Abstract
The mammalian CIP/KIP family of cyclin-dependent kinase (CDK) inhibitors (CKIs) comprises three proteins – p21Cip1/WAF1, p27Kip1, and p57Kip2 – that bind and inhibit cyclin–CDK complexes, which are key regulators of the cell cycle. CIP/KIP CKIs have additional independent functions in regulating transcription, apoptosis and actin cytoskeletal dynamics. These divergent functions are performed in distinct cellular compartments and contribute to the seemingly contradictory observation that the CKIs can both suppress and promote cancer. Multiple ubiquitin ligases (E3s) direct the proteasome-mediated degradation of p21, p27 and p57. This review analyzes recent data highlighting our current understanding of how distinct E3 pathways regulate subpopulations of the CKIs to control their diverse functions.
CIP/KIP CKIs: specificity in degradation
The CIP/KIP (CDK interacting protein/kinase inhibitor protein) family of proteins were identified based on their ability to bind and inhibit cyclin-dependent kinases (CDKs) to regulate the cell cycle [1]. More recently, the CIP/KIP family of CDK-inhibitors (CKIs) were shown to have diverse CDK-independent functions in regulating transcription, apoptosis and the cytoskeleton [2]. An unusually large number (twelve) of ubiquitin protein ligases (E3s) promote degradation of the three mammalian CIP/KIP CKIs. Here, we will focus on the emerging theme that the different E3s regulate distinct CIP/KIP CKI functions, or strategically alter CKI levels at specific points within complex cellular processes such as cell cycle progression.
Ubiquitin-mediated degradation regulates the majority of molecular pathways in eukaryotes [3]. The specificity within the ubiquitin pathway resides with the E3s that bind protein substrates and bring them into close proximity with ubiquitin-conjugating enzymes (E2s) that catalyze the ubiquitylation of the substrate. Poly-ubiquitylation acts as a targeting signal for degradation by the 26S proteasome [3]. The large number of E3s that target the CIP/KIP CKIs makes this an excellent model for understanding how specificity is generated when multiple degradation pathways target the same protein. In this review, we will initially briefly describe the cellular functions of the CIP/KIP CKIs and then summarize information on the individual E3s that regulate the CKIs, focusing on how specificity is achieved in the degradation of sub-pools of CKIs and the impact on distinct CKI functions.
Functions of the CIP/KIP CKIs
Roles of the CIP/KIP CKIs in the cell cycle
Cell cycle progression is driven by the activity of cyclin-dependent kinases (CDKs) in complex with their activating partners, cyclins [1]. CDK inhibitors are critical regulators of cyclin–CDK activities [1]. There are two families of mammalian CKIs: the INK4 family, which specifically inhibits G1 phase cyclin–CDKs [4], and the CIP/KIP family, which interacts with a broader range of cyclin–CDKs to control more aspects of the cell cycle [4].
The three mammalian CIP/KIP CKIs – p21Cip1, p27Kip1 and p57Kip2 – have different roles in cell cycle regulation [5–7]. p21 is induced in response to DNA damage through the activity of the p53 tumor suppressor protein and mediates cell cycle arrest in G1 and G2 phases. During unperturbed cell cycles, p21 oscillates with the highest levels during G1 and G2 phases [8]. p21 is not essential (in a non-redundant manner) for cell cycle progression in mice but is required for proper DNA damage responses [9]. p27 has a major role in cell cycle exit: p27 accumulates upon cell cycle exit, and is rapidly degraded as cells re-enter the cell cycle from quiescence [6]. Mice lacking p27 are larger in size due to increased proliferation resulting from a failure of cells to exit the cell cycle appropriately [9]. p57 is expressed in the mouse embryo in tissue-specific patterns under the control of developmental pathways, which include Notch, MyoD, and BMP-2/6 [2]. Unlike p21 and p27, p57 is required for embryonic development and mice embryos lacking p57 die with failures of tissue differentiation [7,9].
Roles of the CIP/KIP CKIs in transcription
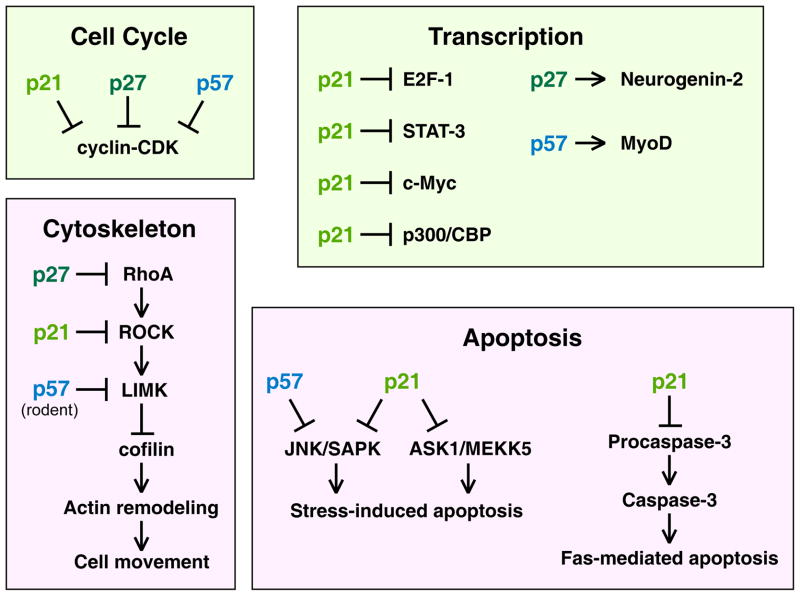
Each of the CKIs has roles in regulating transcription by directly binding to transcriptional regulators (Figure 1). p21 binds to the transcription factors E2F-1, STAT3 and c-Myc to inhibit their transcriptional activities, and binds to p300/CBP to block its transcriptional repressor activity [5]. p27 binds to Neurogenin-2 to stabilize it and thereby promote neuronal differentiation in the cerebral cortex [10]. Finally, p57 binding to MyoD stabilizes it to promote muscle-specific transcription [11].
Figure 1. Diverse cellular functions of the CIP/KIP CKIs.
