Abstract
Synaptic long-term potentiation (LTP) and long-term depression (LTD) have been studied as mechanisms of ocular dominance plasticity in the rat visual cortex. Serotonin (5-hydroxytryptamine, 5-HT) inhibits the induction of LTP and LTD during the critical period of the rat visual cortex (postnatal 3~5 weeks). However, in adult rats, the increase in 5-HT level in the brain by the administration of the selective serotonin reuptake inhibitor (SSRI) fluoxetine reinstates ocular dominance plasticity and LTP in the visual cortex. Here, we investigated the effect of 5-HT on the induction of LTP in the visual cortex obtained from 3- to 10-week-old rats. Field potentials in layer 2/3, evoked by the stimulation of underlying layer 4, was potentiated by theta-burst stimulation (TBS) in 3- and 5-week-old rats, then declined to the baseline level with aging to 10 weeks. Whereas 5-HT inhibited the induction of LTP in 5-week-old rats, it reinstated the induction of N-methyl-D-aspartate receptor (NMDA)-dependent LTP in 8- and 10-week-old rats. Moreover, the selective SSRI citalopram reinstated LTP. The potentiating effect of 5-HT at 8 weeks of age was mediated by the activation of 5-HT2 receptors, but not by the activation of either 5-HT1A or 5-HT3 receptors. These results suggested that the effect of 5-HT on the induction of LTP switches from inhibitory in young rats to facilitatory in adult rats.
Keywords: 5-HT, SSRI, Citalopram, 5-HT2 receptor, Para-chloroamphetamine
INTRODUCTION
Neuromodulators regulate a vast array of brain functions. Serotonin (5-hydroxytryptamine, 5-HT), one of the most important neuromodulators, is involved in higher brain functions, such as cognition and emotional states [1]. Also, 5-HT is involved in the induction of long-term synaptic plasticity, which is a cellular and molecular mechanism of various brain functions such as learning, memory and sensory development [2]. The rat visual cortex receives a huge input of extensively ramified 5-HT fibers from midbrain raphe nuclei [3]. Since 5-HT has enhanced the induction of synaptic long-term potentiation (LTP) and long-term depression (LTD) in the visual cortex of cats [4], 5-HT is proposed as an enabling factor for long-term synaptic plasticity [5]. Because LTP and LTD have been proposed as a mechanism for ocular dominance plasticity in the visual cortex [6,7], despite some controversies [8,9], 5-HT appears to be an important neuromodulator involved in ocular dominance plasticity [10].
In contrast to its effect on cats, 5-HT appears to inhibit the induction of LTP and LTD during the critical period in the visual cortex of rats [11-15]. In rats, 5-HT also inhibits the induction of LTP in various brain regions such as the hippocampus [16], the amygdala [17], and the prefrontal cortex [18]. Since the 5-HT content in the visual cortex increases during the critical period [11,13], the inhibitory effect of 5-HT on the induction of LTP in the visual cortex has been suggested as one of molecular mechanisms for the closure of critical period and a decrease in ocular dominance plasticity of rats [11,12]. However, in recent studies, chronic administration of the selective serotonin reuptake inhibitor (SSRI) fluoxetine has reinstated ocular dominance plasticity and LTP in the visual cortex of adult rats [19,20]. Since the expression patterns of 5-HT receptor subtypes change during postnatal development [4,21], the effect of 5-HT on the induction of LTP and ocular dominance plasticity might differ between young and adult rats. However, comprehensive studies on the effect of 5-HT in the induction of LTP from young to adult rats are not available yet.
Thus, in the present study we studied the acute effect of 5-HT on the induction of LTP in visual cortex slices during development in young and adult rats. We found that, in contrast to the inhibitory effect of 5-HT on the induction of LTP in young rats, 5-HT facilitated the induction of LTP in adult rats at 8 and 10 weeks of age. Thus, the switching effect of 5-HT on the induction of LTP during postnatal development provides new insight on the role of 5-HT in the brain.
