Abstract
Background
To optimize the management of patients with schizophrenia, quantification of treatment effects is crucial. While in research studies, the use of quantitative assessments is ubiquitous; this is not the case in routine clinical practice, creating an important translational practice gap.
Objective
To examine the relevance, methodology, reporting and application of measurement based approaches in the management of schizophrenia.
Methods
We summarize methodological aspects in the assessment of therapeutic and adverse antipsychotic effects in schizophrenia, including definitions and methods of measurement based assessments and factors that can interfere with the valid quantification of treatment effects. Finally, we propose pragmatic and clinically meaningful ways to measure and report treatment outcomes.
Results
While rating scales are ubiquitous in schizophrenia research and provide the evidence base for treatment guidelines, time constraints, lack of familiarity with and/or training in validated assessment tools limits their routine clinical use. Simple, but valid assessment instruments need to be developed and implemented to bridge this research-practice gap. Moreover, results from research trials need to be communicated in clinically meaningful ways. This includes the reporting of effect sizes, numbers-needed-to-treat and -harm, confidence intervals and absolute risk differences. Some important outcomes, such as treatment response, should be reported in escalating intervals using incrementally more stringent psychopathology improvements. Nevertheless, even with quantification, it remains challenging to weigh individual efficacy and adverse effect outcomes against each other and to decide on the targeted/desired improvement or outcome, while also incorporating that in patient-centered and shared decision methods.
Conclusions
Quantification of treatment effects in schizophrenia is relevant for patient management, research, and the evaluation of health care systems. Beyond consensus about meaningful outcome definitions, reporting strategies, pragmatic tool development and implementation, the discovery of novel treatment mechanisms and related biomarkers is hoped to advance measurement based approaches in schizophrenia and thereby improve patient outcomes.
Keywords: Schizophrenia, Measurement, Quantification, Efficacy, Effectiveness, Adverse Effects, Real World
Introduction
Despite multiple advances in the management of schizophrenia, there is still an enormous need to improve our understanding and treatment of patients suffering from schizophrenia. With the development of additional pharmacologic treatment options and the availability of technological tools that are hoped to help personalize treatment1, a careful assessment of treatment effects is needed. First and foremost, this requires the detailed clinical and research assessment of beneficial and adverse effects of the treatment options. Without quantifying these effects, clinicians are hampered when trying to decide on a given treatment strategy. While treatment guidelines are helpful2, 3, 4, 5, 6, 7, 8 in that they synthesize research results across a number of dimensions, these guidelines can only be as accurate, detailed and generalizable as the data they are based on. Moreover, incidence rates of certain treatment effects and group means of rating scale scores can only provide a yard stick for the decision making process. Ultimately, the efficacy and tolerability of a given treatment in a given patient can only be assessed directly. However, to date, measurement based approaches are not used in the routine treatment of schizophrenia. The field needs to develop pragmatic, but meaningful tools that can be used by busy practitioners working under enormous time constraints. The era of electronic medical records makes this even more relevant, as such real world effectiveness assessments can be used in large pragmatic trials. Moreover, data base outcomes research can include more generalizable treatment groups than ordinarily involved in randomized controlled trials and also provides more detailed data than ordinary claims-based research. Nevertheless, to establish measurement based approaches in the treatment of schizophrenia, research approaches need to be adapted to real world settings, simple, but valid and clinically meaningful tools and criteria are needed, and measured outcomes need to be placed in the context of the individual patient. In addition, it is important to have the patient's perspective on what is important to him/her in weighing efficacy and tolerability as well as the relative salience of specific symptoms. Finally, the successful implementation of measurement based principles in psychiatric practice needs to find ways to overcome the not uncommon fragmentation of care of severely mentally ill patients. This includes the orchestration of psychopharmacologic, psychotherapeutic, vocational and social rehabilitative and physical medical care that usually is delivered by different people. In this case, effective communication is key, but quantified assessments can greatly help the integration of goals and identification of areas in need of further improvement and synergy.
Methods
This article summarizes methodological aspects in the assessment of beneficial and adverse effects of antipsychotics in schizophrenia. We provide information about the importance of measurement based approaches in general, and review definitions and methods of such assessments. Furthermore, we address factors that can interfere with the valid quantification of treatment effects in schizophrenia patients and how measurement based outcomes ought to be reported to be clinically meaningful. Although psychosocial interventions are of enormous importance, we focus predominantly on pharmacologic treatments since psychosocial interventions are generally given in conjunction with antipsychotics, which are the cornerstone of management to which other treatments are added.
The Importance of Measurement
Measurement is one of the most critical elements in the diagnosis and clinical management of any illness. We measure the frequency and severity of symptoms as well as their functional consequences in order to make a presumptive diagnosis. We continue to measure disease-related phenomena in the confirmation of our presumptive diagnosis and then some types of measurement become the critical element in evaluating the response to treatment and the overall course of illness. Despite the ubiquitous requirements for some degree of measurement, psychiatry as a field has been remarkable in the lack of consistent clinical training for and application of valid and reliable measurement techniques9. Validity refers to the ability of a diagnosis or clinical assessment to reflect the “reality” of the situation. Is the diagnosis correct according to some “gold standard” or validating criteria? Reliability refers to the characteristics of an instrument or diagnosis and those who use it to arrive at the same conclusion, score, etc. when evaluating the same patient independently. Clinicians can be “wrong” in their measurement/conclusions (poor validity), but still agree with each other (good reliability). Therefore, one has to be careful to distinguish validity and reliability from each other. They are established in very different ways. Moreover, it is also important to realize that a disorder is not defined by symptoms alone, but that either subjective distress and/or functional impairment are required.
Given the fact that there are no objective laboratory or other tests to confirm or measure psychiatric illnesses and that we depend to a large extent on patient and/or informant subjective evaluation of mental sates and behavior, the situation lends itself to considerable room for error, disagreement and lack of adequate documentation. The requirements of clinical research to ensure validity and reliability of diagnosis and clinical assessments of outcome have resulted in the development of numerous diagnostic (structured interviews) and assessment (clinician, patient and informant derived) instruments, but these are very rarely utilized in clinical practice9. There are a variety of obstacles (perceived and real) to implementing quantitative measure in clinical practice, but there are also important benefits to the application of such approaches (Table 1).
Table 1. The Value of and Barriers to Implementing Measurement Based Approaches in Psychiatry.
| Value of Measurement Based Approaches | Barriers to Measurement Based Approaches |
|---|---|
| Contribution to diagnostic process | Inadequate appreciation of benefit |
| Establishing baseline severity | Perceived value of global judgment |
| Providing targets and treatment goals | Time constraints |
| Evaluating the efficacy of treatment | Lack of appropriate instruments |
| Evaluating tolerability and adverse effects | Inadequate training |
| Influencing level of care | Reimbursement concerns |
| Medical record documentation |
Every treatment decision that we make involves concepts like response, remission, relapse, etc. yet these are often used in a very inconsistent fashion without any agreement as to how they should be defined and measured. Clinicians tend to rely on global clinical judgment, which can be difficult to document or replicate. How often do we see a note in a chart which states “patient better, “or “patient has had a relapse,” without any further indication of on what these statements are based. Tremendous emphasis has been appropriately placed on the application of evidenced-based medicine throughout healthcare, but the application of evidence requires an understanding of how the evidence was gathered, how generalizable it is, and to what extent it applies to the patient before us9. All of these issues hinge on the measurements and definitions involved. Below, we review some of these concepts and definitions and discuss their relevance to clinical practice.
Outcomes, Definitions and Methods
Figure 110 outlines common treatment goals and challenges to achieving these goals. While terms like response, resolution of symptoms, remission, recovery, relapse, treatment resistance, etc are commonly used, they are used inconsistently and definitions vary.
Figure 1.
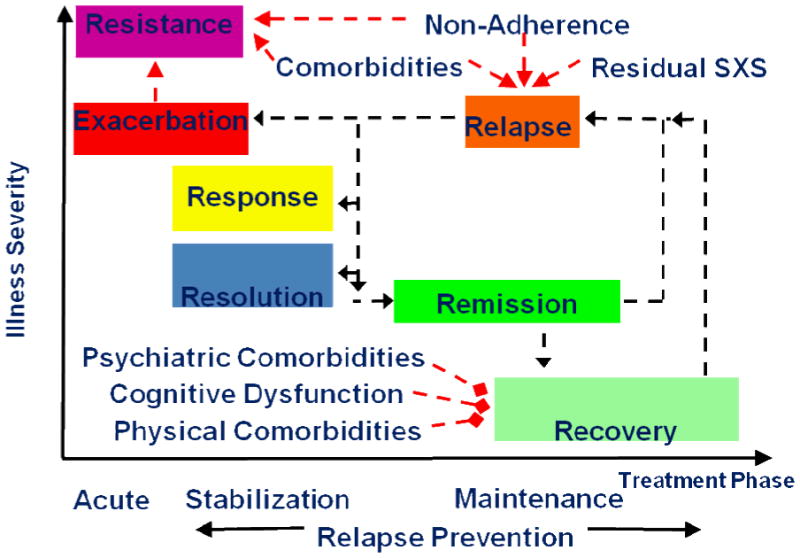
Treatment Goals and Challenges [10]
Adapted from: Kane JM and Correll CU. Dialogues Clin Neurosci. 2010;12(3):345-57 [10]
Treatment Response
After diagnosis and the establishment of a treatment plan, evaluating response to treatment is critical. Response can be considered to be a clinically significant improvement of the psychopathology of a patient, regardless of whether the person continues to have symptoms or not. In clinical trials, thresholds in the sense of a minimum percentage reduction from the initial score on a scale such as Brief Psychiatric Rating Scale11 or the Positive and Negative Syndrome Scale12 are used for this purpose. The problem is that there is no agreement as to which cut-off should be used. In the literature, at least 20%, 30%, 40%, 50% and 60% reduction from the initial score have all been applied, but the clinical meaning of the cutoffs is unclear. Several publications using data from several thousand participants who were rated simultaneously with the BPRS/PANSS and the Clinical Global Impressions Scale13 provided some important insights to this question14, 15, 16, 17, 18.
Equipercentile linking of percentage improvement of the BPRS/PANSS with CGI-improvement score showed that a 25% reduction of the BPRS/PANSS baseline score corresponded roughly to a minimal improvement according to the CGI, while a 50% reduction corresponded to “much improved”. As many acutely ill patients with schizophrenia often respond well to therapy, we concluded that for such patients the 50% cut-off would be a more clinically meaningful criterion than lower cut-offs. On the other hand, in very chronic or treatment resistant patients even a slight improvement might represent a clinically significant effect, justifying the use of the 25% cutoff in treatment refractory patients. Interestingly, the 20% cutoff was indeed initially used in a study of refractory patients19, but was subsequently widely applied in studies of non-refractory subjects.
