Abstract
Mouse genetic approaches when combined with live imaging tools have the potential to revolutionize our current understanding of mammalian biology. The availability and improvement of a wide variety of fluorescent proteins have provided indispensable tools to visualize cells in living organisms. It is now possible to generate genetically modified mouse strains expressing fluorescent proteins in a tissue-specific manner. These reporter-expressing strains make it possible to image dynamic cell behaviors in the context of a living embryo. Since mouse embryos develop within the uterus, live imaging experiments require culture conditions that closely mimic those in vivo. Over the past few decades, significant advances have been made in developing conditions for culturing both pre- and postimplantation stage embryos. In this chapter, we will discuss methods for ex utero culture of preimplantation and postimplantation stage mouse embryos. In particular, we will describe protocols for collecting embryos at various stages, setting up culture conditions for imaging and using laser scanning confocal microscopy to visualize live processes in mouse embryos expressing fluorescent reporters.
Keywords: Mouse embryo, ex utero culture, live imaging, fluorescent protein, time lapse
1. Introduction
Over the past 100 years, mouse genetics has been developed into a powerful system for understanding mammalian biology at the molecular level. The mouse is an excellent model organism to study mammalian biology due to its short gestation period, large litter size, small body size, and resistance to infection.
Unlike many other model organisms such as zebrafish and amphibians, which are readily live imaged as they undergo normal development (1), mouse embryos develop within the uterus, making it necessary to closely mimic conditions in the womb during ex utero culture. In combination with the development of fluorescent labeling techniques and advances in microscope technology, mouse embryos can now be live imaged to visualize developmental processes in vitro.
The characterization and cloning of green fluorescent protein (GFP), originally derived from jellyfish, was awarded the Nobel Prize in chemistry in 2008. Indeed, the discovery and popularization of fluorescent proteins combined with the power of mouse genetics provide attractive tools to follow cells in live organisms (2–5). Subcellular-localized fluorescent proteins such as the human histone H2B fusion protein (H2B–GFP) label active chromatin (Fig. 9.1a), thereby greatly facilitating cell tracking (6–9), while glycosylphosphatidylinositol (GPI) and other membrane-localized fusion proteins help to visualize cell morphology (10–12). The development of spectrally distinct fluorescent proteins such as the cyan and red fluorescent proteins will facilitate labeling and tracking of different cell populations within the embryo (13–16). More information can be obtained by simultaneously visualizing multiple cellular characteristics, such as cell position and cell morphology, which require the use of multiple subcellularly localized labels. To do this, cells can be dual tagged in various spectral combinations so that they express two fluorescent proteins, for example, one at the plasma membrane and a second in the nucleus (17, 18).
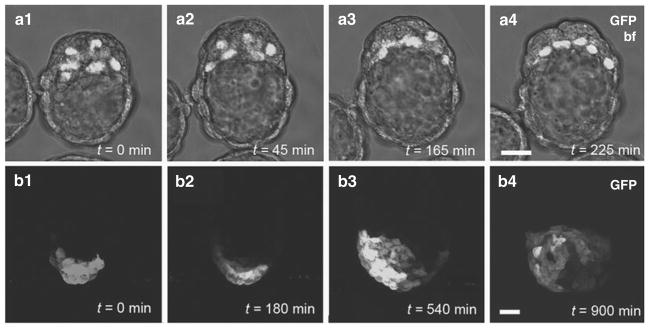
Fig. 9.1.
Examples of 3D time-lapse imaging of mouse embryos. (a1–a4) Primitive endoderm formation in E3.5–E4.5 embryos; nuclear-localized PdgfrαH2B–GFP labeling the primitive endoderm population provides single-cell resolution and facilitates cell tracking. (b1–b4) Anterior visceral endoderm (AVE) migration in E5.5 embryos; cytoplasmic localization of Hex::GFP in AVE cells highlights shapes of migrating cells. All panels represent 3D reconstructions of z-stacks taken during fluorescence time lapse. Note that anterior is to the left in panels b1–b3, and frontal in b4 (as the embryo has rotated 90 degrees counterclockwise). Scale bar: 20 μm.
Lineage-specific expression of fluorescent reporters is an invaluable tool for studying mouse development both in wild-type and mutant embryos and allows for the observation of gene expression in situ in real time (Fig. 9.1) (19–22). Single-cell labeling using fluorescent proteins can be achieved either by injection or electroporation of nucleic acids into individual or groups of cells (23, 24) or by using photomodulatable proteins such as KikGR and activating or converting fluorescent proteins in cells in a region of interest (25).