METHODS
Animal care and slice preparation
Sprague-Dawley rats of both sexes (postnatal 3 to 10 weeks, Orientbio Inc., Korea) were maintained and raised under standard conditions (23±1℃, 12/12 hours light/dark cycle). Animal care and surgical procedures were approved by the Ethics Committee of the Catholic University of Korea and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Visual cortex slices were prepared from 3- to 10-week-old rat as previously described [11]. Briefly, the brains were quickly removed after anesthetization with chloral hydrate (400 mg/kg, i.p.), and were submerged in ice-cold dissection medium. Coronal sections of the occipital cortex (400 µm in thickness) were prepared on a vibrotome (Campden Instruments, Leica, UK) and were allowed to recover in a storage chamber for 40 min at 37℃. The slices were maintained at room temperature prior to recording. The dissection and storage medium consisted of 125 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 2 mM MgSO4, 1.25 mM NaH2PO4, 25 mM NaHCO3, and 10 mM D-glucose, bubbled with 95% O2/5% CO2. The slices were transferred to the submerging chamber for recording and superfused continuously with artificial cerebrospinal fluid (ACSF, 1.5~2 ml/min) containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1.25 mM NaH2PO4, 25 mM NaHCO3, and 10 mM D-glucose, bubbled with 95% O2/5% CO2 at 32~33℃. In some experiments, the slices were incubated with para-chloroamphetamine (PCA, 10 µM) for 2 hr to deplete endogenous 5-HT. Under these experimental conditions, PCA (10 µM) was also included in ACSF throughout the recording.
Electrophysiological recording
The recording electrode pulled from a glass pipette was filled with ACSF (1~3 MΩ) and positioned in layer 2/3 of the primary visual cortex. Field potential (FP) was elicited by a rectangular current pulse (0.2 ms) at a site in the middle of the cortex, by means of a concentric bipolar stimulating electrode (100 µm in diameter, SNE-100, David Kopf). The baseline response was obtained at 30-s intervals for 10 min with a stimulus intensity that yielded half-maximal FP amplitude. Theta-burst stimulation (TBS, 5 bursts at 5 Hz of 10 pulses at 100 Hz) was applied 5 times at 10-s intervals to induce LTP. The FP was recorded for 50 min after the application of TBS. The signals were amplified 1,000-fold, filtered between 0.1 and 3 kHz, and saved to a Pentium PC using the LTP program (v2.3, www.ltp-program.com). The peak amplitude of the FP from the baseline was calculated and used for analysis of the effect of the conditioning stimulation.
Chemicals
D-AP5, NAN-190, 2-methyl-5-hydroxytryptamine (2-me-5-HT), and (±)-2,5-dimethoxy-4-iodoamphetamine (DOI) were purchased from Tocris (Bristol, UK). PCA, 5-HT, (±)-8-hydroxy-2-dipropylaminotetralin (8-OH-DPAT), mesulergine and other chemicals were purchased from Sigma (St. Louis, MO, USA).
Statistical analysis
Data were expressed as the mean±SE. Group comparisons were performed using either paired or unpaired two-tailed Student's t-tests. The level of significance was set at p<0.05.
RESULTS
5-HT inhibits the induction of LTP in young rats
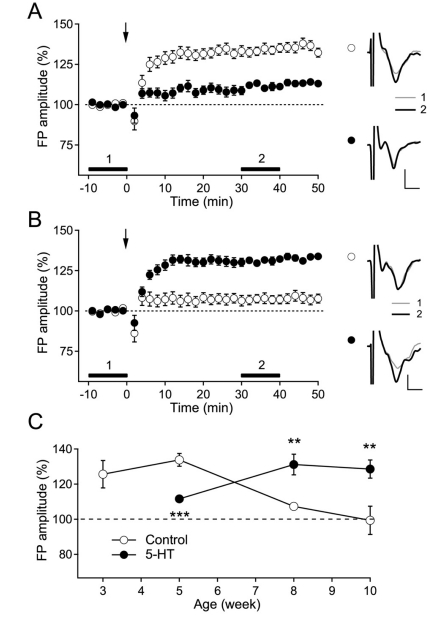
In our previous study, the duration of the stimulation pulse was important for the recruitment of inhibitory circuits and the induction of long-term synaptic plasticity in the supragranular layer of the rat visual cortex [22]. To investigate the inhibitory effect of 5-HT on the induction of LTP in the pathway from layer 4 to layer 2/3 in the present study, we induced LTP in layer 2/3 using a 0.2-ms stimulus pulse, which stably induced LTP during the critical period (postnatal 3~5 weeks in rats). In accordance with our previous study, TBS evoked LTP in slices from 3-(125.6±7.8% of the baseline FP amplitude, n=8, p<0.05) and 5-week-old rats (133.8±3.7% of the baseline FP amplitude, n=10, p<0.001) (Fig. 1A and C). However, although TBS induced a small LTP in 5-week-old rats in the presence of 5-HT (10 µM) (111.6±1.8% of the baseline FP amplitude, n=10, p<0.001), 5-HT significantly inhibited the induction of LTP at this age (p<0.001, compared with control) (Fig. 1C). The magnitudes of LTP and the inhibition by 5-HT were similar to those in our previous studies [11,22]. Thus, we confirmed the inhibitory effect of 5-HT on the induction of LTP, which was induced by the stimulation recruiting less inhibitory circuits, in the pathway from layer 4 to layer 2/3 in the visual cortex of young rats.
