. However, numerous recent studies suggest that, in addition to resident cardiac fibroblasts, adult fibroblast-like cells also originate from endothelial cells (embryonic origin from splanchnopleuric mesoderm) by endothelial-to-mesenchymal transition (EndMT) [1]. EndMT is a common biologic process during embryonic development of the heart and other organs such as lung [2,3]. However, in adults, abnormal activation of EndMT and differentiation of EndMT-derived fibroblast-like cells to collagen producing myofibroblasts play a significant role in the development and progression of fibrosis in organs such as heart and lung [3–8]. EndMT is characterized by endothelial cell disaggregation, morphologic change related to myofibroblast differentiation, and gradual loss of endothelial markers such as CD31, VE cadherin, and vWF, with the gradual appearance of fibroblastic markers such as FSP1, alpha-smooth muscle actin (α-SMA) and collagen. Additionally, different transcription factors such as Snail and β-catenin are also known to participate in the process of EndMT, via suppression of endothelial markers [2,4,6,9]. It is now well documented that elevated transforming growth factor-β (TGF-β) signaling controls endothelial plasticity and plays a significant role in the EndMT process [8]. However, the molecular basis of TGF-β-induced EndMT is poorly understood. Understanding the molecular basis of EndMT and the inhibition of new fibroblast formation from endothelial cells will be an ideal approach to control fibrosis because EndMT-derived fibroblast like cells in the adult myocardium are only associated with pathologic conditions (4,7).
MicroRNAs (miRNAs) are short, highly conserved, RNA sequences comprised of approximately 22-nucleotides, and are involved in epigenetic regulation of eukaryotic gene expression. Aberrant expression levels of several miRNAs are associated with the pathologic conditions of different cardiovascular diseases such as hypertrophy, cardiac fibrosis, arrhythmia, myocardial infarction, heart failure, and cardiomyopathy [10–12]. However, the expression levels of miRNAs and their implication in fibrogenesis via activation of EndMT are still unknown. To better understand the molecular basis of EndMT, we examined the effect of a small molecule inhibitor of transforming growth factor-β (TGF-β) receptor type I kinase (TβRI) on EndMT and presented data showing the efficacy of a small molecule inhibitor of TβRI in blocking cardiac EndMT. Along with EndMT-markers such as α-SMA and involved transcription factors like Snail and β-catenin, the epigenetic regulator of profibrotic signaling ATp300 was also elevated during EndMT. Furthermore, we conducted, for the first time, study of the expression levels of miRNAs by miRNA array in EndMT-derived fibroblast like cells and demonstrated differential expression of several miRNAs during cardiac EndMT. We discuss here the significance of these observations on miRNA and EndMT in the light of cardiac endothelial plasticity and cardiac fibrosis.
2. Materials and Methods
2.1. Mouse cardiac endothelial cells isolation
Mouse cardiac endothelial cells (MCECs) were isolated as described by Lim and Luscinskas (13), with modification. In brief, mouse hearts were collected and minced with scissors. Minced cardiac tissues were treated with collagenase and centrifuged. The cell pellets were resuspended in cold buffer and incubated with anti-mouse CD31 antibody-Dynabeads (Invitrogen, Carlsbad, CA). Dynabead-bound cells were isolated on a magnetic separator, resuspended in media containing 20% fetal bovine serum, and cultured. To increase endothelial cell purity, cells were trypsinized, washed, and incubated with a second endothelial specific antibody (CD102-Dynabeads, Invitrogen). CD102 antibody-Dynabead-bound cells were isolated following the steps used in CD31 antibody–Dynabead mediated isolation. In order to further confirm that the isolated cells were primarily endothelial cells, cell cultures were labeled with diluted fluorescent dye tagged acetylated–low density lipoprotein (Dil-Ac-LDL; Biomedical Technologies, Inc., Stoughton, MA) (10 µg/mL). Fluorescent probe-containing media were removed; cells were washed with probe-free media and visualized via fluorescence microscope.
2.2. Endothelial-to-Mesenchymal Transition
The primary cultures of MCECs were grown in Dulbecco modified Eagle medium with 2% FBS and treated in triplicate for 7 days with TGF-β2 in the presence and absence of small molecule SB431542 (10 µM), a potent inhibitor of TGF-β-receptor I kinase, or PD98059 (25 µM), an inhibitor of MAP kinase kinase (MEK), which inhibits extracellular signal-regulated kinase 1 and 2 (ERK1/2) activation, or vehicle dimethyl sulfoxide (DMSO).
2.3. Cell morphology study
The morphology of controls and TGF-β2 and/or SB431542 and PD98059-treated mouse cardiac endothelial cells was captured by light microscopy (Olympus CKX41 and camera DP70, Center Valley, PA).