Imaging in bright-field differential interference contrast (DIC) has provided useful information about the timing and plane of cell division in early preimplantation embryos (26, 27) or somitogenesis in later stage embryos (28). Fluorescence microscopy, while providing a powerful tool for visualizing whole embryos and subcellular structures, introduces the problem of out-of-focus light depending on the thickness of specimens. This problem is partially resolved by image processing and deconvolution techniques. Recently, confocal microscopy has been used extensively in imaging as it optically sections specimens and eliminates out-of-focus light completely.
Laser scanning confocal microscopy excludes light outside the plane of focus making it possible to optically section a sample, which can then be reconstructed into a 3D image with the appropriate software. Laser point scanning confocal microscopes are most commonly used; however, other variants are also commercially available. Slit-scanning confocals (for example, the Zeiss LSM5LIVE) or Nipkow-type spinning disc confocals (for example, the Perkin Elmer UltraView) allow for increased scan speeds and reduced exposure times and may be preferred for high-speed imaging of rapid processes or for samples that are sensitive to phototoxicity. Multiphoton microscopes also minimize exposure times by illuminating only one focal plane at a time (3, 29). These advanced optical imaging modalities, combined with optimized ex utero embryo cultures and reporter-expressing strains of genetically modified mice, provide powerful tools to live image dynamic cell behaviors in situ in embryos.
2. Materials
2.1. Media
Culture and manipulation media are commercially available from several companies. These media can also be manually prepared in the laboratory.
M2—preimplantation embryo manipulation (Millipore).
KSOM—preimplantation embryo culture (KSOM + AA, Millipore).
95% DMEM/F12 (1:1) (Invitrogen) + 5% newborn calf serum (e.g., Lonza)—postimplantation embryo dissection.
DR100, DR75, or DR50—postimplantation embryo culture (see Fig. 9.2 for specific requirements according to the stage of development): rat serum, diluted in DMEM/F12 (1:1) with GlutaMAX (Invitrogen). (DR100 = pure rat serum, DR75 = 75% rat serum in media, DR50 = 50%).
-
Rat serum (although commercially available, the best results are achieved using homemade serum (30)).
Anesthetize rats (preferably large males) with ether or other volatile gas.
Make an incision in the abdomen and expose the dorsal aorta.
Gently collect blood from the aorta (12–15 mL per rat), using a syringe.
Place the tube with the collected blood on ice.
Euthanize the rat.
Centrifuge the blood for 20 min at 1,300×g.
Collect the supernatant and remove the pellet.
Centrifuge the serum for 10 min at 1,300×g.
Collect the supernatant.
Heat-inactivate the serum for 30 min at 56°C.
Filter the serum with a 0.45-μm filter.
Aliquot the serum and freeze at –80°C for up to 1 year.
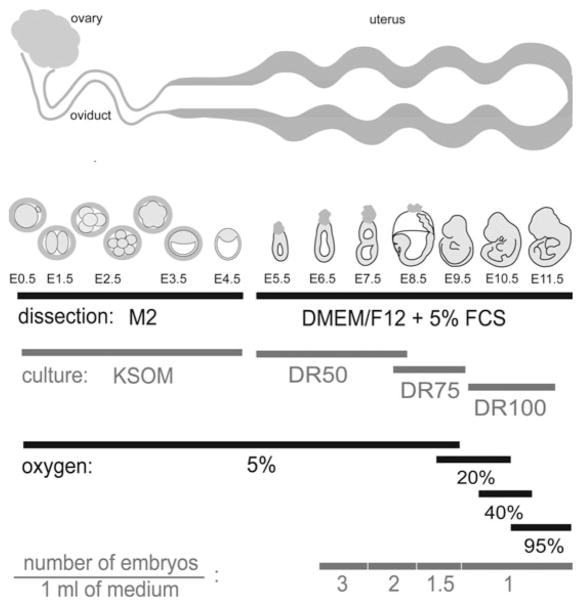
Fig. 9.2.
Schematic representation of time–course of mouse embryonic development. Requirements for dissection media, culture media, and gas content for in vitro culture at each embryonic stage and approximate location of embryos in reproductive tract at each stage of development are included. Note that CO2 concentration is 5% for all stages. Media and gas compositions are the same for both roller and static cultures. DR = rat serum: (DMEM/F12 + GlutaMAX) (see Section 2); embryos not to scale.