Fig. 1.
Switching effect of 5-HT on the induction of LTP in the rat visual cortex. (A) The effect of the TBS of layer 4 (indicated with arrow) on layer 2/3 FP in control slices (open circle, n=10) and in 5-HT-perfused slices (closed circle, n=10) from 5-week-old rats. The right panel shows the representative average traces in a slice marked with corresponding symbols, which were taken at the time period indicated with corresponding numbers. Scales represent 0.4 mV and 2 ms, respectively. (B) The effect of the TBS of layer 4 (indicated with arrow) on layer 2/3 FP in control slices (open circle, n=11) and in 5-HT-perfused slices (closed circle, n=12) from 8-week-old rats. The right panel shows the representative average traces in a slice marked with corresponding symbols, which were taken at the time period indicated with corresponding numbers. Scales represent 0.4 mV and 2 ms, respectively. (C) Age-dependent inhibition and facilitation of layer 2/3 LTP induction induced by TBS to layer 4 in the rat visual cortex. Numbers of the experiments are from 5 to 12. **p<0.01 and ***p<0.001 compared with the corresponding control.
5-HT facilitates NMDA receptor-dependent LTP in adult rats
In contrast with young rats, the magnitude of LTP in the pathway from layer 4 to layer 2/3 declined with age to 106.7±2.8% (n=12) of the baseline FP amplitude for 8-week-old rats (p<0.05) and to 99.4±8.0% (n=5) of the baseline FP amplitude for 10-week-old rats (p=0.94) (Fig. 1B and C). Although it is known that LTP and LTD in the pathway from layer 4 to layer 2/3 is constantly elicited regardless of aging [23], our experimental conditions exhibited an age-dependent decline in the induction of LTP and LTD in this pathway, which might have resulted from the recruitment of a substantial amount of inhibitory components and their postnatal development [22,24]. However, the application of 5-HT into the bath solution reinstated the induction of LTP in 8-(131.1±5.9% of the baseline FP amplitude, n=12, p<0.001) and 10-week-old rats (128.5±5.2% of the baseline FP amplitude, n=7, p<0.01) (Fig. 1C). Thus, these results showed that 5-HT exerts a facilitating effect on the induction of LTP in the pathway from layer 4 to layer 2/3 in the visual cortex of adult rats.
In our previous study, 5-HT specifically inhibited NMDA receptor-dependent LTP in young rats, but not metabotropic glutamate receptor (mGluR)-dependent LTP [11]. We investigated whether the reinstated LTP was dependent on the activation of NMDA receptors. Bath application of the NMDA receptor antagonist D-AP5 (50 µM) in the presence of 5-HT abolished the induction of LTP in 8-week-old rats (106.4±7.6% of the baseline FP amplitude, n=8, p=0.43). Thus, 5-HT specifically facilitated the induction of NMDA receptor-dependent LTP in adult rats.
Endogenous 5-HT facilitates the induction of LTP in adult rats
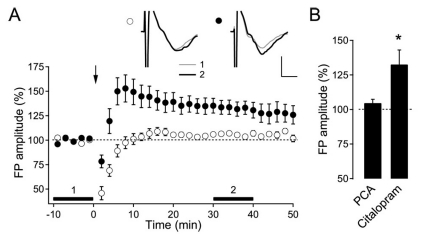
In the next experiment, we tested the effect of pharmacological manipulation of endogenous 5-HT in adult rats. Initially, endogenous 5-HT was depleted by the incubation of slices from 8-week-old rats with PCA (10 µM, 2 hr) [11]. TBS did not induce LTP in 5-HT-depleted slices (104.6±2.8% of the baseline FP amplitude, n=5, p=0.17) (Fig. 2). The SSRI citalopram was used in order to determine the effect of an increased tone of serotonin in the slices [25] on LTP induction. Bath application of citalopram (10 µM) mimicked the effect of 5-HT on the induction of LTP in 8-week-old rats (132.5±10.5% of the baseline FP amplitude, n=6, p<0.05) (Fig. 2). These results suggest that endogenously released 5-HT could facilitate the induction of LTP in the visual cortex of aged rats.