We suggested the value of displaying results on response to treatment in 25% quartiles in a table (Table 2)17, 20, 21 Such a table covers the extreme ranges of patients whose symptoms did not change or worsened during a trial (≤0%BPRS/PANSS reduction), patients who responded at least minimally (25% BPRS/PANSS reduction), patients who were at least much improved (50% BPRS/PANSS reduction) and patients who had exceptionally good responses compared to other participants in such studies (>75% BPRS/PANSS reduction). This methodology of reporting different levels of response has already been adopted22. In this context, it is also reasonable to use cross-sectional “remission” criteria as a measure of response in that this would identify patients who have only mild or absent symptoms on key symptom measures20.
Table 2. Suggested Presentation of Range of Response and of Remission Rates in Clinical Trials 17, 20, 21.
| Presentation of Responder and Remission Rates based on Percentage Change on the Positive and Negative Syndrome Scale (PANSS)12 or the Brief Psychiatric Rating Scale (BPRS)11 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Criteria | Total n | ≤0% PANSS/BPRS reduction | >0% - 24% PANSS/BPRS reduction | 25% - 49% PANSS/BPRS reduction | 50% - 74% PANSS/BPRS reduction | 75% - 100% PANSS/BPRS reduction | Remission* | |
| Intervention Group | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Control Group | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Presentation of Responder and Remission Rates based on Clinical Global Impressions (CGI) Improvement and Severity Scores13 | ||||||||
| CGI-improvement score | Total n | Very much worse | Much worse | Minimally worse | unchanged | Minimally improved | Much improved | Very much improved |
| Score | 7 | 6 | 5 | 4 | 3 | 2 | 1 | |
| Intervention Group | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Control Group | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| CGI-severity score | Total n | Extremely ill | Severely ill | Markedly ill | Moderately ill | Mildly ill | Borderline mentally ill | Normal, not at all ill |
| Score | 7 | 6 | 5 | 4 | 3 | 2 | 1 | |
| Intervention Group | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Control Group | N | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
Many assume that ≥75% BPRS/PANSS reduction is rare in schizophrenia. Nevertheless, in an analysis of 1870 patients in randomized amisulpride trials approximately 25% of the participants reached at least 75% BPRS reduction21. The advantage of such a presentation is that the reader gets an impression of the distribution of the response. Presenting results based on only one cutoff cannot provide such information and the choice of the cutoff remains somewhat arbitrary. It should be noted that when 1-7 scaling of the BPRS/PANSS were used the 18/30 minimum score needs to be subtracted when calculating percentage reduction from baseline21. For the statistical analysis of a clinical trial it is important to choose a primary cutoff a priori to avoid problems of multiple testing. Even if only the Clinical Global Impression Scale13 was used as a response criterion, the results could be shown in a similar fashion presenting the number of participants who were unchanged, minimally improved and much improved; or not ill, mildly ill, severely ill etc. A new version of the CGI that is specific for schizophrenia has recently been developed. The schizophrenia version uses the same items and scores, but provides clear anchors as to what “mildly ill” or “moderately ill” means. Furthermore, there are subscales for positive, negative, depressive and cognitive symptoms using the same scoring system. In contrast to the original CGI, the psychometric properties of the new version have been examined and found to be sufficient23.
In applying the response measure in clinical practice and clinical trials, it is also important to understand the context. For example, we have recently investigated the early response paradigm, in which improvement (or rather lack of improvement) after only one or two weeks is used as a “biomarker” to predict subsequent response. In this context, the threshold that has been used for “response” is a 20% or greater improvement on the PANSS24, but this is early response and should not be confused with ultimate response where such “minimal” improvement would be unacceptable.
Remission
Remission can be defined as a state of absence of significant symptoms. This is an important treatment goal. However, similarly to the different available response criteria, clinical and epidemiological studies on the frequency of remission in schizophrenia were hampered by the lack of a uniformly accepted definition. For example, a series of long-term studies suggested that many patients may be in remission or even recover in the long run [for a review, see25, but any comparison is difficult due to the variety of definitions used. In 2005, American and European expert groups suggested a remission definition for schizophrenia20, which has been adopted by subsequent studies26, 27. According to these criteria, a patient is in remission if eight items of the PANSS12 or corresponding items on the BPRS11 or on the Scale for the Assessment of Positive Symptoms28 or on the Scale for the Assessment of Negative Symptoms29 are rated as “mildly present” or better (Table 3). In addition to the severity criterion there is also a time component which requires that this low level of symptoms must persist for at least six months, although short-term trials may only apply the severity criterion on a cross-sectional basis as previously mentioned.
Table 3. Proposed items for remission criteria as defined by the Remission in Schizophrenia Working Group (Andreasen et al 2005 20).
| Scale for Assessment of Positive Symptoms (SAPS)28 and Scale for Assessment of Negative Symptoms (SANS)29 Items | Positive and Negative Syndrome Scale (PANSS)12 Items | Brief Psychiatric Rating Scale (BPRS)11 Items | |||||
|---|---|---|---|---|---|---|---|
| Dimension of Psychopathology | DSM-IV Criterion | Criterion | Global Rating Item Number | Criterion | Item number | Criterionb | Item number |
| Psychoticism (reality, distortion) | Delusions | Delusions (SAPS) | 20 | Delusions | P1 | Grandiosity | 8 |
| Hallucinations | Hallucinations (SAPS) | 7 | Unusual thought content | G9 | suspiciousness | 11 | |
| Hallucinatory behaviour | P3 | Unusual thought content | 15 | ||||
| Hallucinatory behaviour | 12 | ||||||
|
| |||||||
| Disorganization | Disorganised speech | Positive formal thought disorder (SAPS) | 34 | Conceptual disorganization | P2 | Conceptual disorganization | 4 |
| Grossly disorganised or catatonic behaviour | Bizarre behaviour (SAPS) | 25 | Mannerisms/posturing | G5 | Mannerisms/posturing | 7 | |
|
| |||||||
| Negative symptoms (psychomotor poverty) | Negative symptoms | Affective flattening (SANS) | 7 | Blunted effect | N1 | Blunted affect | 16 |
| Avolition-apathy (SANS) | 17 | Social withdrawal | N4 | No clearly related symptom | |||
| Anhedonia-asociality (SANS) | 22 | Lack of spontaneity | N6 | No clearly related symptom | |||
| Alogia (SANS) | 13 | ||||||
For symptomatic remission, maintenance over a 6-month period of simultaneous ratings of mild or less on all items is required. Rating scale items are listed by item number
Use of BPRS criteria may be complemented by use of the SANS criteria for evaluating overall remission
The PANSS scale is the simplest instrument on which a definition of symptom remission can be practically based.
DSM-IV=Diagnostic and Statistical Manual of Mental Disorders, fourth edition
The rationale for the selection of the eight PANSS items was that they reflect core symptoms that are required according to DSM-IV for the diagnosis of schizophrenia. The rationale for the severity threshold “mildly present at worst” was that such mild symptoms would not interfere with a patient's psychosocial functioning. This definition is also a compromise accounting for the reality of clinical trials. Two analyses of large databases of double-blind trials showed that very few patients reach the clinical state of being fully free of symptoms30, 31, so that a more stringent threshold (“not more than questionable symptoms” or “no symptoms at all”) would not have been clinically realistic. In addition, it was also taken into consideration that there is a dimensional distribution of mild and quasi-psychotic symptoms in a subgroup of the general population, and that – for similar reasons - the remission criteria of other chronic illnesses, such as e.g. polyarthritis, also do not require the complete absence of symptoms.
Advantages and Disadvantages of Response and Remission Criteria
The difference between response and remission is that response based on a percentage BPRS or PANSS reduction from baseline does not provide information on how symptomatic the patient is at endpoint. A reduction on the PANSS from 120 to 60 points is a 50% reduction, as is a change from 80 to 40 points. (In addition, the PANSS is an interval scale, which means that a score of 6 is not necessarily twice as severe as a score of 32.) However, the patient with a score of 60 is far more symptomatic than the patient with a score of 40, although he had an absolute change score of 60 as compared to 40 points. The remission criteria provide information as to where patients end up, i.e., are they still symptomatic? At the same time, the remission criteria do not reflect the amount of change. For example, if at baseline the participants were on the average only mildly ill, many will be in remission at the end of the trial, although there was not a major reduction in symptoms17. Based on the above, we suggest that the best way of reporting symptomatic outcome in schizophrenia trials is to display both measures.
Treatment Resistance
Compared to simple “non-response”, treatment resistance/refractoriness implies a more persistent lack of improvement despite adequate treatment. It is important to emphasize that adequate treatment implies adequate adherence. It is likely that many patients are inappropriately considered to be treatment resistant when they are actually non-adherent33. The definition is at least as complex as that of response and remission. Numerous criteria have been used (Table 4)2, 3, 4, 5, 19, 34, 35, 36. Most often, such criteria focus on positive symptoms, but negative symptoms, affective symptoms, disturbed behavior and cognitive dysfunction can also play a role, because are associated with psychosocial and educational/vocational dysfunction. From a conceptual point of view, such definitions can span a wide range. Such definitions are often used in research. A good example are the criteria applied in a landmark study demonstrating clozapine's superiority compared to chlorpromazine in treatment refractory patients19. Based on history, patients had received in the preceding five years three antipsychotics from two different classes at a dosage of at least 1000 mg/day chlorpromazine equivalents for at least 6 weeks without significant clinical improvement, and without good functioning in the last 5 years (Table 4). Cross-sectionally, the patients had a BPRS total score ≥45, were at least moderately ill according to the CGI and exhibited four at least moderately pronounced BPRS positive symptoms. Prospectively, the patients had not responded to a six-week trial with haloperidol of up to 60 mg/day (non-response was defined as < 20% BPRS reduction and BPRS total score >35 and a CGI-severity score >3). It is also important to acknowledge the attempt of an international study group which described treatment resistance by combining symptoms and social functioning on a scale from 1 (complete remission) to 7 (severe therapy resistance)34.