2.2. Mice
Place one to two female mice in a cage with a single male (to increase efficiency, females can be inspected for estrus before mating). Embryo donors should be at least 6 weeks old.
Check females the following morning for the presence of a vaginal plug. The day of plug detection is counted as embryonic day 0.5 (E0.5) since mating is assumed to have occurred at the midpoint of the dark period.
Dissect out the oviduct or uterus to collect embryos. Embryos at E0.5–E2.5 are found in the oviduct, while later stage embryos remain in the uterus (see Fig. 9.2 for detailed description) (see Note 1).
Stage early postimplantation embryos according to morphological landmarks (31, 32). The time of dissection should not be used as the criterion for staging of embryos, as a range of stages occur even within a single litter of any given age.
2.3. Microscopes
Stereomicroscope with transmitted light and both 20× and 40 × magnification.
Laser scanning inverted microscope with 5×, 10×, 20×, and 40× objectives (for example, PlanApo or PlanNeo objectives). 5× and 10× objectives are usually used dry, 20× objectives are usually used either dry or multi-immersion, and 40× objectives are usually oil or multi-immersion. 5× magnification is used for scanning the field of view to identify and position samples. 10× is used for low-magnification 3D time-lapse image acquisition, and 20× and 40× are used for high-magnification 3D time-lapse imaging. Occasionally a 63× objective may be used for imaging, but in our experience this is too high a magnification for experiments on even the smallest mouse embryos or explants.
Computer workstation with image data acquisition and processing software.
2.4. Embryo Culture
Humidified CO2 incubator.
Roller apparatus (rotating ~30 rpm) in an incubator chamber (37.5°C). A roller culture apparatus providing a constant gas supply is recommended (BTC Engineering, Cambridge, UK).
On-stage environmental chamber that provides a stable temperature and gas content required for embryo culture (Fig. 9.3).
Gas mixtures (CO2/O2/N2; consult Section 3 for appropriate selection).
Watchmaker’s forceps #5 (two pairs, e.g., Roboz) and small surgical scissors (e.g., Roboz) (see Note 2).
35- and 60-mm plastic Petri dishes.
Organ culture dishes—optional (Falcon).
35-mm glass bottom dishes (MatTek) or Lab-tek coverslip bottom chambers.
Mouth pipette: assemble from mouthpiece (HPI Hospital Products Med. Tech., 200 μL tip can be used instead), latex tubing (e.g., latex 1/8″ ID, 1/32″ wall, Fisherbrand), and finely drawn glass Pasteur pipette (e.g., Fisherbrand), using 1,000 μL tip as a connector. Pasteur pipette can be hand-pulled over the flame to a diameter of 1.5× the embryo. Pipettes can be siliconized prior to use to prevent embryos from sticking to the glass.
Plastic transfer pipettes for moving older embryos (e.g., Fisherbrand). These can be cut to accommodate larger embryos.
A 1 mL syringe, 26- or 27.5-gauge needle and blunt 30-gauge needle (cut or blunted with sandpaper or sharpening stone (e.g., Becton Dickinson)).
Embryo-tested lightweight mineral oil (Sigma).
CoverWell perfusion chamber gaskets (Invitrogen).
Human eyelashes, sterilized with 70% ethanol.
Suction holding pipette (optional; e.g., Eppendorf Cell-Tram Air).
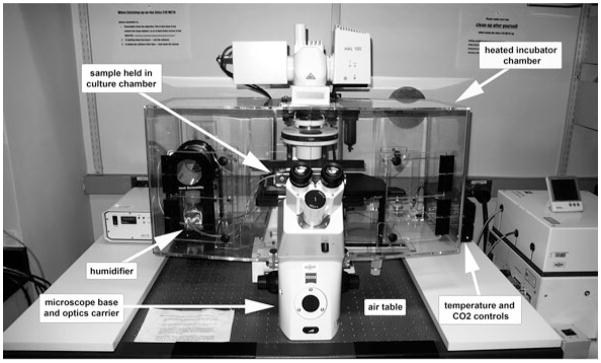
Fig. 9.3.
Microscope setup for live time-lapse imaging. Inverted microscope with environmental chamber provides proper conditions for culture and live imaging of mouse embryos.
3. Methods
3.1. Microscope Setup for Culturing and Imaging Mouse Embryos
For live imaging, it is important to combine conditions that allow for embryonic development closely resembling those in utero with a setup that ensures the best image quality. This can be accomplished using an inverted microscope with an environmental chamber setup (Fig. 9.3) that provides the stable temperature and gas content required for embryo culture.