2.4. Ac-LDL labeling
Mouse cardiac endothelial cells were incubated with or without TGF-β2 in the presence and absence of SB431542 and PD98059 for 7 days. At the end of incubation, cells were incubated with Dil-Ac-LDL (Biomedical Technologies, Inc., Stoughton, MA) (10 µg/mL in culture media) for 3 hours at 37°C followed by wash with PBS and imaging by fluorescence microscope (Olympus CKX41, Center Valley, PA).
2.5. Immunofluorescence
For determination of EndMT and myofibroblast differentiation, control and treated mouse cardiac endothelial cells were washed with phosphate buffered saline (PBS) and fixed with chilled methanol for 7 minutes at −20°C. Fixed cells received blocking buffer and were incubated with fluorescein isothiocyanate (FITC) tagged α-SMA antibody (Sigma, St. Louis, MO) for 1 hour at room temperature and then washed with 1XPBS and photographed by fluorescence microscope (Olympus CKX41, Center Valley, PA).
2.6. Immunoblot analysis
Early passages of primary cultures of mouse cardiac endothelial cells were treated with TGF-β2. After 7-day exposure, cells were harvested and cell lysates were prepared. Equal amounts of protein were loaded on SDS polyacrylamide gel and processed for immunoblot analysis. The protein expression levels of PAI-1, CD31, α-SMA, β-catenin, Snail, p53, downstream TGF-β signaling molecules, pSmad2, p-ERK, total-ERK (T-ERK) and acetyltransferase p300 were measured by immunoblot using a specific antibody raised against α-SMA (Sigma-Aldrich, St. Louis, MO), ATp300, p53, PAI-1 (Santa Cruz Biotech, Inc., Santa Cruz, CA), Snail, β-catenin, pSmad2, ERK (T-ERK) (Cell Signaling, Beverly, MA), pERK (phospho-ERK), (Calbiochem/EMD chemicals, Philadelphia, PA), CD31 (GenScript, Piscataway, NJ) and actin (Abcam, Cambridge, MA).
2.7. Quantitative RT-PCR
Total RNA was extracted from MCECs using Trizol® reagent (Invitrogen, Carlsbad, CA), and precipitated with isopropanol. Complementary DNA (cDNA) was synthesized using qScript™ cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) and then subjected to quantitative RNA polymerase chain reaction (PCR). Briefly, 0.4 µg total RNA from MCECs was mixed with qScript™ cDNA SuperMix and nuclease free water, as per manufacturer’s instructions. Quantitative PCR was conducted by employing Real Time PCR (Bio-Rad Laboratories, Inc., Hercules, CA) using PerfeCTa® SYBR® Green SuperMix (Quanta Bioscience, Gaithersburg, MD) according to manufacturer’s instructions. PerfeCTa® SYBR® Green SuperMix was mixed with forward and reverse primers for Snail, β-catenin, α-SMA and β-actin (Integrated DNA Technologies, Inc., San Diego, CA) to carry out the real time reaction, as per manufacturer’s instructions.
2.8. Micro-RNA Arrays
The levels of microRNA in MCECs and in EndMT derived mesenchymal cells were determined by mouse miRNA array kit following the manufacturer’s (Signosis, Inc., Sunnyvale, CA) instructions. In brief, miRNAs were annealed to oligonucleotide mix by using 5 µg total RNA, mouse oligo mix, array detection oligo, annealing buffer, and nuclease free water. Following this, the miRNA was first incubated at 72°C for 5 minutes and then at 53°C for an additional 90 minutes. Selection of miRNA/oligo hybrids was then performed by first washing the beads with annealing buffer and then adding the bead binding buffer to the annealed miRNA/oligo hybrid mix. This combination was then incubated at 37°C for 30 minutes and washed with wash buffer. Ligation of miRNA-directed oligos was performed using ligation buffer and ligase at 37°C for 90 minutes to form a single molecule. Thereafter, this ligated molecule underwent T7 RNA transcription. After washing, extension buffer was added to the mix followed by PCR, as per manufacturer’s instructions. T7 RNA polymerase was then added to the labeling mix. This mixture was incubated at 37°C for 1 hour. The membrane was pre-hybridized by adding hybridization buffer at 42°C for 30 minutes. After pre-hybridization, the membrane was incubated overnight with transcribed RNA in prewarmed hybridization buffer at 42°C. At completion of incubation, the membrane was washed and rinsed with detection wash buffer. The membrane was then blocked with blocking buffer at 30 minutes at room temperature with shaking. Streptavidin-HRP conjugate was added for 45 minutes, and the membrane was rewashed with detection buffer. Substrate A and B were then added in equal amounts. Following this, the membrane was exposed to Kodak BioMax MR film (Carestream Health, Inc., Rochester, NY) and developed. Spots in MCECs and EndMT membranes were identified using mouse miRNA array chart (Signosis, Inc. Sunnyvale, CA).
