Abstract
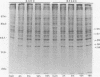
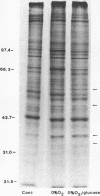
Vascular endothelial cells (EC) are the initial cells within the vascular wall exposed to decreases in blood ambient oxygen concentration. The mechanisms by which they tolerate low levels of oxygen are unknown, but may parallel the response to other cellular stresses, such as heat shock. After 4-8 h of hypoxia, we found a decrease in total protein synthesis in both cultured bovine aortic and pulmonary arterial EC. SDS-PAGE and autoradiographic analysis of [35S]methionine-labeled proteins demonstrated the concomitant induction of a specific set of proteins (Mr 34, 36, 47, and 56 kD) in both cell types. These hypoxia-associated proteins (HAPs) were cell-associated and up-regulated in a time- and oxygen concentration-dependent manner. Comparison of these proteins with heat shock proteins (HSPs) demonstrated that HAPs were distinct from HSPs. EC maintained chronically in 3% O2 continued to synthesize elevated levels of HAPs, yet further up-regulated these proteins when exposed to 0% O2. The presence of five times the normal media glucose concentration did not alter the appearance of HAPs. Hypoxia sensitive renal tubular epithelial cells up-regulated no proteins corresponding to HAPs and were irreversibly damaged within 8 h of exposure to 0% O2. In vitro translation experiments demonstrated that the steady-state level of several mRNAs was higher in the anoxic EC than in normoxic EC and encoded for proteins of Mr 32, 35, 37, 40, and 48 kD that were different from proteins encoded by HSP mRNAs. The induction of HAPs during acute hypoxia and their continued synthesis in chronic hypoxia suggest that HAPs may be important in the maintenance of endothelial cell integrity under conditions of decreased ambient oxygen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Marotti K. R., Whitaker-Dowling P. A. A candidate rat-specific gene product of the Kirsten murine sarcoma virus. Virology. 1979 Nov;99(1):31–48. doi: 10.1016/0042-6822(79)90034-5. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Stoler D. L., Scarcello L. A. Normal fibroblasts responding to anoxia exhibit features of the malignant phenotype. J Biol Chem. 1989 Sep 5;264(25):14885–14892. [PubMed] [Google Scholar]
- Anundi I., de Groot H. Hypoxic liver cell death: critical Po2 and dependence of viability on glycolysis. Am J Physiol. 1989 Jul;257(1 Pt 1):G58–G64. doi: 10.1152/ajpgi.1989.257.1.G58. [DOI] [PubMed] [Google Scholar]
- Carper S. W., Duffy J. J., Gerner E. W. Heat shock proteins in thermotolerance and other cellular processes. Cancer Res. 1987 Oct 15;47(20):5249–5255. [PubMed] [Google Scholar]
- Cummiskey J. M., Simon L. M., Theodore J., Ryan U. S., Robin E. D. Bioenergetic alterations in cultivated pulmonary artery and aortic endothelial cells exposed to normoxia and hypoxia. Exp Lung Res. 1981 Aug;2(3):155–163. doi: 10.3109/01902148109052311. [DOI] [PubMed] [Google Scholar]
- Dwyer B. E., Nishimura R. N., Brown I. R. Synthesis of the major inducible heat shock protein in rat hippocampus after neonatal hypoxia-ischemia. Exp Neurol. 1989 Apr;104(1):28–31. doi: 10.1016/0014-4886(89)90005-8. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Center D. M., Rounds S. Effect of ambient oxygen on cultured endothelial cells from different vascular beds. Am J Physiol. 1987 Oct;253(4 Pt 2):H878–H883. doi: 10.1152/ajpheart.1987.253.4.H878. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Rounds S. Effect of long-term hypoxia on cultured aortic and pulmonary arterial endothelial cells. Exp Cell Res. 1990 Nov;191(1):27–36. doi: 10.1016/0014-4827(90)90031-5. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Weller P. F., Rounds S., Beer D. J., Center D. M. Generation of, lipid neutrophil chemoattractant activity by histamine-stimulated cultured endothelial cells. J Immunol. 1986 Nov 1;137(9):2918–2924. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Induction characteristics of oxygen regulated proteins. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1287–1290. doi: 10.1016/0360-3016(86)90155-0. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Chain E. B. The role of glucose in the survival and 'recovery' of the anoxic isolated perfused rat heart. Biochem J. 1972 Aug;128(5):1125–1133. doi: 10.1042/bj1281125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B., Nieminen A. L., Gores G. J., Lemasters J. J. Irreversible injury in anoxic hepatocytes precipitated by an abrupt increase in plasma membrane permeability. FASEB J. 1988 Feb;2(2):146–151. doi: 10.1096/fasebj.2.2.3342967. [DOI] [PubMed] [Google Scholar]
- Ketis N. V., Hoover R. L., Karnovsky M. J. Effects of hyperthermia on cell survival and patterns of protein synthesis in endothelial cells from different origins. Cancer Res. 1988 Apr 15;48(8):2101–2106. [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg J. I., Mills J. W., Jarrell J. A., Rabito C. A., Leaf A. Protection of cultured renal tubular epithelial cells from anoxic cell swelling and cell death. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5445–5447. doi: 10.1073/pnas.77.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulewitz A. H., Fanburg B. L. The effect of oxygen tension on the in vitro production and release of angiotensin-converting enzyme by bovine pulmonary artery endothelial cells. Am Rev Respir Dis. 1984 Nov;130(5):866–869. doi: 10.1164/arrd.1984.130.5.866. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laszlo A. The relationship of heat-shock proteins, thermotolerance, and protein synthesis. Exp Cell Res. 1988 Oct;178(2):401–414. doi: 10.1016/0014-4827(88)90409-0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol. 1986 May;250(5 Pt 1):C766–C770. doi: 10.1152/ajpcell.1986.250.5.C766. [DOI] [PubMed] [Google Scholar]
- Levine R. A., McCormack J. E., Buckler A., Sonenshein G. E. Transcriptional and posttranscriptional control of c-myc gene expression in WEHI 231 cells. Mol Cell Biol. 1986 Nov;6(11):4112–4116. doi: 10.1128/mcb.6.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Mizzen L. A., Welch W. J. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988 Apr;106(4):1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins: the search for functions. J Cell Biol. 1986 Aug;103(2):321–325. doi: 10.1083/jcb.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz P., Piper H. M., Spahr R., Spieckermann P. G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol. 1984 Jun;115(3):349–361. [PMC free article] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R. Heat shock proteins and protection of proliferation and translation in mammalian cells. Cancer Res. 1984 Nov;44(11):5188–5194. [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Hughes C., Chao C., Cai J., Bartels C., Gessner T., Subjeck J. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasovic S. P., Ramagli L. S., Simonette R. A., Wilson M. J., Rodriguez L. V. Heat-stress proteins of rat lung endothelial and mammary adenocarcinoma cells. Radiat Res. 1987 Apr;110(1):45–60. [PubMed] [Google Scholar]
- Vender R. L., Clemmons D. R., Kwock L., Friedman M. Reduced oxygen tension induces pulmonary endothelium to release a pulmonary smooth muscle cell mitogen(s). Am Rev Respir Dis. 1987 Mar;135(3):622–627. doi: 10.1164/arrd.1987.135.3.622. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]