With the exception of some bright-field contrast microscopy, images can only be acquired through glass coverslips of not more than 1.5 μm thickness (for example, P35G-1.5-14-C MatTek dishes or Lab-Tek coverslip chambers).
Since ultraviolet light and laser beams are harmful to the embryos, imaging conditions should be adjusted to ensure proper development of the embryo. Reducing laser power and exposure time by decreasing the frequency of scans, increasing the size of optical sections, or increasing scan speed can help embryos develop while still allowing for the best quality images.
3.2. Culturing and Imaging Preimplantation Mouse Embryos
At the earliest stages of development, mouse embryos float freely along the mother’s reproductive tract. Therefore, in vitro culture of preimplantation embryos requires the appropriate media, temperature, and gas conditions closely resembling those in the womb. These culture conditions are now largely established, allowing for the proper timing and development of preimplantation stage embryos.
3.2.1. Collection of Preimplantation Mouse Embryos
Before starting the dissection of embryos, equilibrate and pre-warm the KSOM culture media by placing the dish covered with mineral oil for at least 30 min in a humidified incubator at 37.5°C and 5% CO2 in air.
After sacrificing a pregnant female, either (for E0.5–E2.5 embryos) dissect out the oviduct leaving a small part of the distal uterus attached or (for E3.5–E4.5 embryos) remove the entire uterus and place in a drop of pre-warmed M2 media.
Place the dish under a stereomicroscope and flush the oviduct/uterus with pre-warmed M2 media. Use a 1 mL syringe with a 26-gauge needle (uterus) or a blunt 30-gauge needle (for the oviduct, insert the needle in the oviduct infundibulum).
Collect the embryos and transfer into a previously prepared culture dish using a mouth pipette attached to a pulled Pasteur pipette.
3.2.2. Culturing Preimplantation Mouse Embryos
Prepare the culture dish by placing drops (10–100 μL each) of KSOM culture media in the bottom of a 35 mm plastic dish and cover with embryo-tested light mineral oil.
Place the dish for at least 30 min in a humidified incubator at 37.5°C and 5% CO2 in air, to allow for equilibration.
Transfer the embryos into the microdrops under oil. Transfer through several drops, to rinse off residual M2 media. Ideally, culture several embryos together, as a higher density of embryos enhances development.
Embryos in KSOM media should only be removed from the incubator for minimal periods of time as the bicarbonate buffered media quickly changes pH in the air.
Under these conditions, embryos can develop from a zygote to the late blastocyst. If the dissected embryos are at an earlier stage than expected, they can be cultured in vitro until they reach the proper stage without compromising their development, and imaged afterward.
3.2.3. Live Imaging of Preimplantation Mouse Embryos
Preimplantation embryos are live imaged under the same conditions as those for static culture. If the levels of CO2 cannot be reliably maintained, embryos can be imaged short-term at 37.5°C in M2 media instead.
Pre-warm the on-stage incubator to 37.5°C before live imaging; this can take from 30 min to several hours, depending on the incubator.
Prepare the culture dish by placing a drop of KSOM culture media in a glass bottom dish and covering it with embryo-tested light mineral oil (see Note 3).
Place the dish for at least 30 min in a humidified incubator at 37.5°C and 5% CO2 in air to allow for equilibration.
Transfer the embryos into the equilibrated dish. If possible, culture several embryos together even if only one of them is to be imaged. Place the dish on the microscope stage and immediately provide CO2.
Image the embryos. Minimize embryo exposure to laser light by reducing laser power and exposure time, decreasing the frequency of scans, and/or increasing the size of optical sections and scan speed. These adjustments should be determined empirically and will depend on the individual microscope and brightness of the fluorophore. In many cases, 2 μm thick optical sections (up to total of ~100 μm, but see Note 4) taken at 15 min intervals and combined with low laser power give good results.
To prevent embryos from drifting out of the imaging plane, make sure the microscope stage is leveled. The amount of media (too much or too little, especially flat drops) may also affect embryo drifting. Placing several embryos together can help to keep them immobile.
3.3. Culturing and Imaging Postimplantation Mouse Embryos
Around E4.0, mouse embryos start to implant in the uterus and begin to form and expand extraembryonic structures, which provide a physical connection with the mother and help support later development. This makes dissection more difficult and creates a unique challenge for ex utero culture once the mother–embryo connection is irreversibly lost. The methods in this chapter describe protocols for embryo culture up until E9.5 (see Notes 1, 5, and 6).