Table 4. Criteria for treatment resistance according to a selection of authors and guidelines.
| Reference | Definition |
|---|---|
| Kane et al 1988 19 | Historical: no period of good functioning or significant symptomatic relief within preceding 5 years despite at least two courses of antipsychotics (doses:≥ 1000 mg/day chlorpromazine) for 6 weeksCross-sectional: BPRS score ≥ 45, score of ≥ 4 on at least two of the following factors: conceptual disorganization, suspiciousness, hallucinatory behavior, unusual thought contents, CGI score of ≥-4Prospective: 6-week trial of haloperidol- (60mg/day) fails to reduce BPRS by 20% or to below 35, or fails to reduce CGI to below 3 |
| Brenner et al 1990 34 | Seven levels of treatment response incorporating evaluation of symptomatology, personal and social adjustment: level 1, clinical remission; level 2, partial remission; level 3; slight resistance; level 4, moderate resistance; level 5, severe resistance; level 6, refractory; level 7, severely refractory |
| Meltzer 1990 35 | At least in theory every patient who has not fully recovered to his premorbid level of functioning should be regarded as treatment refractory |
| National Institute for Clinical Excellence 2003 3 | Treatment resistance is suggested by a lack of satisfactory clinical improvement despite the sequential use of the recommended doses for 6–8 weeks of at least two antipsychotic drugs, at least one of which should be an atypical. |
| American Psychiatric Association, Lehman et al 2004 2 | Treatment resistance is defined as little or no symptomatic response to multiple (at least two) antipsychotic trials of an adequate duration (at least 6 weeks) and dose (therapeutic range). |
| World Federation of Societies of Biological Psychiatry, Falkai et al 2005 4 | Treatment resistance is assumed if there is either no improvement at all or only insufficient improvement in the target symptoms, despite treatment at the recommended dosage for a duration of at least 6/8 weeks with at least two antipsychotics, one of which should be an atypical antipsychotic. |
| Australian National schizophrenia guideline, McGorry 2005 5 | Two adequate trials (at least 6 weeks of 300–1000 mg in CPZ equivalents) of antipsychotic medication, of which at least one agent should be atypical, should have been conducted. |
| International Pharmacological Algorithm Project 2006 36 | 1) No period of good functioning in previous 5 years; 2) prior non-response to at least 2 antipsychotic drugs of two different chemical classes for at least 4-6 weeks each at doses ≥ 400 mg equivalents of chlorpromazine or 5 mg/day risperidone; 3) moderate to severe psychopathology, especially positive symptoms: conceptual disorganization, suspiciousness, delusions or hallucinatory behavior. IPAP also recommend considering patients as treatment resistant if they exhibit persistent psychotic symptoms, recurrent mood symptoms, repeated suicide attempts or suicidal ideation, uncontrolled aggressive behavior, moderate- severe negative symptoms or moderate-severe cognitive impairment after the adequate treatment mentioned above. |
However, the choice of the specific criteria will depend on the circumstances. For example, the extremely stringent criteria in the study by Kane et al19 were necessary in the context of the reintroduction of a potentially life-threatening antipsychotic drug (i.e., clozapine and its risk for agranulocytosis). Nevertheless, at least in schizophrenia practice guidelines, a certain consensus regarding criteria for treatment resistance seems to emerge (Table 4)2, 3, 4, 5, 19, 34, 35, 36. The American Psychiatric Association guideline2 defines treatment resistance as “little or no symptomatic response to multiple (at least two) antipsychotic trials of an adequate duration (at least 6 weeks) and dose (therapeutic range)”. Other important guidelines such as that by the National Institute for Clinical Excellence (NICE)3, the World Association of Societies of Biological Psychiatry4, or those of other national psychiatric associations present similar definitions (Table 4).
Relapse
Relapse is another important outcome measure, as it often triggers a change in treatment or locus of care. Relapse can be caused by many different factors ranging from comorbid substance abuse to non-adherence, but might also mean that a patient has “broken through” medication. (On some level, a patient who relapses despite taking an adequate dose of antipsychotic medication could be considered to be treatment resistant, but, that is not how the latter term is usually employed.) Since relapse implies an exacerbation or recurrence of symptoms, the critical question is what degree of worsening or what threshold of signs and symptoms is necessary before triggering such a classification? Should the degree of worsening imply a change in functional status or is a purely symptomatic definition sufficient? Relapse is a commonly used outcome measure in clinical trials intended to investigate the efficacy and effectiveness of treatments in the intermediate and long-term management of schizophrenia. Again, the context of use of the term relapse is important, as a trial which includes a placebo arm might have different relapse criteria than an active-active comparator trial in that, from an ethical standpoint, one would want to minimize the risks associated with placebo, while still maintaining the overall goals of the trial. In addition, it is clear that symptoms can wax and wane in schizophrenia without being sufficiently impactful to consider that a relapse has taken place.
The degree of variability in relapse rates seen across studies with similar entry criteria (Table 5) also suggests that patient populations as well as clinician/rater behavior can be different even within the same general protocol design. Although relapse does not necessarily require a change in functional status, many criteria include a change in treatment requirements, intensity of services or locus of care. These in turn are influenced by functional considerations, as are to some extent the severity ratings of specific domains of psychopathology (i.e., a symptom which influences behavior/functioning is a more severe symptom than one which does not). Table 537, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 provides examples of relapse criteria that have been used in recent studies.
Table 5. Relapse Definition in Trials Comparing Oral Second-Generation Antipsychotics to Placebo or to First-Generation Antipsychotics.
| Study | Domains of Relapse Definition | |||
|---|---|---|---|---|
| Hospitalization | CGI 13 | PANSS 12 or BPRS 29 | Clinical Symptoms and Others | |
| Dellva 1997 37; Tran 1998 38; De Sena 2003 39 | hospitalization | - | - | - |
| Cooper 2000 40 a,b,c | hospitalization | CGI-S ↑ ≥2 | 2 of BPRS-P subscale ↑≥2 | - |
| CGI-S ↑ ≥2 | 2 of BPRS-P subscale ↑≥2 | - | ||
| CGI-S ≥6 | - | Need observation if patient is inpatient | ||
| Csernansky 2002 41; Schooler 2005 42 | hospitalization | CGI-C ≥6 | PANSS-T ↑≥25%, or +10 | Self-injury, suicidal/homicidal ideation, violence |
| Arato 2002 43 | - | CGI-C ≥6 | PANSS P7+/-G8 ≥6 in 2 successive days | - |
| Beasley 2003 44 | hospitalization | - | any BPRS-P subscale ≥4 and BPRS specific subscale ↑≥2, or positive subscale↑≥4 | Completed suicide or a serious suicide attempt |
| Piggot 2003 45 | - | CGI-C ≥5 | PANSS P7/G8 ≥5 on 2 successive days or PANSS-T ↑ ≥20% | - |
| Lieberman 2003 46; Green 2006 47 | - | CGI-S ≥4 | Any of PANSS P1, 2, 3, 5, 6 ≥4 | - |
| Lecrubier 2006 48 | hospitalization | - | PANSS-T ↑≥30% | - |
| Peuskins 2007 49 | hospitalization | CGI-C ≥6 | PANSS-T ↑≥30% | Need for additional AP |
| Kramer 2007 50 | hospitalization | CGI-S ≥4 or 3→5 | PANSS-T ↑≥25% or 10 points, or specific PANSS item 3→5 or 4→6 | Self-injury, suicidal/homicidal ideation, violence |
| Gaebel 2007 51 a | - | CGI-C ≥6 | PANSS-P ↑>10 | GAF ↓>20 |
| Kane 2008 52 | hospitalization | CGI-S↑ ≥2 | - | Discontinuation due to lack of efficacy |
| Crespo-Facorro 2010 53 | hospitalization | CGI-S ≥6 and CGI-C ≥6 | Any key BPRS subscale ≥5 | Completed suicide attempt |
BPRS-P=brief psychotic rating scale positive symptoms score; CGI-C/S=clinical global impression change/severity score; GAF=global assessment of functioning; PANSS-P/T=positive and negative symptom scale positive/total score; ↑=increase; ↓=decrease;
patients required to meet all criteria concurrently;
CGI and BPRS criteria both required to be present at 2 consecutive assessments over 3 days;
hospitalization, CGI and BPRS criteria required to be present at the same assessment time point;
all criteria required to be present at two consecutive visits
It is also important to note that in some studies hospitalization is used as a proxy for relapse. Since hospitalization can be influenced by many environmental and health economic factors, those influences must be kept in mind as well when hospitalization is equated with relapse. In addition, not all patients who worsen and get close to a relapse need to be hospitalized, as appropriate treatment changes can be made to avoid this. For example, in a study of patients with recent-onset schizophrenia, which examined the clinical course following antipsychotic discontinuation, 96% of the patients experienced an exacerbation or relapse within 2 years, whereas only 13% were hospitalized54.
Recovery
Recovery is an outcome domain, which much more clearly requires functional measures. This is a term that has taken on particular salience with patients and families - as well it should. Health care providers are at times focused on symptoms and signs to the exclusion of functioning and quality of life. The recovery criteria in schizophrenia are to some extent the most meaningful possible outcome measure. At the same time, however, criteria for recovery are not only influenced by the availability of psychosocial treatments, family and community supports, but also by supportive employment and supportive education as well as available jobs, etc.
Liberman and Kopelewicz55 reviewed different criteria for recovery56, 57, 58, 59 (Table 6). The recovery criteria proposed by Liberman and colleagues57 have been referred to as the UCLA criteria that subsequently have been applied in outcome studies27, 60.
Table 6. Selection of Recovery Criteria in Patients with Schizophrenia.
| Study And Proposed Remission Criteria for Schizophrenia | ||||
|---|---|---|---|---|
| Variable | Harding et al. 1987 56 | Liberman et al. 2002 57 | Torgalsboen et al. 2002 58 | Whitehorn et al. 2002 59 |
| Psychopathology | Symptom free and not taking psychotropic medications | BPRS score of </=4 on all positive and negative psychosis items | No psychiatric hospitalizations for 5 years | PANSS score of </=4 on all scales |
| Psychosocial Functioning | Social life indistinguishable from that of neighbors; holding a job for pay or volunteer | At least-half time work or school; independent management of funds and medications; once weekly socializing with peers | Global Assessment of Functioning (GAF) Score >/=65 | Global Assessment of Functioning (GAF) Score >/=50 |
| Required Duration | Not Listed | 2 Years | 5 Years | 2 Years |
Patient Reported Outcomes
Increasing attention has been focused in recent years on the importance of patient reported outcomes in informing research results and clinical practice61. The U.S. Food and Drug Administration62 defines these measures as “any report coming directly from patients about a health condition and its treatment”. Given the subjective nature of many aspects of psychopathology, it is particularly important in psychiatry to have this perspective. Yet at the same time, in schizophrenia there are concerns about insight, cognitive dysfunction, reality testing and communicative ability, which must be recognized in the acquisition and interpretation of such data. It is also important to recognize the role that such data play in the process of shared decision-making.