A diagram showing primary functions of p21, p27, and p57 in regulating the cell cycle, transcription factors, the actin cytoskeleton, and apoptosis. Boxes are color coded, with light green reflecting functions that occur in the nucleus and magenta reflecting functions that occur in the cytoplasm.
 is an inhibitory symbol, and an arrow is an activating symbol. See text for details.
is an inhibitory symbol, and an arrow is an activating symbol. See text for details.
Roles of CIP/KIP CKIs in apoptosis
The CIP/KIP proteins can block apoptosis through CDK-independent pathways (Figure 1). p21 binds and inhibits JNK1/SAPK kinase and MAPK-kinase-kinase ASK1/MEKK5 to block stress-induced apoptosis [13,14]. p21 phosphorylated by protein kinase A can inhibit Fas-mediated apoptosis by binding to pro-caspase-3 and blocking its processing to an active caspase [15]. These anti-apoptotic activities are attributed to the cytoplasmic pool of p21 [16]. p27 stabilization in response to the removal of growth factors or cellular stress induces autophagy that offers protection against apoptosis [17]. p57 prevents UV- and MEKK1-induced cell death by binding and inactivating the JNK1/SAPK kinase [12]. Conversely, p21 and p57 expression can promote apoptosis; ectopic p21 in human ovarian cancer cells induces the expression of genes that initiate apoptosis [18] and p57 expression enhances staurosporin-induced apoptosis [19].
Roles of the CIP/KIP CKIs in controlling the actin cytoskeleton
All three CIP/KIP CKIs regulate actin cytoskeletal dynamics by modulating the RhoA–ROCK–LIMK–cofilin pathway (Figure 1). In this pathway, the small GTPase RhoA activates Rho-associated kinase (ROCK), which activates LIM kinase (LIMK), which inhibits the actin-depolymerizing protein ADF/cofilin [20]. The CKI-mediated inhibition of the RhoA pathway allows cofilin to become active leading to stress fiber depolymerization with the redistribution of monomeric actin contributing to increased cell motility and morphological changes [21].
Strikingly, each of the CIP/KIP proteins binds and inhibits a different component of the RhoA–ROCK–LIMK pathway: p27 inhibits RhoA; p21 inhibits ROCK; and rodent p57 binding to LIMK facilitates its translocation to the nucleus, which sequesters it from the cytoplasmic RhoA pathway [2]. Interestingly, human p57 binds LIMK1 but does not affect its localization, and instead enhances LIMK activity [22]. The opposite effects of p57 binding to LIMK in humans and rodents may arise from species-specific differences in p57. Rodent p57 binds LIMK via its central region, which contains proline-rich and acidic domains [23], but human p57 lacks these domains [7].
Different CKI functions have opposite effects on cancer
p21, p27, and p57 function as tumor suppressors based on their ability to inhibit cyclin–CDK complexes in the nucleus [2,24,25]. However, unlike other tumor suppressor genes, the complete functional loss of the CKIs is rarely observed in cancers [2,24]. Moreover, there is growing evidence that the CKIs function in the cytoplasm as oncogenes. The accumulation of cytoplasmic p21, p27 and p57 has been reported for many cancers; and p21 and p27 cytoplasmic accumulation has been linked to tumor aggressiveness, metastasis and poor prognosis [2,24,25]. Oncogenic transformation by activated Ras is associated with the accumulation of cytoplasmic p21 and p27, and contributes to the stereotypical loss of stress fibers in Ras-transformed cells [26,27]. Cytoplasmic p21 and p27 function to activate cofilin, and increased cofilin expression is associated with cancer metastasis [28]. Therefore, the cytoplasmic forms of p21 and p27 can promote metastatic cancer through regulation of the actin cytoskeleton and by blocking apoptosis through their anti-apoptotic functions.
The regulation of CIP/KIP CKIs by ubiquitin-mediated proteolysis
The CIP/KIP CKIs have intrinsic molecular flexibility and lack a defined tertiary structure prior to binding to other proteins [29]. This flexibility presumably allows the CKIs to bind a greater number of proteins, acquiring different tertiary structures depending on the protein bound. One strategy that is employed by E3s to provide specificity is to target CKIs only when they are bound to specific proteins (which may induce a specific tertiary structure in the CKI that is recognized). For example, SCFSkp2 specifically recognizes CKIs bound to cyclin–CDK complexes [5] thereby ensuring that only CKIs that are actively regulating the cell cycle are targeted for degradation. The specificity of E3-mediated degradation pathways is also achieved by limiting the levels or activities of E3s to certain cell cycle phases, subcellular locations, or cellular conditions. An extensive number of kinases regulate CKIs to control their function, localization, and stability (Supplemental tables 1 and 2). The phosphorylation events that regulate stability presumably affect the binding of CKIs to E3s. In the sections below, we will focus on the mechanisms that allow particular E3s to target specific sub-pools of CKIs, and the functional consequences of the targeted degradation.
We have classified the E3s into those that have been more extensively studied and those that are not as well characterized. Unless specifically stated, all of the E3s described below pass three minimum criteria for functioning as bona fide E3s for a CKI: 1) the E3 physically associates with the CKI in co-immunoprecipitations; 2) the inactivation of the E3 increases the level of the CKI in vivo; and 3) the E3 can ubiquitylate the CKI in vitro.
E3s with well defined functions in regulating CKIs
SCFSkp2: degradation of p21, p27, and p57 to regulate the cell cycle
The E3 SCFSkp2 is a major regulator of all three CIP/KIP CKIs. SCF complexes are a subgroup of the larger cullin-RING ubiquitin ligase (CRL) family. CRL complexes include a variable substrate-recognition subunit (SRS) that binds the substrate (see Box 1). Skp2 is an F-box protein with leucine-rich repeats that is the SRS for the SCFSkp2 complex. SCFSkp2 (in combination with its co-factor Cks1) targets CKIs that are bound to cyclin E–CDK2 or cyclin A-CDK2 and are phosphorylated by the same cyclin–CDK2 kinases on residues Ser130 (for p21), Thr187 (p27), or Thr310 (p57) [30] (Figures 2,3). SCFSkp2-mediated degradation of the CKIs in late G1 phase and early S phase allows cyclin–CDK activity to increase to promote S phase entry and prevent the re-licensing of DNA replication origins during S phase [31,32]. It should be noted that mice lacking Skp2 are viable with no gross anatomic abnormalities, although their body size is only about two-thirds that of control animals, and a subset of tissues exhibit increased DNA content and centrosome numbers (implying failed mitoses), which can be attributed to a failure to degrade p27 [33].