3.3.1. Collection of Postimplantation Mouse Embryos
Before starting the dissection, equilibrate and pre-warm the culture media by placing the culture dish covered with mineral oil in a humidified incubator at 37.5°C and 5% CO2 in air for at least 1 h.
After sacrificing the female, dissect out the uterus and place in a dish of pre-warmed (25–30°C) dissecting media (DMEM/F12 + 5% FCS; Fig. 9.2).
Place the dish under a stereomicroscope, dissect deciduae out of the uterus, and carefully remove embryos from each decidua using watchmaker’s forceps. For detailed dissection instructions consult (33) (see Note 1).
Remove/reflect Reichert’s membrane from each embryo using watchmaker’s forceps. Great care should be taken to avoid damaging embryos in the process of dissection and to ensure that the ectoplacental cone is left intact. Embryos that have been damaged during dissection should not be used for further culture.
Immediately after dissection, carefully move the embryos into a dish of culture media with a pipette so that only the smallest amount of dissecting media is transferred.
3.3.2. Roller Culture of Postimplantation Mouse Embryos
Roller culture provides the most optimal ex utero conditions for embryonic development at early postimplantation stages. Using this method, embryos are cultured in controlled temperature and gas conditions and are kept in constant motion.
Pre-warm roller culture incubator to 37.5°C before onset of culture.
Mix culture media appropriate for the stage of the embryo (Fig. 9.2). The amount of media required depends on the stage of the embryo.
Equilibrate media with the gas mixture appropriate for the stage of the embryo (Fig. 9.2) at 37.5°C for at least 1 h before culture. For a roller culture apparatus that has a constant gas supply, place a small amount of media in the culture bottle within the machine. If this apparatus is not available, blow gas on the surface of the media using a Pasteur pipette and place in an open dish at 37.5°C.
Move embryos into roller culture bottles with a pipette. Make sure that only the smallest amount of dissecting media is transferred. If necessary, wash embryos in culture media before moving them into culture bottles.
Re-gas the tubes, close tightly, and place in the roller apparatus at 37.5°C.
Re-gas the tubes every 12 h (unless constant gas flow is being provided).
Replace the media with a newly equilibrated mixture after 24 h.
3.3.3. Static Culture and Imaging of Postimplantation Mouse Embryos
Although roller culture provides the best conditions for ex utero development of postimplantation mouse embryos, it is not suitable for time-lapse imaging. For live imaging, embryos are cultured statically, which allows development to proceed for up to 24 h (34). Generally, the conditions for static culture are the same as for roller culture (Fig. 9.2).
Pre-warm the on-stage incubator to 37.5°C before live imaging. This can take from 30 min to several hours, depending on the incubator.
Prepare culture media appropriate for the stage of the embryo.
Prepare the glass bottom culture dish for imaging. Early postimplantation embryos (E5.5–E8.5) are cultured in drops of media covered with embryo-tested light mineral oil (see Notes 4 and 6). Place the culture dish in a humidified incubator at 37.5°C and 5% CO2 in air or appropriate gas mixture if available (see Fig. 9.2) for at least 1 h to pre-warm and equilibrate.
Move embryos into the culture dish with a pipette. Make sure that only the smallest amount of dissecting media is transferred. If necessary, wash embryos in culture media before moving them to the culture dish (see Note 7).
After moving the dish containing the embryos to the microscope stage, immediately provide CO2 (see Note 8).
Image the embryos. Minimize embryo exposure to laser light by reducing laser power and exposure time, decreasing the frequency of scans, and/or increasing the size of optical sections and scan speed. These adjustments will depend on the microscope, brightness of the fluorophore, and developmental stage of the embryo. In many cases, 2 μm optical sections (up to total of ~100 μm, but see Note 4) taken at 15 min intervals and combined with low laser power give good results. Since embryonic development is easily perturbed by culture conditions or phototoxicity, carefully optimize the conditions on wild-type or heterozygous embryos that do not have a defect before proceeding to analyze mutants with phenotypes.
3.3.4. Immobilizing Postimplantation Embryos
For some experiments (35) postimplantation embryos may need to be imaged on their distal or ventral side. This is done by suspending them in cultures using either a suction holding pipette or a modified chamber such as a CoverWell chamber gasket (Fig. 9.4) (35). These gaskets are cut into fragments containing a silicon body attached to a plastic surface with a hole (gaskets of different thicknesses can be used according to embryo size, and the plastic plate can be bent to position the embryo at different angles or to accommodate smaller embryos).