2.9. Micro-RNA qPCR
The levels of miR-125b were measured in mouse cardiac endothelial cells and EndMT derived mesenchymal cells using a miRNA Real-Time PCR Assay Kit (Signosis, Inc. Sunnyvale, CA) according to manufacturer’s instructions. In brief, total RNA along with small RNAs and miRNAs were isolated using Trizol® reagent (Invitrogen, Carlsbad, CA). The miRNA/oligo hybrid was prepared by adding 1 µg RNA to miRNA oligo mix, annealing buffer and nuclease free water and incubating at 72°C for 5 minutes and then at 53°C for 60 minutes. Beads were washed with a magnetic stand using annealing buffer. Following this, beads were subjected to a selection procedure by binding the miRNA/oligo hybrid with binding buffer and incubating it at 37°C for 30 minutes and then washing with wash buffer. Ligase was used to ligate miRNA-directed oligos and form a single molecule. Initially, ligation buffer was added to the beads and then 1 µL of ligase was added before incubating at 37°C for 90 minutes. Beads were then washed and further incubated with nuclease free water at 95°C for 3 minutes to release the ligated molecule. Ligated molecules were used for quantitative PCR reaction as per manufacturer’s instructions by adding SYBR Green PCR Master Mix, miRNA qPCR primer, and DNA polymerase (Signosis, Inc., Sunnyvale, CA).
2.10. Statistical analysis
Data are presented as mean ± SEM. The significance of difference between control and experimental groups were determined by statistical analysis using t-test.
3. Results and Discussion
3.1. TGF-β-receptor kinase inhibitor but not MEK inhibitor blunts TGF-β2-induced EndMT in mouse cardiac endothelial cells (MCECs)
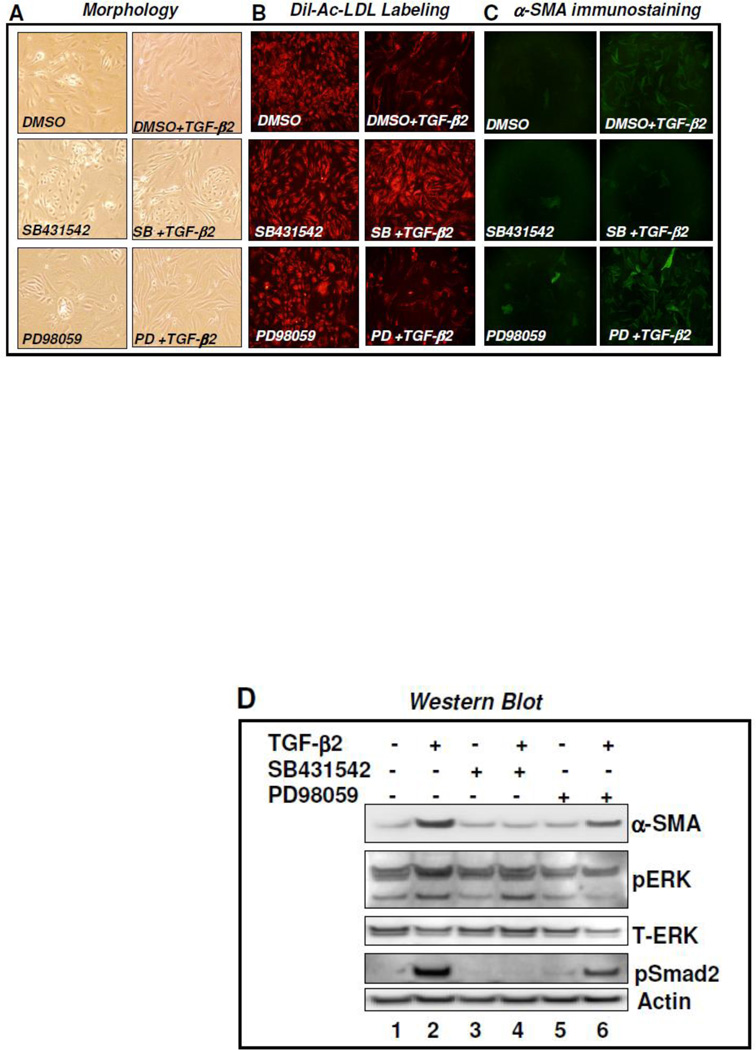
TGF-β2 induces endothelial-to-mesenchymal transition [8]. Here, we investigated the molecular mechanism by which TGF-β2 induces EndMT in primary cultures of MCECs. Exposure of isolated low passage primary cultures of MCECs to TGF-β2 for 7 days altered their morphology from an endothelial polygonal cobblestone-like shape to a more spindle shaped fibroblast-like morphology (Figure 1A, top). Treatment of MCECs with SB431542, a potent inhibitor of TβRI kinase, prevented TGF-β2-induced morphologic transformation (Figure 1A, middle). In contrast, PD98059, an inhibitor of MEK MAPK failed to prevent TGF-β2-induced morphologic changes (Figure 1A, bottom). Only endothelial cells and macrophages are known to uptake acetylated-LDL. Since MCECs were isolated and selected using two endothelial specific antibodies, CD31 and CD102, the cell population was macrophage-free. To further confirm the transition of cardiac endothelial cells to fibroblast-like cells, MCECs were exposed to TGF-β2 for 7 days and were then labeled with Dil-Ac-LDL. Results revealed that in the absence of TGF-β2, cells were labeled with Dil-Ac-LDL as expected. However, in the presence of TGF-β2, cells were unable to uptake Dil-Ac-LDL indicating that MCECs lost the endothelial property and underwent transition (Figure 1B, top). In the presence of TβRI kinase inhibitor SB431542, and not MEK inhibitor PD98059, MCECs preserved Dil-Ac-LDL uptake and thus prevented cell transformation (Figure 1B, middle and bottom).