Traditionally, clinicians have been more focused on the alleviation and control of illness symptoms and disease treatment, rather than on adverse effects, subjective well-being and quality of life63. An emphasis on self report also contributes to enhanced patient self-esteem and a sense of empowerment. In addition to symptoms, adverse effects and quality of life self-report measures can also provide insight into the patient's knowledge of his/her illness and the treatments being prescribed and into other areas, such as access, quality of care, health system issues, etc.
It is beyond the scope of this paper to review the various constructs involved in the generation of these instruments or the various characteristics of specific instruments. In addition, further work needs to be done to better understand the relationship between clinician-rated and patient- rated outcomes in order to inform their most appropriate utilization and interpretation. It would also be important to determine the value of these measures as predictors of various aspects of outcome including treatment acceptance and adherence as well as overall response, relapse, long-term outcomes, tolerability of adverse effects, etc.
Table 7 provides a selected overview of relevant self report instruments that have been used in studies of patients with schizophrenia64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89. Some scales take relatively little time to complete, making them amenable to being used in the waiting room of mental health clinics and doctor's offices. As always, the choice and interpretation of specific measures will be influenced by the goals of the study or data collection, the characteristics of the patient population, phase and severity of illness, etc.
Table 7. Selected Self Report Measures Scales for Patients with Schizophrenia.
| Domains | Name | Reference | # of items | Administration Time |
|---|---|---|---|---|
| Symptom | Brief Symptom Inventory (BSI) | Derogatis 1992 64 | 53 | 10 |
| Symptom Checklist-90-R (SCL-90-R) | Derogatis 1983 65 | 90 | 15 | |
| Hamilton Program for Schizophrenia Voices Questionnaire (HPSVQ) | Van Lieshout and Goldberg. 2007 66 | 9 | 10 | |
| Insight Scale | Birchwood et al.1994 67 | 32 | 10 | |
| Attitude towards treatment/Care | Drug Attitudes Inventory (DAI-30/10) | Awad 1993 68; Hogan 1983 69 | 30/10 | 15/5 |
| Camberwell Asssessment of Need (CAN) | Phelan et al. 1995 70 | 22 | 15 | |
| Camberwell Asssessment of Need Short Appraisal Schedule (CANSAS) | Slade et al. 1999 71 | 22 | 5 | |
| Verona Service Satisfactioni Scale (VSSS) | Ruggeri and Dall'Agnola. 1993 72 | 82 | 30 | |
| Scale To Assess the Therapeutic Relationship (STAR) | McGuire-Snieckus et al. 2007 73 | 12 | 10 | |
| Recovery | Mental Health Recovery Measure (MHRM) | Yound and Bullock 2003 74 | 30 | 15 |
| Recovery Assessment Scale (RAS) | Giffort et al.1995 75 | 41 | 30 | |
| QOL/subjective well-being (for psychiatric disease) | Lancashire Quality of Life Profile (LQOLP) | Oliver et al. 1997 76 | 105 | 45 |
| Personal Evaluation of Transitions in Treatment (PETIT) | Voruganti and Awad. 2002 77 | 30 | 5 | |
| Quality of Life Questionnaire in Schizophrenia (S-QoL) | Auquier et al. 2003 78 | 41 | 15 | |
| Subjective Wellbeing under Neuroleptic Treatment Scale (SWN original/short form) | Naber 1995 79; Naber et al. 2001 80 | 38/20 | 20/10 | |
| QOL (generic) | World Health Organization Quality of Life Assessments (WHOQOL-100/BRIEF) | WHOQOL group 1998 81 | 100/26 | 45/10 |
| 36-Item Short Form Health Survey (SF-36) | Ware and Sherbourne 1992 82 | 36 | 15 | |
| EQ-5D | EuroQOL group 190 83 | 5 items + 1 visual analogue scale | 5 | |
| Sheehan Disability Scale (SDS) | Sheehan et al. 1983 84 | 3 visual analogue scales + 2 items | 3 | |
| Others | Psychological Stress Index (PSI) | Tso et al. 2011 85 | 18/9 | 10/5 |
| Self Esteem Scale | Rosenberg 1965 86 | 10 | 10 | |
| Sense of Coherence Scale (SOC) | Antonovsky 1987 87 | 29 | 20 | |
| Knowledge About Schizophrenia Questionnaire (KASQ) | Ascher-Svanum 1999 88 | 25 | 20 | |
| Approaches to Schizophrenia Communication Self-Report (ASC-SR) | Approaches to Schizophrenia Communication (ASC) Steering Group 2001 89 | 18 | 10 |
Clinical Relevance of Outcomes in Schizophrenia
In order to establish the clinical relevance of treatment effects, clinicians should carefully assess and quantify efficacy and adverse effects using direct questioning of patients (see below) and, as much as is practical, also using rating scales. In research studies, validated scales should always be employed, both for therapeutic as well as for adverse effect outcomes. Moreover, adherence and attitudes toward medications that can be used to identify patients at high risk for non-adherence, such as the Drug Attitude Inventory69 should be considered, as non-adherence affects both efficacy and tolerability outcomes.
In addition to selecting the most appropriate assessment tools for the patient's condition and setting, a clinically meaningful display of the data beyond statistical significance should be mandatory in research reports. This includes the reporting of 95% confidence intervals or interquartile ranges around point estimates, as well as the calculation of effect sizes and numbers-needed-to-treat/harm (NNT/NNH). In general, effect sizes of 0.2 or less are considered not clinically relevant, 0.5 is a medium effect size and effect sizes of 0.8 and above are considered large90. Point estimates with non-overlapping 95% confidence intervals are considered significantly different. Moreover, to establish clinically meaningful effect sizes for categorical outcomes of benefit or harm, NNTs/NNHs should be calculated as the inverse of the absolute risk difference (i.e., 1 divided by the delta between the proportion of patients having a certain outcome in one group compared to the other group). NNTs/NNHs of 1-3 represent a large effect size, 4-6 are medium and 7-10 are small91. Furthermore, Likert-type scales that provide ordinal, rather than nominal, data are frequently used in psychiatry. Although each ordinal data point can be converted into a numeric value for ease of data entry (e.g., the CGI scale goes from 1-7), it is important to note that these are not continuous data and that nonparametric, not parametric, statistical tests should be employed in the analysis of such results.
Notwithstanding these general principles, the translation of such mathematical quantification into clinical relevance is not straightforward. This complication is due to the fact that effect sizes for certain efficacy or adverse effect outcomes might be weighted rather differently based on how critical the improvement or intolerability for a given individual is, i.e., how much it affects subjective well-being, quality of life, functioning, health and longevity. Such risk-benefit evaluation must be made on a case-by-case basis and evaluations might change over time, even for the same physician or patient and his/her family92.
Clinical Measurement of Efficacy in Schizophrenia
A number of rating scales and assessment batteries have been validated that are used frequently in clinical pharmacology trials in schizophrenia. While the use of efficacy rating scales is commonplace in research settings, there exists a big research-practice gap regarding the routine implementation of measurement based principles in clinical care. As mentioned above, this is due to a variety of factors, with time constraints and lack of familiarity and training being among the most important ones. Thus, the field, administrators and regulators need to decide which outcome measures are most appropriate that can be realistically implemented in routine clinical practice. Ideally, abbreviated, pragmatic but meaningful scales should be developed and field tested to identify those that could become standard of care.
We propose that, at a minimum, the Clinical Global Impressions (CGI) severity and improvement scale 13 and the Global Assessment of Functioning (GAF) scale93 should be used and documented at each clinical visit (Table 8). While the administration of the entire BPRS11 or PANSS12 is likely not practical in most busy clinical settings, the assessment and documentation of the eight items from the PANSS (or the equivalent items from the BPRS) that are used to define remission in patients with psychosis20 is a reasonable expectation, as a clinical interview should focus on each of these items anyway (i.e., delusions, hallucinations, social withdrawal/anhedonia), with four if these being merely observational (formal thought disorder, bizarre behavior, alogia and blunted affect) (Table 3). Nevertheless, training on the anchored assessment needs to be provided.
Table 8. Commonly Used Rating Scales To Assess Key Efficacy and Adverse Effect Outcomes in Patients with Schizophrenia.
| Efficacy | Adverse Effects | ||
|---|---|---|---|
| Domain | Commonly Used Rating Scale | Domain | Commonly Used Rating Scale |
| Global Outcome | Clinical Global Impressions (CGI) Severity and Improvement Scale 13 Global Assessment of Functioning (GAF) Scale 93 | General | Udvalg for Kliniske Undersøgelser (UKU) 128 Treatment Emergent Side Effect Scale (TESS) 13 |
| General Psychopathology (including Positive and Negative Symptoms) | Brief Psychiatric Rating Scale (BPRS) 11 Positive and Negative Syndrome Scale (PANSS) 12 | Sedation | Agitation-Calmness Evaluation Scale (ACES) 134 |
| Positive Symptoms | Scale for the Assessment of Positive Symptoms (SAPS) 28 | Sexual | Arizona Sexual Experience Scale (ASEX) 135 |
| Negative Symptoms | Scale for the Assessment of Negative Symptoms (SANS) 29 | EPS overall | Simpson-Angus Rating Scale for Extrapyramidal side-effects 125Extrapyramidal Symptom Ratings Scale (ESRS)126 |
| Aggression/Agitation | PANSS Excited Component subscale or PANSS/BPRS “hostility” item11, 12 | Akathisia | Barnes Akathisia Rating Scale (BARS) 124 |
| Depression | Calgary Depression Rating Scale 116 Hamilton Depression Rating Scale (HAM-D) 117 Montgomery-Asberg Depression rating Scale (MADRS) 118 | Dyskinesia | Abnormal Involuntary Movement Scale (AIMS) 127 |
| Anxiety | Hamilton Anxiety Rating Scale (HAM-A) 119 | ||
| Quality of Life | Heinrich Carpenter Quality of Life Scale 120 | ||
In addition, the clinical assessment should also include the specific PANSS (or BPRS) item “hostility”, as this item has been shown to be predictive of overt physical aggression (or violence) against other persons. For example, in schizophrenia patients in the CATIE trial, for each unit increase on the 7-point rating of hostility, the odds of serious violence increased significantly by a factor of 1.694. Hostility and related violent behaviors are clinically relevant since they constitute a frequent reason for hospital admission (being reflected in some criteria for relapse), delay discharge, and increase the burden of illness for families and caregivers. Finally, assessing hostility and violence in schizophrenia has important treatment implications95, 96, 97, 98.