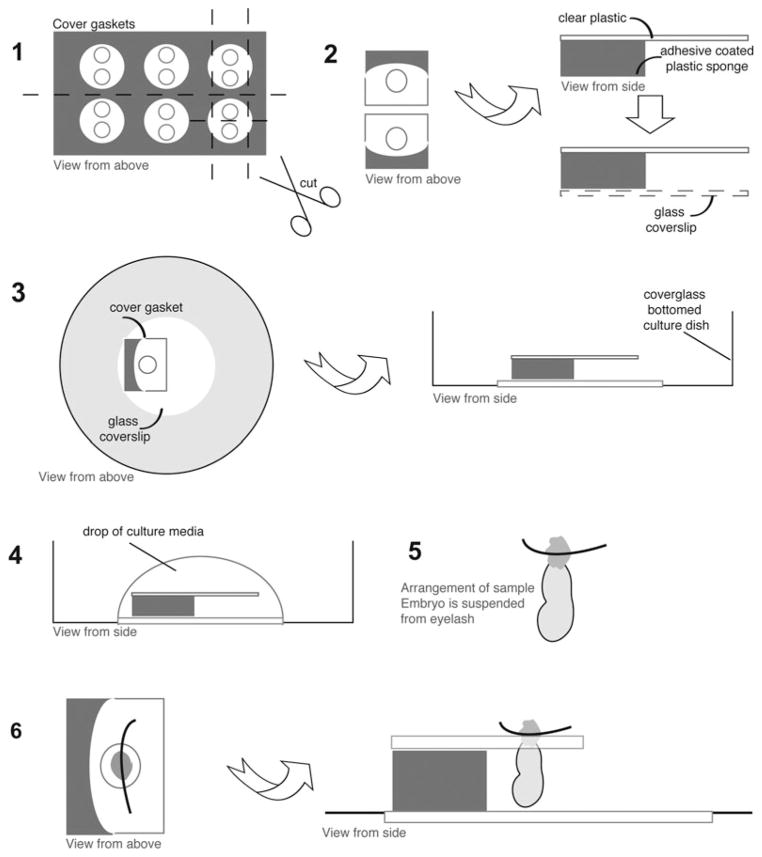
Fig. 9.4.
Immobilizing postimplantation embryos using chamber gaskets. (1) CoverWell chamber gaskets are cut into small pieces, each containing a silicon body attached to a plastic surface with a hole. (2) Bottom plastic surface is removed. (3) Such prepared piece of the gasket is placed on a glass bottom dish, silicone part down, and placed on a hot surface for better adhesion. (4) Culture media added to the dish. (5) Ectoplacental cone of the embryo is pierced with an eyelash. (6) The embryo is suspended in the hole of the gasket.
Adhere a pre-cut piece of the gasket to a glass bottom dish and place the dish on a hot surface to facilitate adhesion by melting of the plastic (Fig. 9.4, steps 1–3)
Add culture media to the dish (Fig. 9.4, step 4).
Pierce the ectoplacental cone of the embryo with an eyelash and suspend the embryo in the hole of the gasket. The embryo should hang from the plastic plate (Fig. 9.4, steps 5–6).
Cover the media with light mineral oil.
Move the embryos to the microscope stage incubator set at 37.5°C and supplied with CO2.
Image the embryos.
Acknowledgments
We thank our laboratory colleagues for helping perfect the techniques detailed in this chapter. Work in our laboratory is supported by the National Institutes of Health (RO1-HD052115 and RO1-DK084391), NYSTEM, and The Starr Foundation.
Footnotes
It is imperative to work quickly and efficiently. Prolonged time on the bench adversely affects embryos and compromises their subsequent culture. Therefore, a balance needs to be struck between speed and care. If you have more than one litter to dissect, sacrifice females one at a time.
It is recommended to use a set of coarse tools for the dissection of the uterus from the mouse, then one set of less pristine watchmaker’s forceps (#5s) to remove the decidua from the uterus, and a second set of pristine watchmaker’s forceps (#5s) for the dissection of embryos from the decidua. Removal of Reichert’s membrane requires particularly fine forceps, which can be sharpened whenever necessary with a sharpening stone.