Figure 1. Molecular basis of EndMT.
A–D. TβR1 kinase inhibitor SB431542 blocks TGF-β2-induced EndMT: Mouse cardiac endothelial cells (MCECs) were treated with DMSO or SB431542 (SB) or PD98059 (PD) in the presence and absence of TGF-β2 for 7 days. Cell morphology was studied by light microscopy (A); receptor-mediated high endocytosis of Dil-Ac-LDL by primary cultures of MCECs was studied by fluorescence microscopy (B), and immunostaining of cells was performed using FITC tagged α-SMA (myofibroblast marker) antibody (C); MCECs were treated with DMSO or SB431542 (SB) or PD98059 (PD) in the presence and absence of TGF-β2 for 7 days and cell lysates were prepared on three plates and pooled. Equal amounts of proteins were subjected to western blot analysis with α-SMA, p-ERK, T-ERK, pSmad2 and actin (loading control) (D).
To further confirm the negative influence of TβRI kinase inhibitor on the transition of endothelial cells to fibroblast-like cells, cells were immunostained with anti-α-SMA antibody. While untreated MCECs were α-SMA negative, almost 95% of TGF-β2 treated cells were positively stained with α-SMA indicating that TGF-β2-induced EndMT and EndMT-derived fibroblast-like cells were differentiated to myofibroblasts (Figure 1C, top). However, treatment of MCECs with TGF-β-receptor I kinase inhibitor SB431542 (Figure 1C middle), not MEK inhibitor PD98059 (Figure 1C bottom), completely blocked TGF-β2-induced EndMT as evidenced by the lack of α-SMA positive cells in the presence of TGF-β2. These observations were further confirmed by western blot analysis. Results revealed that TGF-β2 induced the expression of α-SMA proteins and TβRI kinase inhibitor SB431542 completely blocked the TGF-β2-induced elevated expression of α-SMA and EndMT (Figure 1D, compare lane 4 [SB+TGF-β2] with lanes 2 [DMSO+TGF-β2]). In contrast, MEK-inhibitor, PD98059 failed to block TGF-β2-induced α-SMA expression and cardiac EndMT (Figure 1D, compare lane 6 [PD+TGF-β2] with lane 2 [DMSO+TGF-β2]) and lane 4 [SB+TGF-β2]). Therefore, these results are consistent with the results obtained in morphology (Figure 1A), Ac-LDL labeling (Figure 1B) and immunostaining (Figure 1C) studies using mouse cardiac endothelial cells and EndMT-derived fibroblast-like cells. Kinase-specific inhibitory effects of SB431542 and PD98059 on TβR1 kinase and MEK-MAPK were confirmed by western blot analysis. Results revealed that while the levels of actin (loading control) remain unaltered, phopshorylation of Smad2 and ERK1/2-MAPK were inhibited by SB431542 (lane 4) and PD98059 (lane 6) respectively (Figure 1D). Upon 7 days exposure of MCECs to PD98059, the level of total ERK was also decreased to some extent compared to loading control actin. Nevertheless, in the absence of ERK-MAPK, TGF-β2 was able to induce EndMT as characterized by the presence of elevated level of α-SMA protein. These results collectively suggest that TβRI kinase and Smad-dependent downstream signaling pathway may play a significant role in the pathogenesis of fibrosis via activation of cardiac EndMT. Most importantly, the results of the present study, along with those of a recent study using umbilical cord endothelial progenitor cells [14], revealed that a small molecule inhibitor of TβRI kinase can efficiently block endothelial plasticity and EndMT.