Box 1. Overview of the modular structure of CRL ubiquitin ligases.
CRL complexes are modular E3s that utilize variable substrate-binding components. CRLs constitute the largest family of E3s. CRL complexes utilize cullin proteins as a scaffold. There are eight human cullins: CUL1, CUL2, CUL3, CUL4A, CUL4B, CUL5, CUL7, and CUL9 (formerly PARC) [72–74]. Each cullin forms a set of modular CRL complexes that is named based on the cullin (e.g. CRL2 complexes contain CUL2); the exception is CUL1-based complexes, which are denoted SCF for the components Skp1, CUL1, and F-box proteins. A RING-H2 finger protein binds to the C-terminus of the cullin; this is RBX1/ROC1 for most cullins, and RBX2/ROC2 for CRL5 complexes [73,74]. RBX1/ROC1 binds to the ubiquitin-conjugating enzyme (E2). When the CRL complex is in the active conformation (which occurs upon conjugation of the ubiquitin-like Nedd8 protein to the cullin) then RBX1/ROC1 extends from the cullin on a flexible linker domain. When the E2 binds to RBX1/ROC1, the flexible linker allows greater mobility to position ubiquitin onto the substrate or the growing poly-ubiquitin chain [75] (see diagram). For most CRL complexes, an adaptor protein binds to the N-terminus of the cullin. The following adaptor proteins are utilized in CRL complexes: Skp1 (for SCF and CRL7 complexes); Elongin C (CRL2 and CRL5); and DDB1 (CRL4) [72–74]. The adaptor protein binds to the substrate-recognition subunit (SRS); and the SRS binds substrates. In CRL3 complexes, SRSs bind directly to the N-terminus of CUL3. Of central importance, the SRSs are variable components, and different SRSs bind to the same core CRL components to form different E3 complexes with different subcellular localizations that target the degradation of different substrates to regulate different cellular processes. SRSs are typified by the motif through which the SRS binds to the adaptor or cullin: F-box proteins are SRSs for SCF and CRL7 complexes (with the F-box motif binding to the adaptor); VHL-box proteins for CRL2; BTB/POZ-domain proteins for CRL3; WDXR proteins for CRL4; and SOCS-box proteins for CRL5 [72,73,76]. Multiple SRSs for each class of CRL complex are present in metazoan species; in humans there are: 69 F-box proteins (SCF complexes) [77]; 18 VHL-box proteins (CRL2) [78,79]; 53 BTB/POZ-box proteins (CRL3) [79]; between 50 and 90 WDXR-box proteins (CRL4) [80]; and 21 SOCS-box proteins (CRL5) (with SOCS1 functioning in a CRL2 complex, not a CRL5 complex) [76,78,79,81]. The large numbers of SRSs allow CRL complexes to regulate a wide range of cellular processes.
Box 1 Figure.

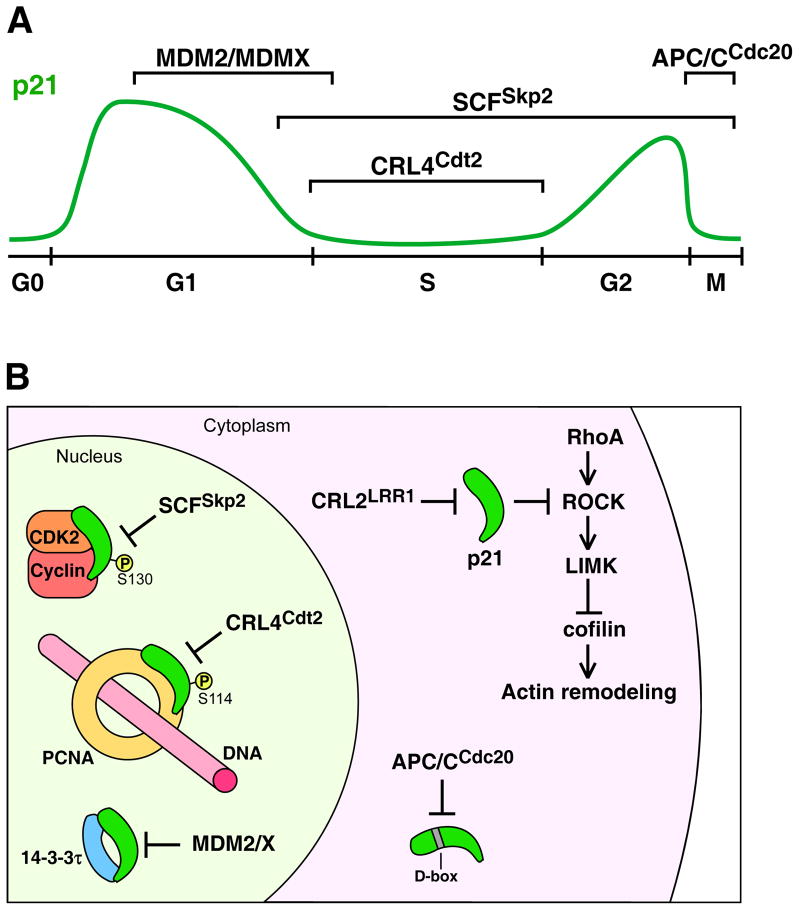
Figure 2. Ubiquitin ligase (E3) pathways that target the degradation of p21Cip1.