Cognitive deficits are a core feature of schizophrenia and appear to be particularly related to poor functional outcomes99, 100, 101, 102. Although the development of interventions to improve the cognitive deficits in schizophrenia has become a major target, antipsychotics have minimal effects and, to date, no selective treatment has been identified. Key areas of the cognitive deficits in schizophrenia include: attention/vigilance, processing speed working memory, verbal memory, visual memory, reasoning and problem solving, executive functioning and social cognition99. A number of neurocognitive test batteries exist (Table 9) 99, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115. However, these formal tests are usually labor and time intensive and require special training for the administration and scoring. While the mini mental status examination can be used to assess gross cognitive abnormalities, it is too crude to be useful for the assessment of deficits that are below the level of those observed in delirium or dementia. As part of the general mental status examination, a number of attention, memory and reasoning capabilities are crudely assessed by use of the serial 7 (or 3) subtraction, 5 minute 3-word recall, the request to link words through overarching categorical similarities and explain proverbs. Beyond this, few parent, teacher and/or clinician reports/questionnaires are available 114 or under development 115 that could prove to be helpful to provide an office based opportunity to assess cognitive deficits cross-sectionally and over time, which could include beneficial or adverse medication effects on clinically relevant, real world cognitive functioning.
Table 9. Cognitive Test Batteries and Questionnaires.
| Test | Cognitive Domains | Administration Time | Pros/Cons |
|---|---|---|---|
| Neuropsychological Tests | |||
| Measurement and Treatment to Improve Cognition in Schizophrenia (MATRICS) 99 | Speed of processing | 60 min | Interview-based rating scale Strong correlation with functional outcome |
| Attention/vigilance | |||
| Working memory | |||
| Verbal learning | |||
| Visual learning | |||
| Reasoning/problem solving | |||
| Social cognition | |||
| Clinical Global Impression of Cognition in Schizophrenia (CGI-CogS) 104 | Speed of processing | 30 min | Interview-based rating scale Strong correlation with functional outcome |
| Attention/vigilance | |||
| Working memory | |||
| Verbal learning | |||
| Visual learning | |||
| Reasoning/problem solving | |||
| Social cognition | |||
| CogState Schizophrenia Battery 105, 106 | Speed of processing | 35 min | Valid measurement of MATRICS cognitive domains Designed for clinical trial use |
| Attention/vigilance | |||
| Working memory | |||
| Verbal learning | |||
| Visual learning | |||
| Reasoning/problem solving | |||
| Social cognition | |||
| CogState 12-Minute Battery 107 | Executive function | 12 min | Designed for clinical trial use |
| Psychomotor function | |||
| Visual attention | |||
| Visual learning | |||
| Schizophrenia Cognition Rating Scale (SCoRS) 108 | Speed of processing | 12–14 min for each of 3 interviews (patient, informant, and interviewer) | Excellent correlations with performance and functional outcomes Appropriate for clinical use |
| Attention/vigilance | |||
| Working memory | |||
| Verbal learning | |||
| Visual learning | |||
| Reasoning/problem solving | |||
| Repeatable Battery for Assessment of Neuropsychological Status (RBANS) 109, 110 | Immediate memory | 25–45 min | Lacks measures of motor, executive, and working memory Appropriate for clinical use |
| Visuospatial/constructional ability | |||
| Language | |||
| Attention | |||
| Delayed memory | |||
| Brief Assessment of Cognition in Schizophrenia (BACS) 111 | Reasoning/problem solving | <35 min | Appropriate for clinical use |
| Verbal fluency | |||
| Attention | |||
| Verbal memory | |||
| Working memory | |||
| Motor speed | |||
| Brief Cognitive Assessment Tool for Schizophrenia (B-CATS) 112 | Attention | 10–11 min | Appropriate for clinical setting |
| Language | |||
| Processing speed | |||
| 5-minute Digit Symbol Coding Task 113 | Processing speed | 5 min | Limited to 1 domain Appropriate for clinical setting |
| Questionnaires/Interviews | |||
| Behavior Rating Inventory of Executive Functioning (BRIEF) 114 | Behavioral Regulation Index (BRI): Inhibit, Shift, and Emotional Regulation subdomains. | 10-15 minutes (as per authors) | Scale consists of a 86 questions real-world task related Parent Form and 86 questions Teacher Form. Questions are answered as “never, “sometimes”, “often”. |
| Metacognition Index (MCI): Initiate, Working, Memory, Plan/Organize, Organization of Materials, and Monitor subdomains. | |||
| Global Executive Composite (GEC) = BRI + MCI | |||
| New York Assessment of Adverse Cognitive Effects of Neuropsychiatric Treatment (NY-AACENT) 115 | Working memory | 5-7 minutes | Clinician interview integrates informant data, patient self-report (separate forms) and indirect assessment based on real-life observations/impact. 7 probes/items rated “not present”, “a little bit bothersome”, “somewhat bothersome”, “quite bothersome”, “very bothersome” (Note: validation underway). Appropriate for clinical use |
| Attention/Vigilance | |||
| Verbal Learning/Memory | |||
| Visual Learning/Memory | |||
| Reasoning & Problem Solving | |||
| Speed of Processing | |||
| Social Cognition |
Regarding related psychopathology, such as depression and anxiety, at a minimum, a global, two-item assessment of “observed” and “reported” severity of these domains should be ascertained and reported using a 10-point visual analogue scale or a Likert-like scale (none, mild, moderate severe, extreme). Neuro-vegetative signs (appetite, sleep, activity level) should also be part of a regular clinical interview and should be quantified in the same way. Areas of high clinical importance for the safety of the patients and of others, i.e., suicidality and homicidality should also always be inquired about. It is recommended that medical records be equipped with such simple rating tools and that clinicians have to fill out these scales before being able to move on to another page when using electronic medical records. Obviously, in settings with more time and depending on the aim, formal rating scales can also be employed for depression116, 117, 118 and anxiety119.
While quality of life and functional outcomes are increasingly relevant as treatment aims, there are currently no simple tools to measure these outcomes, as available interview-based scales are lengthy [e.g., 120] and simpler self reports [e.g., 84] may lack detail and be too insensitive. Thus, qualitative statements about who the patient lives and interacts with, how many times per week social contacts take place outside of the immediate family, whether the patient can provide self care and what the voluntary or paid employment status is should be inquired about and recorded at regular time intervals. These areas are useful to assess, as they overlap with proposed psychosocial recovery criteria reviewed above57. Furthermore, to document treatment decisions, inefficacy in specific domains should be recorded as a justification to switch or augment any given treatment. Finally, adherence also needs to be inquired about and quantified, so that efficacy patterns can be evaluated more objectively.
Clinical Measurement of Adverse Effects in Schizophrenia
Antipsychotic treatment is associated with a wide range of acute and long-term side-effects that can impact on psychiatric and physical health, adherence, subjective well-being and quality of life121. However, identifying side-effects and attributing them to a particular drug can be difficult because patients are frequently on more than one medication and cannot always describe the onset and circumstances of their experiences in detail. Some patients with schizophrenia are not even aware that certain experiences can be a drug effect, requiring counseling when initiating treatment. Some domains, such as sexual side-effects and constipation, are less readily volunteered and patients may only report these side effects when directly questioned about them122.
Moreover, it can be difficult to distinguish some adverse effects from illness symptoms, e.g. Parkinsonism from negative symptoms or depression, and akathisia from agitation123. Side-effects are best assessed by using a standardized rating scale and a number of global and specific rating scales are available (Table 8). Some measure specific side-effects, such as the Barnes Aktathisia Ratings scale for assessing akathisia124, the Simpson Angus Scale125, Extrapyramidal Symptom Rating Scale 126 or the Abnormal Involuntary Movement scale127, whereas others, such as the Udvalg for Kliniske Undersøgelser (UKU) scale or the Treatment Emergent Side Effect Scale (TESS)13, provide an overview of side-effects128. Side-effects are not simple dichotomous variables and their presence differs among subjects. Therefore, rating scales should include thorough anchors for the score of each item. Moreover, the severity, attribution to a given medication and onset and offset need to be captured. Furthermore, rating scales generally only assess the presence and severity of a side-effect, but not whether the side-effect is subjectively bothersome or associated with functional impairment, although quality of life is more affected by the subjective feeling of a side-effect than the number of side-effects129. Some patients feel sedation as a bothersome side-effect whereas others are not particularly bothered by it and may even see this as a desired effect. Both past and present side-effects have a negative impact on compliance which should encourage psychiatrists' to do their best to avoid, monitor and manage adverse effects in order to optimize treatment outcomes130.
In clinical trials, most frequently adverse effect are assessed by means of general, open ended and unstructured questioning, rather than by rating scales. This is supposedly done to reduce the background noise of symptoms not associated with a given treatment or not reaching the level of subjective relevance. However, some symptoms might have been relevant and are not reported due to cognitive difficulties or feelings of shame, or lack of knowledge that a symptom could be related to a medication. Moreover, in many trials, the absence of rating scale assessed side effects is used as a justification to solely report frequencies, but not statistically analyze and compare them against control conditions. This has remained the case in regulatory trials performed to gain approval or indications. This is an obvious bias against a state-of-the art assessment of adverse effects that are not treated the same way as efficacy outcomes. In fact, a recent systematic review of adverse effect reporting in 167 antipsychotic clinical trials in schizophrenia-spectrum disorder patients published in English between January 2002 and July 2007 with available efficacy and/or adverse effect reporting found that safety and tolerability data were collected and reported in mostly non-standardized ways, which does not allow a fair and meaningful comparison of the relative risk profiles of individual antipsychotics131. Across these studies, EPS and weight gain were most frequently assessed, but a minority of studies included reporting of metabolic abnormalities, negative subjective experiences and sexual dysfunction. Published rating scales were frequently used to evaluate EPS, but systematic methods were rarely applied to any other treatment-emergent problems. Moreover, the definition of individual adverse effects and the method of reporting were inconsistent131.