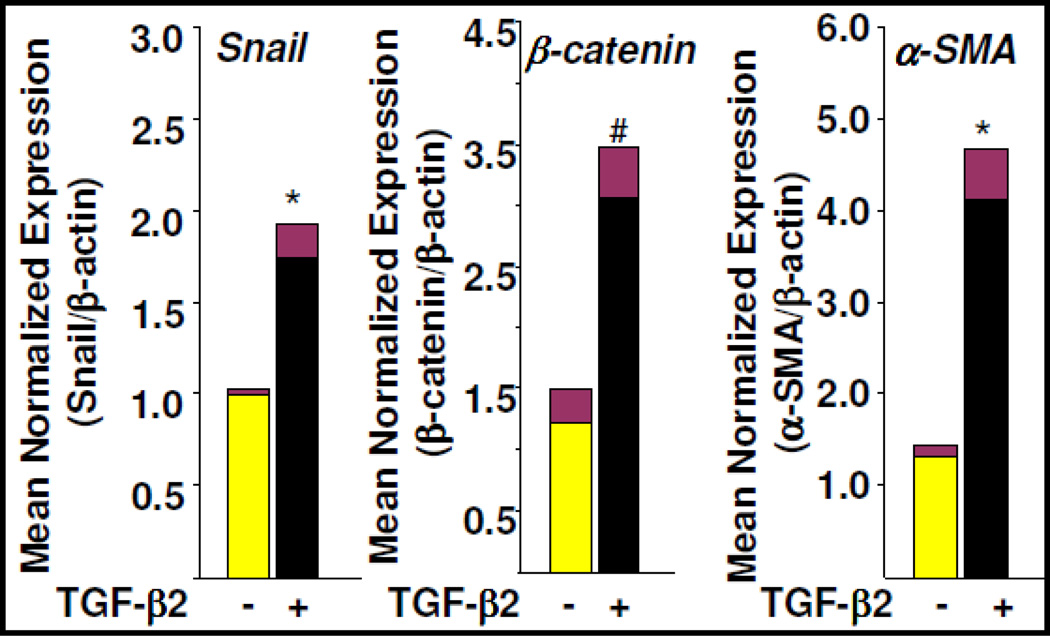
The transcription factors Snail and β-catenin are known to inhibit expression of endothelial markers including vascular endothelial (VE)-cadherin and are involved in the TGF-β-induced EndMT process [8]. As a positive control of the cardiac EndMT process, we examined the expression levels of these known transcription factors during EndMT of cardiac endothelial cells. Results revealed that the mRNA and protein expression levels of Snail and β-catenin were elevated in EndMT-derived fibroblast-like cells (Figure 2 and Figure 3). Elevation of Snail and β-catenin during EndMT of MCECs were consistent with previous findings [2,8,9]. However, the levels of β-catenin expression in cardiac EndMT-derived fibroblast-like cells were not significantly different from MCECs. β-catenin, a major effector in canonical Wnt-signaling pathway, is activated during EndMT and contributing to heart cushion formation. Furthermore, EndMT is inhibited in mice that are deficient for β-catenin, and β-catenin-deficient endothelial cells are unable to transform into α-SMA positive cells in response to TGF-β[2]. TGF-β-activated Smads can cooperate with β-catenin and mediate the crosstalk between TGF-β and Wnt-signaling pathways [2]. Importantly, Smad3 is required for transcriptional activation of β-catenin as evidenced by the observation that the levels of β-catenin is significantly lower in Smad3 null cells compared to wildtype cells (15). Snail, a zinc finger transcription factor, is upregulated by TGF-β2 which is dependent on activation of Smad, MEK, PI3K and p38 MAPK (8), and is required for TGF-β2-induced EndMT of embryonic stem cell–derived endothelial cells. Importantly, overexpressed Snail alone can augment EndMT [9] and its expression is elevated in idiopathic pulmonary fibrosis [16]. In our study, the levels of α-SMA mRNA [Fig. 2] were significantly elevated in TβR1 kinase-dependent EndMT-derived fibroblast-like cells consistent with the results obtained in fluorescence microscopy and western blot analysis (Figure 1C,D), thus supporting the notion that EndMT-derived fibroblast-like cells undergo myofibroblast differentiation (Figure 2, Figure 1D, Figure 3 and Figure 4D). Additionally, Smad-dependent regulation of α-SMA during EndMT as described in the present study is also consistent with a recent observation demonstrating that during gingival fibroblast-myofibroblast transition, the regulation of α-SMA expression by TGF-β is Smad-dependent (17).
Figure 2. Differential expression of EndMT marker and regulators during EndMT.
MCECs were treated with TGF-β2 for 7 days and total RNA was isolated. mRNA levels of Snail (left), β-catenin (middle) and α-SMA (right) were determined by qPCR using gene specific primers. Data were analyzed using q-gene software. Note: *denotes P<0.05 vs. MCECs #denotes P=0.06 vs. MCECs. Yellow bar represents MCECs; Black bar represents EndMT-derived fibroblast-like cells.