A) A diagram of the p21 protein level (green line) through the cell cycle, starting from G0 phase, with brackets denoting the intervals of the cell cycle in which particular E3s regulate p21 levels. While many cells have low levels of p21 during G0 phase (shown), some cells express high levels of p21 in G0 phase (see references in [71]). B) A diagram showing the context of nuclear and cytoplasmic E3–p21 interactions. In the nucleus MDM2/MDMX targets the degradation of p21 in the context of both p21 and MDM2/MDMX binding to the 14-3-3τ protein. SCFSkp2 (with co-factor Cks1) targets p21 that is bound to cyclin–CDK2 and is phosphorylated on Ser130. CRL4Cdt2 targets p21 bound to chromatin-associated PCNA and phosphorylated on Ser114. In the cytoplasm, CRL2LRR1 targets p21 that inhibits ROCK to inactivate the RhoA–ROCK–LIMK pathway, which negatively regulates ADF/cofilin and actin cytoskeleton remodeling. APC/CCdc20 targets p21 for degradation at prometaphase via recognition of the p21 D-box motif. Note that APC/CCdc20 is active during mitosis when the nuclear envelope has disassembled and therefore nucleoplasm and cytoplasm are mixed. All E3s may not function within the same cells. See text for details
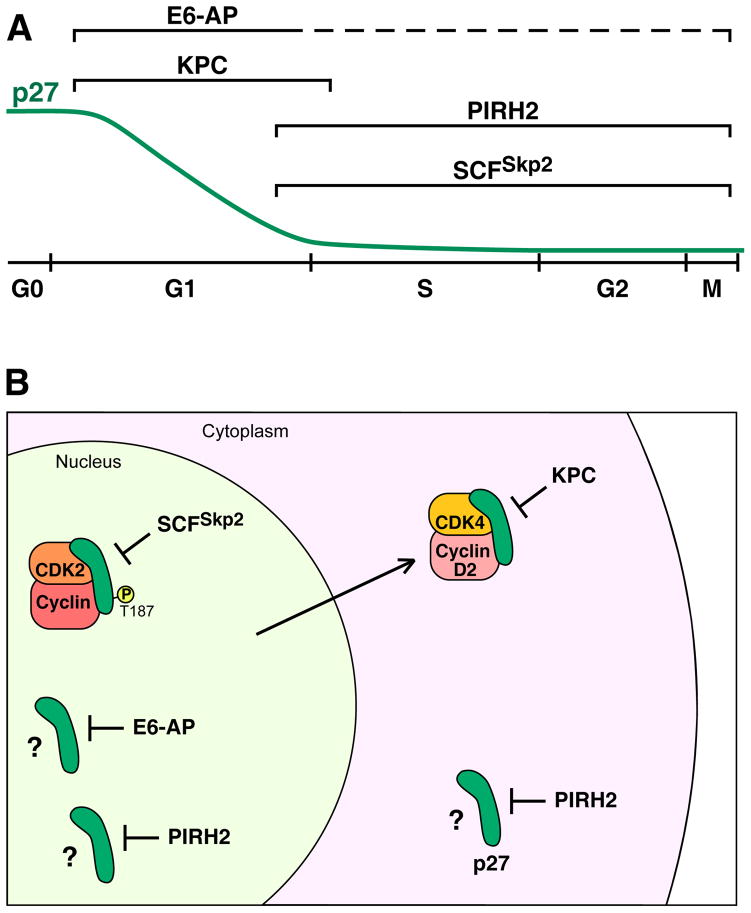
Figure 3. Ubiquitin ligase (E3) pathways that target the degradation of p27Kip1.
A) A diagram of the p27 protein level (green line) through the cell cycle, initiating from G0 phase, with brackets denoting the intervals of the cell cycle in which particular E3s regulate p27 levels. The dashed line for E6-AP denotes the absence of information on when it is active during the cell cycle. B) A diagram showing the context of nuclear and cytoplasmic E3–p27 interactions. In the nucleus, SCFSkp2 targets p27 that is bound to CDK2–cyclin and is phosphorylated on Thr187. E6-AP targets nuclear p27, but the context is not known (e.g., is p27 bound to another protein or modified by a PTM?); denoted by a question mark. PIRH2 targets p27 in both the nucleus and the cytoplasm, but the context is not known. KPC targets p27 that has been translocated to the cytoplasm from the nucleus. One mechanism of translocation that is linked to KPC-mediated degradation is the binding of nuclear p27 to cyclin D2–CDK4/6, which is translocated to the cytoplasm in response to the phosphorylation of cyclin D2. All E3s may not function within the same cells. See text for details.
SCFSkp2 is regulated so that it reduces CKI levels beginning in late G1 and early S phase. To accomplish this temporal regulation, Skp2 protein levels are kept low in G1 phase by degradation catalyzed by the E3 APCCdh1 [34]. Skp2 levels rise in late G1 phase and early S phase [35] coincident with the inactivation of APCCdh1 and the phosphorylation of Skp2 by cyclin–CDK2 to protect it from APCCdh1 [36,37]. A second mechanism for temporal regulation is that Skp2 binding to CKIs depends on the phosphorylation of the CKI by S phase cyclin–CDK2 complexes, which become active in late G1 phase [38]. CIP/KIP CKIs regulate cyclin–CDK functions when they are in the nucleus [24], and Skp2 and its cofactor Cks1 are nuclear localized in many cells, which further restricts the subcellular pool of CKIs that is targeted [39–41]. One additional regulatory mechanism for the SCFSkp2-mediated degradation of p27 involves the tyrosine kinases ABL, SRC, and LYN. The phosphorylation of p27 on Tyr74 and Tyr88 by these kinases loosens the interaction between p27 and cyclin–CDK2 to allow the cyclin–CDK2 to more readily phosphorylate p27 on Thr187 (which is required for its recognition by Skp2) [42,43].
CRL4Cdt2: degradation of p21 in S phase to prevent DNA re-replication
CRL4Cdt2 is a CRL E3 that is formed with the cullin CUL4 and the SRS Cdt2/L2DTL. CRL4Cdt2 targets substrates, including p21, that bind PCNA (proliferating cell nuclear antigen) that has been loaded onto chromatin during S phase or upon DNA damage [44] (Figure 2). PCNA is the sliding clamp that increases the processivity of DNA polymerases Polδ and Polε during DNA replication [45]. CRL4Cdt2 recognizes a specialized PIP box (PCNA interacting protein box) motif of p21 when it is bound to PCNA [46]. Additionally, p21 must be phosphorylated on Ser114 by GSK3β to be recognized by CRL4Cdt2 [44] (Supplemental Table 1).