We propose that, at a minimum, key adverse areas that should be inquired about in patients treated with antipsychotics (sedation, EPS, dyskinesia, sexual functioning) should be quantified either along a 10-point visual analogue scale or using a Likert-like scale (none, mild, moderate severe, extreme). Furthermore, Parkinsonian side effects and abnormal involuntary movements should be measured directly at least twice per year, using the Simpson Angus Scale125 or the ESRS126, the Barnes Akathisia Scale124 and the AIMS127 (Table 8). This takes less than 5 minutes when both the clinician and patient are familiar with this assessment. In addition, cardiometabolic indices, such as body weight, body mass index, waist circumference and fasting glucose and lipids, should be measured and documented at currently recommended time intervals132. Implementation of monitoring guidelines can be difficult133, but low-cost, strategic and administrative interventions can help increase guideline compliance. Finally, as for efficacy, to document treatment decisions, intolerability in specific areas should be recorded as a justification to switch or augment any given treatment.
Summary and Conclusions
Quantification of treatment effects has relevance for patient management, research, and overall health care. Optimized treatment of schizophrenia aims for improvements in symptoms, subjective well being and functioning, while minimizing adverse effects that interfere with treatment success. Clinical decision making requires the standardized definition of treatment goals and outcomes, and the quantification of efficacy, adverse effects and overall effectiveness to inform what degree of improvement in target domains is sufficient, what type and severity of adverse effects are still acceptable and what functional outcomes are sought. Rational decisions about dose adjustments, when to stop a medication, when to switch or augment treatments, etc require measurement based approaches. While rating scales are ubiquitous in schizophrenia research, time constraints, lack of familiarity with and training in validated assessment tools has limited their routine use in clinical practice. Easy to use but meaningful rating scales need to be developed and implemented to bridge the gap between lengthy rating scales used in research trials and mostly unstructured, qualitative assessments employed in clinical practice. Moreover, results from research trials providing the evidence base that guide practice need to be communicated in clinically meaningful ways. This includes going beyond the mere reliance on statistical significance. Pragmatic quantification should always include the reporting of effect sizes, numbers-needed-to-treat and -harm for meaningful categorical outcomes, confidence intervals, and absolute risk differences. Some important outcomes, such as treatment response, should be reported in escalating intervals using incrementally stringent psychopathology improvements.
Nevertheless, even despite quantification, it remains a challenge to weigh individual efficacy and adverse effect outcomes against each other and to decide on the targeted maximum improvement or outcome. Subjective, patient-based ratings and shared decision making need to be integrated with measurement based approaches. Finally, beyond consensus about meaningful outcome definitions, reporting strategies and pragmatic tool development and implementation, the discovery of novel treatment mechanisms and biomarkers is hoped to further advance measurement based approaches in schizophrenia and improve patient outcomes in the near future.
Acknowledgments
Supported in part by the NIMH Advanced Center for Services and Intervention Research, The Zucker Hillside Hospital (P30MH090590).
Footnotes
Financial Disclosures: Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, IntraCellular Therapies, Ortho-McNeill/Janssen/J&J, Merck, Novartis, Otsuka, Pfizer, and Sepracor/Sunovion. He has received grant support from the Feinstein Institute for Medical Research, the National Institute of Mental Health (NIMH), and the National Alliance for Research in Schizophrenia and Depression (NARSAD) and Ortho-McNeill/Janssen/J&J.
Dr. Kishimoto has received speaker's honoraria from Banyu, Eli Lilly, Dainippon Sumitomo, Janssen, Novartis, Otsuka and Pfizer. He has received grant support from the Byoutaitaisyakenkyukai Fellowship (Fellowship of Astellas Foundation of Research on Metabolic Disorders) and Eli Lilly Fellowship for Clinical Psychopharmacology.
Dr. Kane has been a consultant to Astra-Zeneca, Janssen, Pfizer, Eli Lilly, Bristol-Myers Squibb, Dainippon Sumitomo/Sepracor/Sunovion, Johnson & Johnson, Otsuka, Vanda, Proteus, Takeda, Targacept, IntraCellular Therapies, Merck, Lundbeck, Novartis, Roche, Rules Based Medicine, Sunovion and has received honoraria for lectures from Otsuka, Eli Lilly, Esai, Boehringer-Ingelheim, Bristol-Myers Squibb, and Janssen. He has received grant support from The National Institute of Mental Health.
Dr. Nielsen has received research grants from H. Lundbeck, Pfizer and Chempaq for clinical trials and received speaking fees from Bristol-Myers Squibb, Astra Zeneca, Janssen & Cilag, Lundbeck and Eli-Lilly.
References
- 1.Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. Journal of Clinical Psychiatry. 2010;71(9):1115–24. doi: 10.4088/JCP.10r06264yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J, McIntyre JS, Charles SC, Altshuler K, Cook I, Cross CD, Mellman L, Moench LA, Norquist G, Twemlow SW, Woods S, Yager J, Gray SH, Askland K, Pandya R, Prasad K, Johnston R, Nininger J, Peele R, Anzia DJ, Benson RS, Lurie L, Walker RD, Kunkle R, Simpson A, Fochtmann LJ, Hart C, Regier D. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2):1–56. [PubMed] [Google Scholar]
- 3.National Institute for Clinical Excellence. Schizophrenia: Full national guideline on core interventions in primary and secondary care. Royal College of Psychiatrists. 2003 [Google Scholar]
- 4.Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Moller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) - Guidelines for biological treatment of schizophrenia, part 1: Acute treatment of schizophrenia. World J BiolPsychiatry. 2005;6(3):132–191. doi: 10.1080/15622970510030090. [DOI] [PubMed] [Google Scholar]
- 5.McGorry PD. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry. 2005;39(1-2):1–30. doi: 10.1080/j.1440-1614.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 6.Moore TA, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Essock SM, Finnerty M, Marder SR, Miller del D, McEvoy JP, Robinson DG, Schooler NR, Shon SP, Stroup TS, Miller AL. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68(11):1751–62. doi: 10.4088/jcp.v68n1115. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, Himelhoch S, Fang B, Peterson E, Aquino PR, Keller W. Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB. Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94–103. doi: 10.1093/schbul/sbp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correll CU, Kishimoto T, Kane JM. Randomized Controlled Trials in Schizophrenia: Opportunities, Limitations and Novel Trial Designs. Dialogues Clin Neurosci. 2011;13(2):155–72. doi: 10.31887/DCNS.2011.13.2/ccorrell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane JM, Correll CU. Pharmacologic treatment of schizophrenia. Dialogues Clin Neurosci. 2010;12(3):345–57. doi: 10.31887/DCNS.2010.12.3/jkane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:790–812. [Google Scholar]
- 12.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–275. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Guy W. Clinical Global Impression in ECDEU assessment manual for psychopharmacology, revised (DHEW Publ No ADM 76-338) National Institute of Mental Health; Rockville, MD: p. 1976. [Google Scholar]
- 14.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. Clinical implications of BPRS scores. Br J Psychiatry. 2005;187:363–371. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- 15.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: Clinical implications. Neuropsychopharmacology. 2006;31(10):2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 17.Leucht S, Kane JM. Measurement based psychiatry: definitions of response, remission, stability and relapse in schizophrenia. J Clin Psychiatry. 2006;67:1813–1814. doi: 10.4088/jcp.v67n1120. [DOI] [PubMed] [Google Scholar]
- 18.Levine SZ, Rabinowitz J, Engel R, Etschel E, Leucht S. Extrapolation between measures of symptom severity and change: An examination of the PANSS and CGI. Schizophr Res. 2008;98(1-3):318–22. doi: 10.1016/j.schres.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Kane JM, Honigfeld G, Singer J, Meltzer H and the Clozaril Collaborative study group. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 20.Andreasen N, Carpenter W, Kane J, Lasser R, Marder S, Weinberger D. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;62:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 21.Leucht S, Davis JM, Engel RR, Kane JM, Wagenpfeil S. Defining 'response' in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacology. 2007;32:1903–1910. doi: 10.1038/sj.npp.1301325. [DOI] [PubMed] [Google Scholar]
- 22.Boter H, Peuskens J, Libiger J, Fleischhacker WW, Davidson M, Galderisi S, Kahn RS EUFEST study group. Effectiveness of antipsychotics in first-episode schizophrenia and schizophreniform disorder on response and remission: an open randomized clinical trial (EUFEST) Schizophr Res. 2009 Dec;115(2-3):97–103. doi: 10.1016/j.schres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Haro JM, Kamath SA, Ochoa S, Novick D, Rele K, Fargas A, Rodriguez MJ, Rele R, Orta J, Kharbeng A, Araya S, Gervin M, Alonso J, Mavreas V, Lavrentzou E, Liontos N, Gregor K, Jones PB. The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand. 2003;107:16–23. doi: 10.1034/j.1600-0447.107.s416.5.x. [DOI] [PubMed] [Google Scholar]
- 24.Kinon BJ, Lei C, Ascher-Svanum H, Stauffer VL, Kollack-Walker S, Wei Z, Kapur S, Kane JM. Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology. 2010;35:581–590. doi: 10.1038/npp.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leucht S, Lasser R. The concepts of remission and recovery in schizophrenia. Pharmacopsychiatry. 2006;39(5):161–170. doi: 10.1055/s-2006-949513. [DOI] [PubMed] [Google Scholar]
- 26.van Os J, Burns T, Cavallaro R, Leucht S, Peuskens J, Helldin L, Bernardo M, Arango C, Fleischhacker W, Lachaux B, Kane JM. Standardized remission criteria in schizophrenia. Acta Psychiatrica Scandinavica. 2006;113(2):91–95. doi: 10.1111/j.1600-0447.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 27.Emsley R, Chiliza B, Asmal L, Lehloenya K. The concepts of remission and recovery in schizophrenia. Curr Opin Psychiatry. 2011;24(2):114–21. doi: 10.1097/YCO.0b013e3283436ea3. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City: p. 1984. [Google Scholar]
- 29.Andreasen Nancy C. Scale for the Assessment of Negative Symptoms (SANS) Br J Psychiatry. 1989;155(Suppl 7):53–58. [PubMed] [Google Scholar]
- 30.Beitinger R, Lin J, Kissling W, Leucht S. Comparative remission rates of schizophrenic patients using various remission criteria. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1643–1651. doi: 10.1016/j.pnpbp.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Leucht S, Beitinger R, Kissling W. On the concept of remission in schizophrenia. Psychopharmacology. 2007;194(4):453–461. doi: 10.1007/s00213-007-0857-1. [DOI] [PubMed] [Google Scholar]
- 32.Obermeier M, Schennach-Wolff R, Meyer S, Möller HJ, Riedel M, Krause D, Seemüller F. Is the PANSS used correctly? a systematic review. BMC Psychiatry. 2011;18(11):113. doi: 10.1186/1471-244X-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkis H. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2007;30(3):511–33. doi: 10.1016/j.psc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Brenner HD, Dencker SJ, Goldstein MJ, Hubbard JW, Keegan DL, Kruger G, Kulhanek F, Liberman RP, Malm U, Midha KK. Defining treatment refractoriness in schizophrenia. Schizoph Bull. 1990;16(4):551–561. doi: 10.1093/schbul/16.4.551. [DOI] [PubMed] [Google Scholar]
- 35.Meltzer HY. Commentary: defining treatment refractoriness in schizophrenia. Schizophr Bull. 1990;16(4):563–565. doi: 10.1093/schbul/16.4.563. [DOI] [PubMed] [Google Scholar]
- 36.The International Psychopharmacology Algorithm Project. [Accessed: 09.02.2011];2006 http://www.ipap.org/index.php.