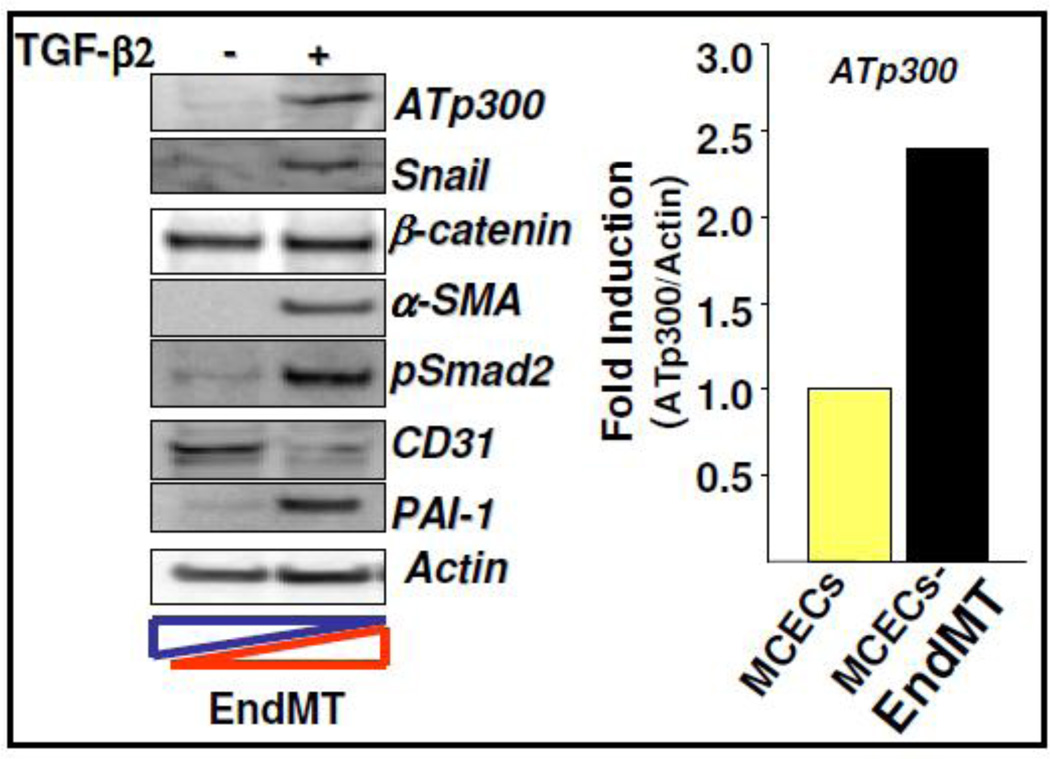
Figure 3. Epigenetic regulator ATp300 is upregulated during EndMT.
MCECs were treated with TGF-β2 for 7 days and cell lysates were subjected to western blot using ATp300, Snail, β-catenin, PAI-1, α-SMA (mesenchymal markers), CD31 (endothelial marker), pSmad2 (TGF-β-signal transducer), and actin antibodies (left panel). The levels of ATp300 were quantified and presented as fold induction of ATp300 relative to actin in EndMT-derived fibroblast-like cells compared to MCECs (right panel). Yellow bar represents MCECs; Black bar represents EndMT-derived fibroblast-like cells.
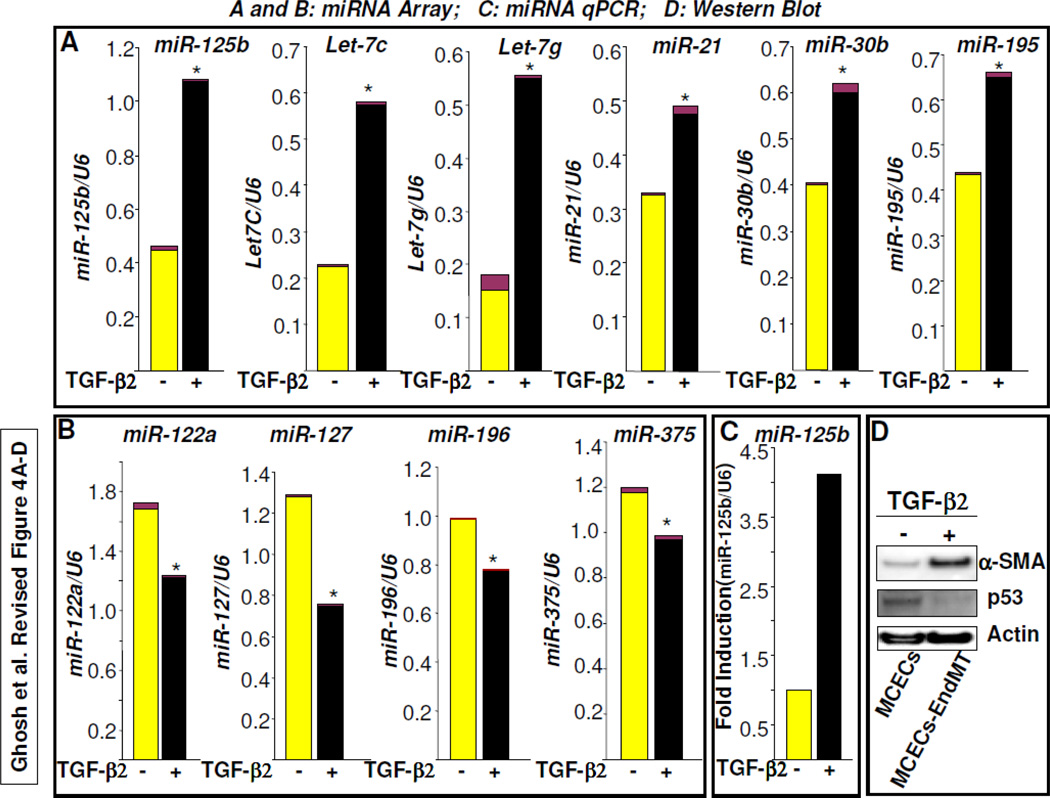
Figure 4. Differential expression of microRNAs during EndMT.