It has been shown that p21 binding to PCNA can inhibit PCNA’s interactions with DNA polymerases, however this mechanism has not been established in vivo [47]. Rather, in HeLa cells, the perdurance of p21 in S phase that occurs upon inactivation of CRL4Cdt2 contributes to DNA re-replication [48]. The degradation of p21 by CRL4Cdt2 (acting redundantly with SCFSkp2) acts to prevent DNA re-replication by promoting the nuclear export of the replication-licensing factor Cdc6 during S phase, which occurs in response to cyclin–CDK-mediated phosphorylation [48,49]. CRL4Cdt2 therefore targets the degradation of p21 during S phase to prevent the over-replication of genomic DNA by preventing Cdc6 from re-loading the replicative helicase onto replication origins.
APC/CCdc20: degradation of p21 during mitosis
APC/CCdc20 (anaphase promoting complex/cyclosome with activating subunit Cdc20) is a multisubunit E3 that degrades proteins during mitosis to allow mitotic transitions [50] (Box 2). APC/CCdc20 becomes active during mitosis and targets the degradation of substrates with specific motifs, most notably the destruction box (D-box) motif. During the cell cycle, the level of p21 increases as cells progress from S phase to G2 phase [8,51] (Figure 2). APC/CCdc20 degrades p21 (which contains a D-box) during prometaphase, earlier than most of its other substrates [51] (Figure 2). This degradation allows the full activation of mitotic cyclin-CDK1 complexes during mitosis, which is important for subsequent mitotic events.
Box 2. Overview of the structure of APC/C ubiquitin ligases.
The Anaphase Promoting Complex/Cyclosome is a multisubunit complex that is distantly related to CRL complexes but contains more subunits (13 core subunits in humans) [50]. The APC2 subunit has homology to cullins and similarly functions as a scaffold for the complex; and the APC11 subunit is a RING finger protein with homology to RBX1/ROC1 [50]. The core components of human APC/C are: APC1–8, 10, 11, 13, 16, and CDC26. Active APC/C complexes include one of two activating subunits, Cdc20/Fzy (Fizzy) or Cdh1/Fzr (Fizzy-related), which act as SRSs to target specific substrates for degradation. The APC/CCdc20 complex is active in mitosis and is critical for progression through the metaphase-to-anaphase transition, while the APC/CCdh1 complex is active in late mitosis and G1 phase and is important for mitotic exit and G1 maintenance [50].
MDM2 and MDMX: ubiquitin-independent degradation of p21
MDM2 and MDMX are closely related monomeric RING E3s that promote the proteasome-mediated degradation of p21, but strikingly without ubiquitylation of p21 [52–54]. To accomplish this, MDM2/MDMX binds to both p21 and the proteasome to bring them together [53,54]. MDM2/MDMX targets the degradation of p21 in G1 and early S phases [52,53] (Figure 2). The 14-3-3τ protein binds to MDM2, p21, and the C8 subunit of the 20S proteasome to promote MDM2-mediated p21 degradation [55]. In the absence of 14-3-3τ, MDM2, or MDMX, cells arrest in G1 phase in a p21-dependent manner [53,55]. The nuclear pool of p21 is specifically targeted for degradation because, while MDM2, MDMX, and 14-3-3τ are present in both the nucleus and cytoplasm, 14-3-3τ only binds to p21 in the nucleus [55]. MDM2 and MDMX therefore target nuclear p21 to promote the G1-to-S phase transition.
CRL2LRR1: degradation of p21 to control the actin cytoskeleton and motility
Human CRL2LRR1 regulates the cytoplasmic form of p21, which controls the actin cytoskeleton [56] (Figure 2). Loss of the SRS LRR1 in human cells produces increased cytoplasmic p21 that inhibits the RhoA–ROCK–LIMK pathway. This leads to the activation of the actin depolymerizing protein cofilin, which causes the disassembly of actin stress fibers, the formation of cell extensions, and increased cell motility. Therefore, human CRL2LRR1 normally acts to reduce the level of cytoplasmic p21 to keep cells in a non-motile state. The regulation of cytoplasmic p21 by CRL2LRR1 in human cells has no effect on the cell cycle (which is regulated by nuclear p21) [56]. The cytoplasmic localization of LRR1 is likely to be a major contributor to the specificity of CRL2LRR1 for the cytoplasmic pool of p21, especially in light of the fact that in C. elegans germ cells, LRR1 is instead nuclear localized and regulates nuclear p21 to promote G1 progression [56]. It is currently unknown whether there is a coordination of CRL2LRR1 with phosphorylations of p21 that determine cytoplasmic localization.
KPC: degradation of p27 during the G0-to-G1 phase transition
p27 is important for cell cycle exit when cells enter the quiescent G0 phase [6,38]. As cells re-enter the cell cycle from G0, p27 levels fall dramatically [6]. This degradation relies on the E3 KPC (Kip1 ubiquitylation-promoting complex). KPC is composed of the RING finger protein KPC1 in combination with KPC2, which contains a ubiquitin-like domain and two ubiquitin-associated domains [57]. In G0 arrested cells, p27 is nuclear localized, while KPC is located in the cytoplasm. During early G1 phase, p27 is exported from the nucleus to the cytoplasm, where KPC targets it for degradation [57]. One mechanism for p27 nuclear export to allow KPC-mediated degradation is via p27 binding to cyclin D2–CDK4/6, which is translocated to the cytoplasm during G1 phase (in response to the phosphorylation of cyclin D2) [58].
The inactivation of KPC by itself has little effect on cell cycle progression, however, co-inactivation of KPC and Skp2 inhibits the G0 to S phase transition, indicating that the two E3s act redundantly to allow cell cycle re-entry [57]. p27 is also exported from the nucleus in response to binding to COP9/Signalosome (CSN) components, including Jab1/CSN5 [59]. This export similarly leads to p27 degradation in the cytoplasm [59], but it is currently unclear if KPC or another E3 is responsible for the degradation.
SCFFBL12: degradation of p57 to prevent osteoblast differentiation
Cell cycle exit is a requirement for the terminal differentiation of cells. In embryonic tissues, p57 is expressed at the highest levels as tissues undergo terminal differentiation [7]. Mice embryos lacking p57 exhibit failures in the differentiation of multiple tissues [7]. In osteoblasts, p57 is targeted for degradation by the SCFFBL12 complex to prevent the differentiation of these cells [60]. The SRS component FBL12 is induced in response to TGFβ1 signaling and is required for the TGFβ1-mediated decrease in p57 levels [60,61]. Inhibition of FBL12 leads to osteoblast differentiation [60]. The SCFFBL12-mediated degradation of murine p57 requires that it is phosphorylated on Thr329 by cyclin–CDK2 (this is equivalent to Thr310 in human p57, the same phosphorylation site recognized by SCFSkp2) [60]. This requirement suggests that SCFFBL12 (like SCFSkp2) targets the pool of p57 that regulates the cell cycle.