If zona-free embryos are to be cultured (for example, after embryo manipulation), coat the glass bottom dish with a small amount of 2% agarose to avoid sticking of the embryo to the dish. At E4.0 mouse embryos begin hatching from the zona pellucida and changing their shape in the process. These changes may cause the embryo to move and obscure the visualization of processes being imaged. To overcome these movements during live imaging, the zona pellucida can be removed beforehand.
Embryos at E9.5 can be dissected out of their yolk sac for live imaging. Although it is feasible to culture mouse embryos in vitro beyond E10.5 (36), the size of the embryo and thickness of its tissues make imaging extremely difficult. However, recent reports using multiphoton excitation for live imaging in neonatal and adult mice (37, 38) suggest that the same technique can be used in older mouse embryos to a depth of up to 1,000 μm (39).
Successful roller culture of midgestational mouse embryos (E10.5) free of yolk sac and amnion has been reported in serum-free media (36).
For imaging at later stages, it is recommended to dissect out the region of interest (such as the ureteric buds (21) or pancreas (40)) and image as an explant culture.
Transferring older embryos into oil-covered media may be a problem due to the surface tension of the media and a relatively large diameter of the pipette being used. To address this issue, the media can be equilibrated prior to embryo dissection in an organ culture dish in a humidified incubator. Just before starting the culture, place a drop of the equilibrated media in a glass bottom dish, transfer the embryos into the drop, and cover with mineral oil.
It is necessary to replace water in the incubator humidifier bottle periodically, as the water can become contaminated and affect embryo development. The bottle should be rinsed with 70% ethanol and refilled with sterile water.
References
- 1.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 2008;322:1065–1069. doi: 10.1126/science.1162493. [DOI] [PubMed] [Google Scholar]
- 2.Megason SG, Fraser SE. Digitizing life at the level of the cell: high-performance laser-scanning microscopy and image analysis for in toto imaging of development. Mech Dev. 2003;120:1407–1420. doi: 10.1016/j.mod.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Hadjantonakis AK, Dickinson ME, Fraser SE, Papaioannou VE. Technicolour transgenics: imaging tools for functional genomics in the mouse. Nat Rev Genet. 2003;4:613–625. doi: 10.1038/nrg1126. [DOI] [PubMed] [Google Scholar]
- 4.Nowotschin S, Eakin GS, Hadjantonakis AK. Live-imaging fluorescent proteins in mouse embryos: multi-dimensional, multi-spectral perspectives. Trends Biotechnol. 2009;27:266–276. doi: 10.1016/j.tibtech.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson ME. Multimodal imaging of mouse development: tools for the postgenomic era. Dev Dyn. 2006;235:2386–2400. doi: 10.1002/dvdy.20889. [DOI] [PubMed] [Google Scholar]
- 6.Hadjantonakis AK, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser ST, Hadjantonakis AK, Sahr KE, Willey S, Kelly OG, Jones EA, Dickinson ME, Baron MH. Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice. Genesis. 2005;42:162–171. doi: 10.1002/gene.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plusa B, Hadjantonakis AK, Gray D, Piotrowska-Nitsche K, Jedrusik A, Papaioannou VE, Glover DM, Zernicka-Goetz M. The first cleavage of the mouse zygote predicts the blastocyst axis. Nature. 2005;434:391–395. doi: 10.1038/nature03388. [DOI] [PubMed] [Google Scholar]
- 9.Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee JM, Pirity MK, Lackan CS, Long JZ, Kondoh G, Takeda J, Hadjantonakis AK. In vivo imaging and differential localization of lipid-modified GFP-variant fusions in embryonic stem cells and mice. Genesis. 2006;44:202–218. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi X, Hadjantonakis AK, Wu Z, Hyink D, Costantini F. A transgenic mouse that reveals cell shape and arrangement during ureteric bud branching. Genesis. 2009;47:61–66. doi: 10.1002/dvg.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec (Hoboken) 2009;292:333–341. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjantonakis AK, Macmaster S, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long JZ, Lackan CS, Hadjantonakis AK. Genetic and spectrally distinct in vivo imaging: embryonic stem cells and mice with widespread expression of a monomeric red fluorescent protein. BMC Biotechnol. 