- 37.Dellva MA, Tran P, Tollefson GD, Wentley AL, Beasley CM., Jr Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatr Serv. 1997;48(12):1571–7. doi: 10.1176/ps.48.12.1571. [DOI] [PubMed] [Google Scholar]
- 38.Tran PV, Dellva MA, Tollefson GD, Wentley AL, Beasley CM., Jr Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. Br J Psychiatry. 1998;172:499–505. doi: 10.1192/bjp.172.6.499. [DOI] [PubMed] [Google Scholar]
- 39.de Sena EP, Santos-Jesus R, Miranda-Scippa A, Quarantini LC, Oliveira IR. Relapse in patients with schizophrenia: a comparison between risperidone and haloperidol. Rev Bras Psiquiatr. 2003;25:220–223. doi: 10.1590/s1516-44462003000400007. [DOI] [PubMed] [Google Scholar]
- 40.Cooper SJ, Butler A, Tweed J, Welch C, Raniwalla J. Zotepine in the prevention of recurrence: a randomised, double-blind, placebo-controlled study for chronic schizophrenia. Psychopharmacology (Berl) 2000;150(3):237–43. doi: 10.1007/s002130000452. [DOI] [PubMed] [Google Scholar]
- 41.Csernansky JG, Mahmoud R, Brenner R. A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med. 2002;346:16–22. doi: 10.1056/NEJMoa002028. [DOI] [PubMed] [Google Scholar]
- 42.Schooler N, Rabinowitz J, Davidson M, Emsley R, Harvey PD, Kopala L, McGorry PD, Van HI, Eerdekens M, Swyzen W, De Smedt G. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162:947–953. doi: 10.1176/appi.ajp.162.5.947. [DOI] [PubMed] [Google Scholar]
- 43.Arato M, O'Connor R, Meltzer HY ZEUS Study Group. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207–15. doi: 10.1097/00004850-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Beasley CM, Jr, Sutton VK, Hamilton SH, Walker DJ, Dossenbach M, Taylor CC, Alaka KJ, Bykowski D, Tollefson GD Olanzapine Relapse Prevention Study Group. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol. 2003;23(6):582–94. doi: 10.1097/01.jcp.0000095348.32154.ec. [DOI] [PubMed] [Google Scholar]
- 45.Pigott TA, Carson WH, Saha AR, Torbeyns AF, Stock EG, Ingenito GG Aripiprazole Study Group. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–56. doi: 10.4088/jcp.v64n0910. [DOI] [PubMed] [Google Scholar]
- 46.Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, Kahn R, McEvoy J, Perkins D, Sharma T, Zipursky R, Wei H, Hamer RM. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 47.Green AI, Lieberman JA, Hamer RM, Glick ID, Gur RE, Kahn RS, McEvoy JP, Perkins DO, Rothschild AJ, Sharma T, Tohen MF, Woolson S, Zipursky RB HGDH Study Group. Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophr Res. 2006;86(1-3):234–43. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Lecrubier Y, Quintin P, Bouhassira M, Perrin E, Lancrenon S. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6-month double-blind controlled clinical trial. Acta Psychiatr Scand. 2006;114(5):319–27. doi: 10.1111/j.1600-0447.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 49.Peuskens J, Trivedi J, Malyarov S, Brecher M, Svensson O, Miller F, Persson I, Meulien D. Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomized, placebo-controlled trial in clinically stable patients. Psychiatry (Edgmont) 2007;4(11):34–50. [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer M, Simpson G, Maciulis V, Kushner S, Vijapurkar U, Lim P, Eerdekens M. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2007;27(1):6–14. doi: 10.1097/JCP.0b013e31802dda4a. Erratum in: J Clin Psychopharmacol. 2007;27(3):258. [DOI] [PubMed] [Google Scholar]
- 51.Gaebel W, Riesbeck M, Wolwer W, Klimke A, Eickhoff M, von Wilmsdorff M, Jockers-Scherubl MC, Kuhn KU, Lemke M, Bechdolf A, Bender S, Degner D, Schlosser R, Schmidt LG, Schmitt A, Jager M, Buchkremer G, Falkai P, Klingberg S, Kopcke W, Maier W, Hafner H, Ohmann C, Salize HJ, Schneider F, Moller HJ. Maintenance treatment with risperidone or low-dose haloperidol in first-episode schizophrenia: 1-year results of a randomized controlled trial within the German Research Network on Schizophrenia. J Clin Psychiatry. 2007;68:1763–1774. doi: 10.4088/jcp.v68n1116. [DOI] [PubMed] [Google Scholar]
- 52.Kane JM, Lauriello J, Laska E, Di Marino M, Wolfgang CD. Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol. 2008;28:S29–S35. doi: 10.1097/JCP.0b013e318169cca7. [DOI] [PubMed] [Google Scholar]
- 53.Crespo-Facorro B, Perez-Iglesias R, Mata I, Caseiro O, Martinez-Garcia O, Pardo G, Ramirez-Bonilla M, Pelayo-Teran JM, Vazquez-Barquero JL. Relapse prevention and remission attainment in first-episode non-affective psychosis. A randomized, controlled 1-year follow-up comparison of haloperidol, risperidone and olanzapine. J Psychiatr Res. 2010;(45):763–769. doi: 10.1016/j.jpsychires.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fogelson DL, Bartzokis G, Aravagiri M. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry. 2001;158(11):1835–42. doi: 10.1176/appi.ajp.158.11.1835. [DOI] [PubMed] [Google Scholar]
- 55.Liberman RP, Kopelowicz A. Recovery from schizophrenia: a concept in search of research. Psychiatr Serv. 2005;56(6):735–42. doi: 10.1176/appi.ps.56.6.735. [DOI] [PubMed] [Google Scholar]
- 56.Harding CM, Brooks GW, Ashikaga T, et al. The Vermont longitudinal study of persons with severe mental illness: II. Long-term outcome of subjects who retrospectively met DSM-III criteria for schizophrenia. American Journal of Psychiatry. 1987;144:727–735. doi: 10.1176/ajp.144.6.727. [DOI] [PubMed] [Google Scholar]
- 57.Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. International Review of Psychiatry. 2002;14:256–272. [Google Scholar]
- 58.Torgalsboen AK, Rund BR. Lessons learned from three studies of recovery from schizophrenia. International Review of Psychiatry. 2002;14:312–317. [Google Scholar]
- 59.Whitehorn D, Brown J, Richard J, et al. Multiple dimensions of recovery in early psychosis. International Review of Psychiatry. 2002;14:273–283. [Google Scholar]
- 60.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2004;161:473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 61.McCabe R, Saidi M, Priebe S. Patient-reported outcomes in schizophrenia. Br J Psychiatry. 2007;50(Suppl 1):S21–8. doi: 10.1192/bjp.191.50.s21. [DOI] [PubMed] [Google Scholar]
- 62.U.S. Food and Drug Administration. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. FDA; 2006. Guidance for Industry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bobes J, García-Portilla P, Sáiz PA, Bascarán T, Bousoño M. Quality of life measures in schizophrenia. Eur Psychiatry. 2005;20(Suppl 3):S313–7. doi: 10.1016/s0924-9338(05)80182-8. [DOI] [PubMed] [Google Scholar]
- 64.Derogatis LR. The Brief Symptom Inventory (BSI); Administration, Scoring, and Procedures. manual-II. Clinical Psychometric Research. (2nd) 1992 [Google Scholar]
- 65.Derogatis LR. SCL-90R Administration, Scoring and Procedures Manual. Clinical Psychometric Research. 1983 [Google Scholar]
- 66.Van Lieshout RJ, Goldberg JO. Quantifying self-reports of auditory verbal hallucinations in persons with psychosis. Can J Behav Sci. 2007;39:73–7. [Google Scholar]
- 67.Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89(1):62–7. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 68.Awad AG. Subjective response to neuroleptics in schizophrenia. Schizophr Bull. 1993;19(3):609–18. doi: 10.1093/schbul/19.3.609. [DOI] [PubMed] [Google Scholar]
- 69.Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177–83. doi: 10.1017/s0033291700050182. [DOI] [PubMed] [Google Scholar]
- 70.Phelan M, Slade M, Thornicroft G, Dunn G, Holloway F, Wykes T, Strathdee G, Loftus L, McCrone P, Hayward P. The Camberwell Assessment of Need: the validity and reliability of an instrument to assess the needs of people with severe mental illness. Br J Psychiatry. 1995;167(5):589–95. doi: 10.1192/bjp.167.5.589. [DOI] [PubMed] [Google Scholar]
- 71.Slade M, Thornicroft G, Loftus L, et al. CAN: Camberwell Assessment of Need. A Comprehensive Needs Assessment Tool for People with Severe Mental Illness. Gaskell. 1999 [Google Scholar]
- 72.Ruggeri M, Dall'Agnola R. The development and use of the Verona Expectations for Care Scale (VECS) and the Verona Service Satisfaction Scale (VSSS) for measuring expectations and satisfaction with community-based psychiatric services in patients, relatives and professionals. Psychol Med. 1993;23(2):511–23. doi: 10.1017/s0033291700028609. [DOI] [PubMed] [Google Scholar]
- 73.McGuire-Snieckus R, McCabe R, Catty J, Hansson L, Priebe S. A new scale to assess the therapeutic relationship in community mental health care: STAR. Psychol Med. 2007;37(1):85–95. doi: 10.1017/S0033291706009299. [DOI] [PubMed] [Google Scholar]
- 74.Yound S, Bullock W. Illness Management and Measure (MHRM) in Outcomes Research. Ohio Department of mental Health, Ohio Coordinating Center for Excellence for Illness and Recovery. :2003. [Google Scholar]
- 75.Giffort D, Schmook A, Woody C, et al. Construction of a Scale to Measure Consumer Recovery. Illinois Office of mental Health. :1995. [Google Scholar]
- 76.Oliver JP, Huxley PJ, Priebe S, Kaiser W. Measuring the quality of life of severely mentally ill people using the Lancashire Quality of Life Profile. Soc Psychiatry Psychiatr Epidemiol. 1997;32(2):76–83. doi: 10.1007/BF00788924. [DOI] [PubMed] [Google Scholar]
- 77.Voruganti LN, Awad AG. Personal evaluation of transitions in treatment (PETiT):a scale to measure subjective aspects of antipsychotic drug therapy in schizophrenia. Schizophr Res. 2002;56(1-2):37–46. doi: 10.1016/s0920-9964(01)00161-x. [DOI] [PubMed] [Google Scholar]
- 78.Auquier P, Simeoni MC, Sapin C, Reine G, Aghababian V, Cramer J, Lançon C. Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: the S-QoL. Schizophr Res. 2003;63(1-2):137–49. doi: 10.1016/s0920-9964(02)00355-9. [DOI] [PubMed] [Google Scholar]