E3s with less well defined functions and regulation
p53RFP: possible E3 for degradation of p21 upon DNA damage
p53RFP (p53-inducible RING-finger protein) targets the degradation of p21 upon DNA damage. p53RFP contains a RING-IBR-RING domain, a Golgi transport motif, and a transmembrane domain [62]. Both p53RFP and p21 are transcriptionally induced in response to DNA damage. p53RFP overexpression or inactivation has a relatively modest effect on p21 levels [62]. When p53RFP is induced after DNA damage, it acts to reduce the extent of G1 arrest and to promote apoptosis [62]. p53RFP can induce apoptosis through a mechanism that is independent of its E3 activity; and p53RFP has been proposed to switch cells from p53-induced growth arrest (by targeting p21 for degradation) to p53-induced apoptosis (which p53RFP promotes) [63]. It is currently unclear whether p53RFP regulates a particular sub-pool of p21.
MKRN1: degradation of p21 to control apoptosis
The E3 MKRN1 (Makorin RING Finger Protein 1) targets p21 and p53 for degradation [64]. Under normal conditions, p53 is the preferential substrate for MKRN1, while upon DNA damage, MKRN1 primarily targets p21 for degradation. MKRN1 promotes apoptosis in response to DNA damage, and it has been proposed that its degradation of p21 helps to induce apoptosis [64]. However, further work is needed to clarify which subcellular pool of p21 is targeted by MKRN1, and the pathway by which it promotes apoptosis.
PIRH2: degradation of p27 in the nucleus and cytoplasm
PIRH2 (p53-inducible protein with RING H2 domain) is a monomeric RING finger E3 that targets p27 for degradation in both the nucleus and cytoplasm [65]. PIRH2 is expressed in late G1 through the remainder of the cell cycle. The loss of PIRH2 increases p27 levels from late G1 phase through G2 phase (Figure 3). Inactivation of PIRH2 leads to the accumulation of p27 in both the nucleus and cytoplasm. Inactivation of PIRH2 causes a G1 phase arrest [65], but this has not been shown to occur as a consequence of elevated p27 levels, and could conceivably arise from the loss of other PIRH2 functions. Overall, the purpose of PIRH2 regulation of the nuclear and cytoplasmic pools of p27 is unclear.
E6-AP: negative regulation of p27 transcription and protein turnover
UBE3A/E6-AP (E6-associated protein) functions independently as both an E3 and as a transcriptional co-repressor [66]. E6-AP negatively regulates the level of p27 only in the nucleus despite the fact that the E6-AP protein is both nuclear and cytoplasmic [67]. E6-AP negatively regulates the level of p27 in G1 phase [67], but it is unclear if it regulates p27 in other cell cycle phases (Figure 3). Inactivation of E6-AP increases the level of both p27 protein and p27 mRNA; and the relative contributions of transcriptional regulation vs. protein degradation are not established [67]. Inactivation of E6-AP decreases the number of cells in S phase and increases apoptosis in cultured cells, but it is unclear to what extent the accumulation of p27 contributes to these phenotypes. Mice lacking E6-AP are viable, although with reduced fertility [66], suggesting that E6- AP is not, by itself, essential for cell cycle progression.
CUL4-based E3(s) promoting p27 degradation: SRS to be identified
Human CUL4A has been implicated in the degradation of p27. In mouse and human mammary epithelium and in MEFs, Wnt signaling induces the expression of CUL4A and reduces p27 levels via a CUL4A-dependent mechanism [68]. The p27 degradation occurs in nuclei and promotes S-phase progression [68]. CUL4A and p27 co-precipitate, suggesting that CUL4A directly targets p27; although in vitro ubiquitylation has not yet been reported [68,69]. Two different studies show different requirements for Skp2 in p27 degradation linked to CUL4A. In mammary epithelium and MEFs, p27 degradation linked to CUL4A occurs independently of both Skp2 and phosphorylation of Thr187 of p27 (which is required for Skp2 binding). In contrast, another study found that CUL4 degrades p27 in a Skp2-dependent manner [70]. The observation that Skp2 co-precipitated with CUL4A led to the suggestion that Skp2 may act as the SRS for a CRL4Skp2 complex [70]. The current biochemical evidence is not sufficient to confirm that Skp2 functions as a CRL4 SRS. Until the CRL4 SRS(s) that bind p27 are identified (potentially confirming Skp2 and/or identifying the Skp2-independent SRS) it will be difficult to understand how the degradation is regulated.
Concluding Remarks
The number of E3s that target the CIP/KIP CKIs for degradation is exceptionally large. Our current understanding of the functions of the E3s is based on the context of the degradation (e.g. targeting a subpool of CKIs based on CKI subcellular localization, cell cycle phase, or in response to DNA damage, signal transduction, or binding to a particular protein); as well as on the phenotype that results from the failure of the E3 to degrade the CKI. From these considerations, we can assign the E3s to different functional categories. At least seven of the E3s appear to regulate the cell cycle functions of the CKIs: SCFSkp2; CRL4Cdt2; MDM2/MDMX; APC/CCdc20; KPC; SCFFBL12, and CUL4-based E3(s). One E3, CRL2LRR1 targets the cytoplasmic pool of p21 that controls the actin cytoskeleton and cell motility. Two E3s, p53RFP and MKRN1, promote apoptosis upon DNA damage, at least in part by degrading p21. Currently there is insufficient information to assign a function for the CKI degradation mediated by PIRH2, which degrades both nuclear and cytoplasmic pools of p27, and E6-AP, which degrades nuclear p27 but also regulates p27 expression.