2005;5:20. doi: 10.1186/1472-6750-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 17.Nowotschin S, Eakin GS, Hadjantonakis AK. Dual transgene strategy for live visualization of chromatin and plasma membrane dynamics in murine embryonic stem cells and embryonic tissues. Genesis. 2009;47:330–336. doi: 10.1002/dvg.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trichas G, Begbie J, Srinivas S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 2008;6:40. doi: 10.1186/1741-7007-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol. 2004;287:H1561–1569. doi: 10.1152/ajpheart.00081.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kwon GS, Hadjantonakis AK. Eomes::GFP—a tool for live imaging cells of the trophoblast, primitive streak, and telencephalon in the mouse embryo. Genesis. 2007;45:208–217. doi: 10.1002/dvg.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Kwon GS, Hadjantonakis AK. Transthyretin mouse transgenes direct RFP expression or Cre-mediated recombination throughout the visceral endoderm. Genesis. 2009;47:447–455. doi: 10.1002/dvg.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meilhac SM, Adams RJ, Morris SA, Danckaert A, Le Garrec JF, Zernicka-Goetz M. Active cell movements coupled to positional induction are involved in lineage segregation in the mouse blastocyst. Dev Biol. 2009;331:210–221. doi: 10.1016/j.ydbio.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perea-Gomez A, Meilhac SM, Piotrowska-Nitsche K, Gray D, Collignon J, Zernicka-Goetz M. Regionalization of the mouse visceral endoderm as the blastocyst transforms into the egg cylinder. BMC Dev Biol. 2007;7:96. doi: 10.1186/1471-213X-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowotschin S, Hadjantonakis AK. Use of KikGR a photoconvertible green-to-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos. BMC Dev Biol. 2009;9:49. doi: 10.1186/1471-213X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutherland AE, Speed TP, Calarco PG. Inner cell allocation in the mouse morula: the role of oriented division during fourth cleavage. Dev Biol. 1990;137:13–25. doi: 10.1016/0012-1606(90)90003-2. [DOI] [PubMed] [Google Scholar]
- 27.Lehtonen E. Changes in cell dimensions and intercellular contacts during cleavage-stage cell cycles in mouse embryonic cells. J Embryol Exp Morphol. 1980;58:231–249. [PubMed] [Google Scholar]
- 28.Flint OP, Ede DA. Cell interactions in the developing somite: in vitro comparisons between amputated (am/am) and normal mouse embryos. J Embryol Exp Morphol. 1982;67:113–125. [PubMed] [Google Scholar]
- 29.Dickinson ME, Simbuerger E, Zimmermann B, Waters CW, Fraser SE. Multiphoton excitation spectra in biological samples. J Biomed Opt. 2003;8:329–338. doi: 10.1117/1.1583734. [DOI] [PubMed] [Google Scholar]
- 30.Copp AJ, Cockroft DL. Postimplantation Mammalian Embryos. A Practical Approach. IRL; Oxford: 1990. [Google Scholar]
- 31.Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- 32.Theiler K. The House Mouse, Atlas of Embryonic Development. Springer; New York, NY: 1989. [Google Scholar]
- 33.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo, A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. [Google Scholar]
- 34.Jones EA, Crotty D, Kulesa PM, Waters CW, Baron MH, Fraser SE, Dickinson ME. Dynamic in vivo imaging of postimplantation mammalian embryos using whole embryo culture. Genesis. 2002;34:228–235. doi: 10.1002/gene.10162. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev Cell. 2007;13:884–896. doi: 10.1016/j.devcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Moore-Scott BA, Gordon J, Blackburn CC, Condie BG, Manley NR. New serum-free in vitro culture technique for midgestation mouse embryos. Genesis. 2003;35:164–168. doi: 10.1002/gene.10179. [DOI] [PubMed] [Google Scholar]
- 37.Keller-Peck CR, Walsh MK, Gan WB, Feng G, Sanes JR, Lichtman JW. Asynchronous synapse elimination in neonatal motor units: studies using GFP transgenic mice. Neuron. 2001;31:381–394. doi: 10.1016/s0896-6273(01)00383-x. [DOI] [PubMed] [Google Scholar]
- 38.Cahalan MD, Parker I, Wei SH, Miller MJ. Real-time imaging of lymphocytes in vivo. Curr Opin Immunol. 2003;15:372–377. doi: 10.1016/s0952-7915(03)00079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theer P, Hasan MT, Denk W. Two-photon imaging to a depth of 1000 micron in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt Lett. 2003;28:1022–1024. doi: 10.1364/ol.28.001022. [DOI] [PubMed] [Google Scholar]
- 40.Puri S, Hebrok M. Dynamics of embryonic pancreas development using real-time imaging. Dev Biol. 2007;306:82–93. doi: 10.1016/j.ydbio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]