MCECs were treated with TGF-β2 for 7 days and total RNA was extracted from three plates and pooled. Equal amounts of RNA were subjected to miRNA array (A,B) and qPCR analysis (C). The levels of individual miRNA expression relative to U6 expression were quantified and presented as mean ± SEM. Note: * denotes P<0.05 vs. MCECs. The levels of miR-125b, Let7C, Let-7g, miR-21, miR-30b and miR-195 in miRNA array were upregulated during EndMT (A); the levels of miR-122a, miR-127, miR-196, and miR-375 were downregulated during EndMT (B). The levels of miR-125b in MCECs and TGF-2-induced EndMT-derived cells were further determined by microRNA qPCR and data were presented as fold induction of miR-125b relative to U6 expression in EndMT-derived fibroblast-like cells compared to MCECs (C). Total protein extracted from MCECs (control) and EndMT-derived fibroblast-like cells were subjected to western blot using antibodies against p53, α-SMA and actin. Note: while EndMT marker α-SMA was upregulated, the miR-125b target gene p53 protein level was significantly decreased (D).
3.2. The level of acetyltransferase p300 is elevated during EndMT
Acetyltransferase p300 (ATp300) is an essential epigenetic regulator of TGF-β-induced profibrotic responses and is significantly elevated in fibrotic tissues [18]. As EndMT-derived fibroblast-like cells contribute to cardiac fibrosis and the profibrotic cytokine TGF-β controls endothelial plasticity [4–6,8], we examined the levels of ATp300 during EndMT. Results from western blot analysis revealed that the protein level of ATp300 was significantly elevated during EndMT that was characterized by the downregulation of endothelial marker CD31 and upregulation of mesenchymal markers such as α-SMA, Snail and β-catenin, profibrotic markers such as PAI-1 and TGF-β signal transducer pSmad2 (Figure 3). These results indicate that epigenetic regulator ATp300 may play an important role in endothelial plasticity and the specific inhibition of acetyltransferase activity of ATp300 may be an ideal approach to control EndMT and the pathogenesis of cardiac fibrosis.
3.3. Differential expression of microRNAs during EndMT
MicroRNAs (miRNAs) are a group of small highly conserved RNA molecule sequences comprised of approximately 22 nucleotides. MiRNAs control the downregulation of numerous direct target genes. Indirectly, miRNAs also upregulate many genes via suppression of their repressor molecules. TGF-β is known to control the expression levels of different cardio-pathologic and cardio-physiologic miRNAs [10–12] and TGF-β-induced EndMT plays an important role in cardiac fibrosis [4]. Here, we determined the expression levels of miRNAs during EndMT of MCECs. We performed microRNA array of total RNA isolated from MCECs and EndMT-derived fibroblast-like cells. MicroRNA array data revealed that while specific miRNAs such as miR-125b, Let-7c, Let-7g, miR-21, miR-30b and miR-195 were significantly elevated during EndMT (Figure 4A), the levels of several miRNAs including miR-122a, miR-127, miR-196, and miR-375 were significantly downregulated (Figure 4B). Interestingly, the levels of many of these miRNAs are known to be deregulated in different cardiovascular diseases, such as hypertrophy, cardiac fibrosis, arrhythmia, myocardial infarction, heart failure, and cardiomyopathy [10–12]. Thus the expression levels of specific miRNAs are associated with the diagnosis and prognosis for specific diseases. However, several miRNAs were unaltered in EndMT-derived fibroblast-like cells compared to MCECs (data not shown). To validate the microRNA array data, the expression levels of miR-125b in EndMT-derived fibroblast-like cells and MCECs were further confirmed by miRNA qPCR analysis (≈4-fold increase in EndMT-derived fibroblast vs. MCECs) (Figure 4C). One of the major targets of miR-125b is cellular p53 [19]. Previously, we demonstrated that cellular p53 antagonizes TGF-β-induced profibrotic responses [18] and interestingly, the results of the present study revealed that the levels of cellular p53 was significantly downregulated during EndMT of cardiac endothelial cells (Figure 4D) which was characterized by significantly elevated level of α-SMA, a bonafide marker of EndMT-derived fibroblast-like cells (Figure 4D). Therefore, it is possible that elevated levels of miR-125b downregulates p53 and thus the level of profibrotic signaling is increased in the absence of p53, a known negative modulator of TGF-β-induced profibrotic signaling (18). The elevated levels of Let-7c and miR-21 have also been reported in acquired heart diseases [10,11]. Specific suppression of upregulated miRNA or specific overexpression of downregulated miRNA may be a viable approach to blocking induced EndMT. While this article was under revision, Kumarswamy et al. [20] reported that miRNA-21 contributes partially to EndMT in human umbilical vein endothelial cells (HUVEC). Importantly, our miRNA array data confirm that miR-21 is upregulated during EndMT of cardiac endothelial cells as well. However, the present findings suggest that other miRNAs may also contribute to EndMT and the pathogenesis of cardiac fibrosis. Further in vivo study is required to establish the role of these miRNAs in EndMT and in the progression of cardiac fibrogenesis. Such studies are now in progress in our laboratory.