As noted above, the CKIs are inherently unstructured prior to binding other proteins, and this can be used to allow specificity in targeting certain CKI populations. E3s that target CKIs when they are complexed with particular proteins will by default target only a subset of CKIs that are likely to be engaged in specific cellular functions. SCFSkp2 and CRL2Cdt2 are known to employ this mechanism (targeting CKIs bound to cyclin-CDK and PCNA, respectively). However, given the utility of this mechanism, we expect that other E3s will also recognize CKIs when bound to specific proteins, and in particular, we expect that E3s will exist that target CKIs bound to transcription factors (none of which are currently identified). We also expect additional E3s that mediate p57 degradation during development to allow regulation in specific tissues in response to particular developmental signals (similar to the regulation of p57 by SCFFBL12 in osteoblasts in response to TGFβ1). We predict that the total number of E3s that target the CIP/KIP CKIs, while already substantial, will increase in the future.
The stability of p21 and p27 are regulated by phosphorylation on multiple residues (Supplemental tables 1 and 2). Many of these phosphorylation sites stabilize or destabilize the CKIs, but for most of these, the relevant E3(s) have not been identified. Given the role of phosphorylation in both stabilizing and destabilizing CKIs, it is expected that specific phosphorylation events enhance E3 specificity by promoting or blocking the binding of specific E3s to the phosphorylated CKI. Uncovering how phosphorylation events (and the signal-transduction pathways that control them) are linked to E3-mediated degradation will allow further integration of these pathways into developmental and homeostatic contexts. There is still much to learn about how degradation pathways control these versatile, multifunctional proteins, which are poised to act at the crossroads of the cell cycle, transcription, apoptosis, and the cytoskeleton.
Supplementary Material
Acknowledgments
We thank members of the Kipreos laboratory for critical comments on the manuscript. We apologize to those authors whose work we could not cite due to space limitations. Work in our lab is supported by grants from NIH/NIGMS (R01GM074212 and R01GM055297).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 2.Besson A, et al. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 5.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu IM, et al. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 7.Pateras IS, et al. p57KIP2: “Kip” ing the cell under control. Mol Cancer Res. 2009;7:1902–1919. doi: 10.1158/1541-7786.MCR-09-0317. [DOI] [PubMed] [Google Scholar]
- 8.Dulic V, et al. Nuclear accumulation of p21Cip1 at the onset of mitosis: a role at the G2/M-phase transition. Mol Cell Biol. 1998;18:546–557. doi: 10.1128/mcb.18.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama K. Cip/Kip cyclin-dependent kinase inhibitors: brakes of the cell cycle engine during development. Bioessays. 1998;20:1020–1029. doi: 10.1002/(SICI)1521-1878(199812)20:12<1020::AID-BIES8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen L, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynaud EG, et al. Stabilization of MyoD by direct binding to p57(Kip2) J Biol Chem. 2000;275:18767–18776. doi: 10.1074/jbc.M907412199. [DOI] [PubMed] [Google Scholar]
- 12.Chang TS, et al. p57KIP2 modulates stress-activated signaling by inhibiting c-Jun NH2-terminal kinase/stress-activated protein Kinase. J Biol Chem. 2003;278:48092–48098. doi: 10.1074/jbc.M309421200. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1) Mol Cell. 2003;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 14.Asada M, et al. Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki A, et al. Procaspase 3/p21 complex formation to resist fas-mediated cell death is initiated as a result of the phosphorylation of p21 by protein kinase A. Cell Death Differ. 2000;7:721–728. doi: 10.1038/sj.cdd.4400706. [DOI] [PubMed] [Google Scholar]
- 16.Dotto GP. p21(WAF1/Cip1): more than a break to the cell cycle? Biochim Biophys Acta. 2000;1471:M43–56. doi: 10.1016/s0304-419x(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q, et al. Transcriptional regulation during p21WAF1/CIP1-induced apoptosis in human ovarian cancer cells. J Biol Chem. 2002;277:36329–36337. doi: 10.1074/jbc.M204962200. [DOI] [PubMed] [Google Scholar]
- 19.Vlachos P, et al. The cell cycle inhibitor p57(Kip2) promotes cell death via the mitochondrial apoptotic pathway. Cell Death Differ. 2007;14:1497–1507. doi: 10.1038/sj.cdd.4402158. [DOI] [PubMed] [Google Scholar]
- 20.Narumiya S, et al. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlachos P, Joseph B. The Cdk inhibitor p57(Kip2) controls LIM-kinase 1 activity and regulates actin cytoskeleton dynamics. Oncogene. 2009;28:4175–4188. doi: 10.1038/onc.2009.269. [DOI] [PubMed] [Google Scholar]
- 23.Yokoo T, et al. p57Kip2 regulates actin dynamics by binding and translocating LIM-kinase 1 to the nucleus. J Biol Chem. 2003;278:52919–52923. doi: 10.1074/jbc.M309334200. [DOI] [PubMed] [Google Scholar]
- 24.Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. doi: 10.1017/S1462399408000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, et al. p57: A multifunctional protein in cancer (Review) Int J Oncol. 2010;36:1321–1329. doi: 10.3892/ijo_00000617. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, et al. Disruption of TGF-beta growth inhibition by oncogenic ras is linked to p27Kip1 mislocalization. Oncogene. 2000;19:5926–5935. doi: 10.1038/sj.onc.1203991. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Helfman DM. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem. 2004;279:1885–1891. doi: 10.1074/jbc.M306968200. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, et al. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7:429–440. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galea CA, et al. Regulation of cell division by intrinsically unstructured proteins: intrinsic flexibility, modularity, and signaling conduits. Biochemistry. 2008;47:7598–7609. doi: 10.1021/bi8006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010:9. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Jin J. DNA replication licensing control and rereplication prevention. Protein Cell. 2010;1:227–236. doi: 10.1007/s13238-010-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pospiech H, et al. The initiation step of eukaryotic DNA replication. Subcell Biochem. 2010;50:79–104. doi: 10.1007/978-90-481-3471-7_5. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama K, et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 34.Bashir T, et al. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 35.Carrano AC, et al. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 36.Lukas J, Bartek J. Cell division: the heart of the cycle. Nature. 2004;432:564–567. doi: 10.1038/432564a. [DOI] [PubMed] [Google Scholar]