- 79.Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. Int Clin Psychopharmacol. 1995;(Suppl 3):133–8. [PubMed] [Google Scholar]
- 80.Naber D, Moritz S, Lambert M, Pajonk FG, Holzbach R, Mass R, Andresen B. Improvement of schizophrenic patients' subjective well-being under atypical antipsychotic drugs. Schizophr Res. 2001;50(1-2):79–88. doi: 10.1016/s0920-9964(00)00166-3. [DOI] [PubMed] [Google Scholar]
- 81.The WHOQOL Group. The World Health Organization Quality of Life Assessment (the WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–09. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 82.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 83.EuroQol Group. EuroQol – A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 84.Sheehan DV. The Anxiety Disease. New York: Bantam Books; p. 1983. [Google Scholar]
- 85.Tso IF, Grove TB, Taylor SF. Self-assessment of psychological stress in schizophrenia: Preliminary evidence of reliability and validity. Psychiatry Res. 2011 Jul 23; doi: 10.1016/j.psychres.2011.07.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg M. Society and the Adolescent Self-Image. Princeton University Press; 1965. [Google Scholar]
- 87.Atonovsky A. Unravelling the Mystery of Health: How People manage Stress and Stay Well. Jossey-Bass; 1987. [Google Scholar]
- 88.Ascher-Svanum H. Development and validation of a measure of patients' knowledge about schizophrenia. Psychiatr Serv. 1999;50(4):561–3. doi: 10.1176/ps.50.4.561. [DOI] [PubMed] [Google Scholar]
- 89.Weiden PJ, Miller AL. Which side effects really matter? Screening for common and distressing side effects of antipsychotic medications. J Psychiatr Pract. 2001;7(1):41–7. doi: 10.1097/00131746-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 91.Citrome L. Compelling or irrelevant? Using number needed to treat can help decide. Acta Psychiatr Scand. 2008;17:412–419. doi: 10.1111/j.1600-0447.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 92.Glick ID, Correll CU, Altamura CA, Marder SR, Csernansky JG, Weiden PJ, Leucht S, Davis JM. Long-term efficacy and effectiveness of antipsychotic medications for schizophrenia: a data-driven, personalized, clinical approach. J Clin Psychiatry. doi: 10.4088/JCP.11r06927. in press. [DOI] [PubMed] [Google Scholar]
- 93.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd. Washington DC: 1987. [Google Scholar]
- 94.Swanson JW, Swartz MS, Van Dorn RA, Elbogen EB, Wagner HR, Rosenheck RA, Stroup TS, McEvoy JP, Lieberman JA. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry. 2006;63(5):490–499. doi: 10.1001/archpsyc.63.5.490. [DOI] [PubMed] [Google Scholar]
- 95.Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer JP, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility in treatment-resistant patients with schizophrenia and schizoaffective disorder. Psychiatric Services. 2001:521510–1514. doi: 10.1176/appi.ps.52.11.1510. [DOI] [PubMed] [Google Scholar]
- 96.Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622–629. doi: 10.1001/archpsyc.63.6.622. [DOI] [PubMed] [Google Scholar]
- 97.Lindenmayer JP, Liu-Seifert H, Kulkarni PM, Kinon BJ, Stauffer V, Edwards SE, Chen L, Adams DH, Ascher-Svanum H, Buckley PF, Citrome L, Volavka J. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry. 2009;70(7):990–996. doi: 10.4088/JCP.08m04221. [DOI] [PubMed] [Google Scholar]
- 98.Volavka J, Czobor P, Derks EM, Bitter I, Libiger J, Kahn RS, Fleischhacker WW. Efficacy of antipsychotic drugs against hostility in the European First-Episode Schizophrenia Trial (EUFEST) J Clin Psychiatry. 2011;72(7):955–961. doi: 10.4088/JCP.10m06529. [DOI] [PubMed] [Google Scholar]
- 99.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 9:3–8. [PubMed] [Google Scholar]
- 100.Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19(3):365–84. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A, Jones PB, Doody GA, Kapur S, Murray RM. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry. 2010;167(1):78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- 102.Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients' real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–11. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kraus MS, Keefe RS. Cognition as an outcome measure in schizophrenia. Br J Psychiatry Suppl. 2007;50:s46–51. doi: 10.1192/bjp.191.50.s46. [DOI] [PubMed] [Google Scholar]
- 104.Ventura J, Cienfuegos A, Boxer O, Bilder R. Clinical global impression of cognition in schizophrenia (CGI-CogS): reliability and validity of a co-primary measure of cognition. Schizophr Res. 2008;106(1):59–69. doi: 10.1016/j.schres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 105.Pietrzak RH, Olver J, Norman T, Piskulic D, Maruff P, Snyder PJ. A comparison of the CogState Schizophrenia Battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31(7):848–59. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- 106.http://www.cogstate.com/go/clinicaltrials/our-test-batteries/schizophrenia-battery
- 107.http://www.cogstate.com/go/clinicaltrials/our-test-batteries/early-phase-battery
- 108.Keefe RS, Poe M, Walker TM, Kang JW, Harvey PD. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163(3):426–32. doi: 10.1176/appi.ajp.163.3.426. [DOI] [PubMed] [Google Scholar]
- 109.Wilk CM, Gold JM, Bartko JJ, Dickerson F, Fenton WS, Knable M, Randolph C, Buchanan RW. Test-retest stability of the Repeatable Battery for the Assessment of Neuropsychological Status in schizophrenia. Am J Psychiatry. 2002;159(5):838–44. doi: 10.1176/appi.ajp.159.5.838. [DOI] [PubMed] [Google Scholar]
- 110.Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156(12):1944–50. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- 111.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2-3):283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 112.Hurford IM, Marder SR, Keefe RS, Reise SP, Bilder RM. A brief cognitive assessment tool for schizophrenia: construction of a tool for clinicians. Schizophr Bull. 2011;37(3):538–45. doi: 10.1093/schbul/sbp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–42. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 114.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6(3):235–8. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- 115.Opler M, Antonius D, Correll CU. New York Assessment of Adverse Cognitive Effects of Neuropsychiatric Treatment (NY-AACENT) Unpublished Rating Instrument [Google Scholar]
- 116.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247–51. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 117.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 119.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 120.Heinrichs DW, Hanlon TE, Carpenter WT., Jr The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388–98. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 121.Correll CU. Balancing efficacy and safety in the treatment with antipsychotics. CNS Spectr. 2007;12(10)(Suppl 17):12–20. 35. doi: 10.1017/s1092852900026298. [DOI] [PubMed] [Google Scholar]
- 122.Kelly DL, Conley RR. Sexuality and schizophrenia: a review. Schizophr Bull. 2004;30(4):767–79. doi: 10.1093/oxfordjournals.schbul.a007130. [DOI] [PubMed] [Google Scholar]
- 123.Chouinard G. Interrelations between psychiatric symptoms and drug-induced movement disorder. J Psychiatry Neurosci. 2006;31(3):177–80. [PMC free article] [PubMed] [Google Scholar]
- 124.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 125.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 126.Chouinard G, Ross-Chouinard A, Annable L, Jones B. Extrapyramidal Symptom Rating Scale. Can J Neurol Sci. 1980;7:234. [Google Scholar]
- 127.Guy W. Abnormal Involuntary Movement Scale (AIMS), ECDEU Assessment Manual for Psychopharmacology. NIMH, Psychopharmacology Research Branch, Division of Extramural Research Programs. 1976:218–22. [Google Scholar]
- 128.Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 129.Ritsner M, Ponizovsky A, Endicott J, Nechamkin Y, Rauchverger B, Silver H, et al. The impact of side-effects of antipsychotic agents on life satisfaction of schizophrenia patients: a naturalistic study. Eur Neuropsychopharmacol. 2002;12(1):31–8. doi: 10.1016/s0924-977x(01)00128-6. [DOI] [PubMed] [Google Scholar]
- 130.Lambert M, Conus P, Eide P, Mass R, Karow A, Moritz S, et al. Impact of present and past antipsychotic side effects on attitude toward typical antipsychotic treatment and adherence. Eur Psychiatry. 2004;19(7):415–22. doi: 10.1016/j.eurpsy.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 131.Pope A, Adams C, Paton C, Weaver T, Barnes TR. Assessment of adverse effects in clinical studies of antipsychotic medication: survey of methods used. Br J Psychiatry. 2010;197(1):67–72. doi: 10.1192/bjp.bp.109.070961. [DOI] [PubMed] [Google Scholar]
- 132.De Hert M, Bobes J, Cetkovich-Bakmas M, Cohen D, Leucht S, Maj M, Ndetei DM, Newcomer JM, Uwakwe R, Asai I, Möller HJ, Gautam S, Detraux J, Correll CU. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, and recommendations at the system and individual level. World Psychiatry. 2011;10(2):138–51. doi: 10.1002/j.2051-5545.2011.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline Concordant Monitoring of Metabolic Risk in People with Mental Ill Health: Systematic Review and Meta-Analysis of Screening Practices. Psychol Med. 2011 Aug 10;:1–23. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- 134.Meehan K, Zhang F, David S, Tohen M, Janicak P, Small J, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol. 2001;21(4):389–97. doi: 10.1097/00004714-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 135.McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]