4. Conclusions
In conclusion, our present study reveals for the first time that i) microRNAs which are dysregulated in cardiovascular diseases, are differentially regulated during cardiac EndMT compared to cardiac endothelial cells; ii) The level of cellular p53, a target of miR-125b and a negative modulator of TGF-β-induced profibrotic signaling, is significantly downregulated during EndMT; iii) The epigenetic regulator ATp300, an essential coactivator of profibrotic signaling, is significantly elevated during EndMT; and iv) A synthetic small molecule inhibitor of TβRI kinase, but not ERK1/2 MAPK inhibitor, significantly blocks cardiac EndMT. Therefore, downstream epigenetic regulators, miRNAs and ATp300 may accelerate TβRI-kinase-induced cardiac EndMT and promote profibrotic signaling in injured heart. Pharmacologic agents that restrict the progression of EndMT targeting epigenetic regulators, miRNAs or ATp300, may be an ideal approach in preventing and treating cardiac fibrosis.
Highlights.
A small molecule inhibitor of TβRI-kinase (prevents Smad phosphorylation and activation), but not MEK inhibitor (prevents ERK phosphorylation), blocks cardiac endothelial-to-mesenchymal transition (EndMT)
The epigenetic regulator ATp300 is significantly elevated during cardiac EndMT
Several miRNAs, the epigenetic regulators, are differentially regulated during cardiac EndMT. The level of cellular p53, a target of miR-125b, is downregulated during EndMT
Epigenetic regulators, specific miRNA and ATp300, may be an ideal target to controlling cardiac EndMT and EndMT-derived fibroblast-like cells-contributed cardiac fibrosis
Acknowledgements
This work was supported by grants from NIH-NHLBI (HL051387 and 1P01HL108795-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeisberg EM, Kalluri R. Circ. Res. 2010;107:1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. J. Cell. Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 5.Goumans MJ, van Zonneveld AJ, ten Dijke P. Trends Cardiovasc. Med. 2008;18:293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK, Bradham WS, Gleaves LA, De Taeye B, Murphy SB, Covington JW, Vaughan DE. Circulation. 2010;122:1200–1209. doi: 10.1161/CIRCULATIONAHA.110.955245. [DOI] [PubMed] [Google Scholar]
- 7.Krenning G, Zeisberg EM, Kalluri R. J. Cell. Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Meeteren LA, Ten Dijke P. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokudo T, Suzuki Y, Yoshimatsu Y, Yamazaki T, Watabe T, Miyazono K. J. Cell. Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 10.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 11.Latronico MV, Catalucci D, Condorelli G. Physiol. Genomics. 2008;34:239–242. doi: 10.1152/physiolgenomics.90254.2008. [DOI] [PubMed] [Google Scholar]
- 12.Mishra PK, Tyagi N, Kumar M, Tyagi SC. J. Cell. Mol. Med. 2009;13:778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim YC, Luscinskas FW. In: Cell-Cell Interaction: Methods and Protocols: Methods in Molecular Biology. Colgan SP, editor. Vol. 341. Totowa, NJ: Humana Press Inc.; 2006. pp. 141–154. [Google Scholar]
- 14.Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC. Cardiovasc Res. 2010;86:506–515. doi: 10.1093/cvr/cvq012. [DOI] [PubMed] [Google Scholar]
- 15.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 16.Jayachandran A, Königshoff M, Yu H, Rupniewska E, Hecker M, Klepetko W, Seeger W, Eickelberg O. Thorax. 2009;64:1053–1061. doi: 10.1136/thx.2009.121798. [DOI] [PubMed] [Google Scholar]
- 17.Sobral LM, Montan PF, Zecchin KG, Martelli-Junior H, Vargas PA, Graner E, Coletta RD. J Periodontol. 2011;82:642–651. doi: 10.1902/jop.2010.100510. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh AK, Vaughan DE. Frontiers in Biosci. 2011;E4:1556–1570. doi: 10.2741/e480. [DOI] [PubMed] [Google Scholar]
- 19.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T. Arterioscler Thromb Vasc Biol. 2012;32:361–369. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]