- 37.Rodier G, et al. Phosphorylation of Skp2 regulated by CDK2 and Cdc14B protects it from degradation by APC(Cdh1) in G1 phase. EMBO J. 2008;27:679–691. doi: 10.1038/emboj.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guardavaccaro D, Pagano M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol Cell. 2006;22:1–4. doi: 10.1016/j.molcel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Lee JG, Kay EP. Involvement of two distinct ubiquitin E3 ligase systems for p27 degradation in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:189–196. doi: 10.1167/iovs.07-0855. [DOI] [PubMed] [Google Scholar]
- 40.Lisztwan J, et al. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 1998;17:368–383. doi: 10.1093/emboj/17.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodier G, et al. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 2001;20:6672–6682. doi: 10.1093/emboj/20.23.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu I, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimmler M, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 44.Abbas T, Dutta A. CRL4Cdt2: master coordinator of cell cycle progression and genome stability. Cell Cycle. 2011;10:241–249. doi: 10.4161/cc.10.2.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moldovan GL, et al. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Havens CG, Walter JC. Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell. 2009;35:93–104. doi: 10.1016/j.molcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Havens CG, Walter JC. Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 25:1568–1582. doi: 10.1101/gad.2068611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y, et al. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 2008;22:2507–2519. doi: 10.1101/gad.1703708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, et al. C. elegans CUL-4 prevents rereplication by promoting the nuclear export of CDC-6 via a CKI-1-dependent pathway. Curr Biol. 2007;17:966–972. doi: 10.1016/j.cub.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLean JR, et al. State of the APC/C: organization, function, and structure. Crit Rev Biochem Mol Biol. 2011;46:118–136. doi: 10.3109/10409238.2010.541420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amador V, et al. APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell. 2007;27:462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jin Y, et al. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 2003;22:6365–6377. doi: 10.1093/emboj/cdg600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin Y, et al. MDMX promotes proteasomal turnover of p21 at G1 and early S phases independently of, but in cooperation with, MDM2. Mol Cell Biol. 2008;28:1218–1229. doi: 10.1128/MCB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, et al. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem. 2004;279:16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, et al. 14-3-3Tau regulates ubiquitin-independent proteasomal degradation of p21, a novel mechanism of p21 downregulation in breast cancer. Mol Cell Biol. 2010;30:1508–1527. doi: 10.1128/MCB.01335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starostina NG, et al. CRL2(LRR-1) targets a CDK inhibitor for cell cycle control in C. elegans and actin-based motility regulation in human cells. Dev Cell. 2010;19:753–764. doi: 10.1016/j.devcel.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamura T, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 58.Susaki E, et al. Cyclin D2 translocates p27 out of the nucleus and promotes its degradation at the G0–G1 transition. Mol Cell Biol. 2007;27:4626–4640. doi: 10.1128/MCB.00862-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomoda K, et al. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–2310. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, et al. A new ubiquitin ligase involved in p57KIP2 proteolysis regulates osteoblast cell differentiation. EMBO Rep. 2008;9:878–884. doi: 10.1038/embor.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimori S, et al. Smad-mediated transcription is required for transforming growth factor-beta 1-induced p57(Kip2) proteolysis in osteoblastic cells. J Biol Chem. 2001;276:10700–10705. doi: 10.1074/jbc.M007499200. [DOI] [PubMed] [Google Scholar]
- 62.Ng CC, et al. p53RFP, a p53-inducible RING-finger protein, regulates the stability of p21WAF1. Oncogene. 2003;22:4449–4458. doi: 10.1038/sj.onc.1206586. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, et al. The p53-inducible E3 ubiquitin ligase p53RFP induces p53-dependent apoptosis. FEBS Lett. 2006;580:940–947. doi: 10.1016/j.febslet.2005.09.105. [DOI] [PubMed] [Google Scholar]
- 64.Lee EW, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J. 2009;28:2100–2113. doi: 10.1038/emboj.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hattori T, et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 2007;67:10789–10795. doi: 10.1158/0008-5472.CAN-07-2033. [DOI] [PubMed] [Google Scholar]
- 66.Ramamoorthy S, Nawaz Z. E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nucl Recept Signal. 2008;6:e006. doi: 10.1621/nrs.06006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra A, et al. UBE3A/E6-AP regulates cell proliferation by promoting proteasomal degradation of p27. Neurobiol Dis. 2009;36:26–34. doi: 10.1016/j.nbd.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Miranda-Carboni GA, et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121–3134. doi: 10.1101/gad.1692808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li B, et al. Cul4A targets p27 for degradation and regulates proliferation, cell cycle exit, and differentiation during erythropoiesis. Blood. 2006;107:4291–4299. doi: 10.1182/blood-2005-08-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bondar T, et al. Cul4A and DDB1 associate with Skp2 to target p27Kip1 for proteolysis involving the COP9 signalosome. Mol Cell Biol. 2006;26:2531–2539. doi: 10.1128/MCB.26.7.2531-2539.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perucca P, et al. Loss of p21 CDKN1A impairs entry to quiescence and activates a DNA damage response in normal fibroblasts induced to quiescence. Cell Cycle. 2009;8:105–114. doi: 10.4161/cc.8.1.7507. [DOI] [PubMed] [Google Scholar]
- 72.Skaar JR, et al. PARC and CUL7 form atypical cullin RING ligase complexes. Cancer Res. 2007;67:2006–2014. doi: 10.1158/0008-5472.CAN-06-3241. [DOI] [PubMed] [Google Scholar]
- 73.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 74.Bosu DR, Kipreos ET. Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div. 2008;3:7. doi: 10.1186/1747-1028-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duda DM, et al. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamura T, et al. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin J, et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahrour N, et al. Characterization of Cullin-box sequences that direct recruitment of Cul2-Rbx1 and Cul5-Rbx2 modules to Elongin BC-based ubiquitin ligases. J Biol Chem. 2008;283:8005–8013. doi: 10.1074/jbc.M706987200. [DOI] [PubMed] [Google Scholar]
- 79.Bennett EJ, et al. Dynamics of cullin-RING ubiquitin ligase network revealed by systematic quantitative proteomics. Cell. 2010;143:951–965. doi: 10.1016/j.cell.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson S, Xiong Y. CRL4s: the CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci. 2009;34:562–570. doi: 10.1016/j.tibs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamizono S, et al. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.