Fig. 2.
Effect of endogenous 5-HT on the induction of LTP in 8-week-old rats. (A) Average response to TBS applied at the indicated time (arrow) in PCA-incubated (open circle, n=5) and citalopram-treated slices (closed circle, n=6). Insets show the average FP recording taken from a representative experiment at the indicated time period with corresponding numbers. Scales represent 0.4 mV and 2 ms, respectively. (B) Summary plot of the LTP induction in PCA- and citalopram-treated slices from 8-week-old rats (n=5 and n=6, respectively). *p<0.05 compared with the baseline.
Effect of specific 5-HT receptor agonists and antagonists on the induction of LTP
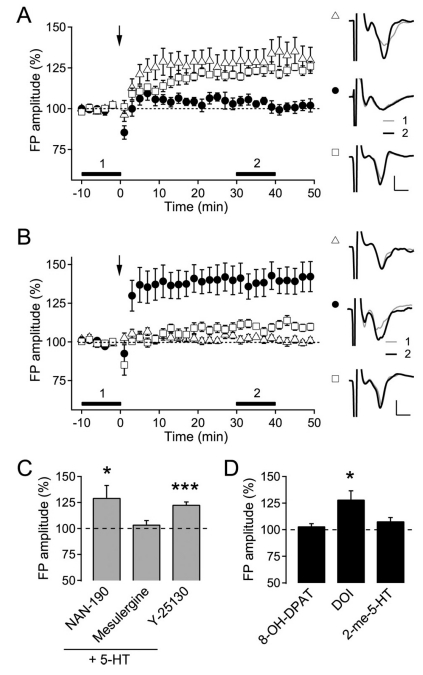
Membrane receptors for 5-HT comprise at least 14 subtypes from 5-HT1 to 5-HT7 receptors [26], among which 5-HT1A, 5-HT2 and 5-HT3 receptors are reportedly expressed in the rat visual cortex [21,25,27]. Thus, in the following experiment we tested which 5-HT receptor subtypes are involved in the facilitation of LTP in adult rats using specific antagonists (Fig. 3A and C) and agonists (Fig. 3B and D) for 5-HT1A, 5-HT2 and 5-HT3 receptors. Co-application of the specific 5-HT1A receptor antagonist NAN-190 (10 µM) with 5-HT did not inhibit the induction of LTP (129.5±11.8% of the baseline FP amplitude, n=7, p<0.05). The specific 5-HT3 receptor antagonist Y-25130 (10 µM) did not change the magnitude of LTP facilitated by 5-HT (122.9±2.6% of the baseline FP amplitude, n=5, p<0.001). In contrast, co-application of the 5-HT2 receptor antagonist mesulergine (10 µM) with 5-HT abolished the induction of LTP (103.8±3.8% of the baseline FP amplitude, n=6, p=0.36). These results suggest that the activation of 5-HT2 receptors might be involved in the facilitation of LTP in the visual cortex of aged rats.
Fig. 3.
Effect of 5-HT receptor antagonists and agonists on the induction of LTP in 8-week-old rats. (A) The effect of 5-HT receptor antagonists on layer 2/3 LTP induced by TBS to layer 4 (indicated with arrow) in 8-week-old rats. NAN-190 (10 µM, n=7, open triangle), mesulergine (10 µM, n=6, closed circle) and Y-25130 (10 µM, n=5, open square) were co-applied into the bath with 5-HT (10 µM). The right panel shows the representative average traces in a slice marked with corresponding symbols, which were taken at the time period indicated with corresponding numbers. Scales represent 0.4 mV and 2 ms, respectively. (B) The effect of 5-HT receptor agonists on layer 2/3 LTP induced by TBS to layer 4 (indicated with arrow) in 8-week-old rats. 8-OH-DPAT (10 µM, n=7, open triangle), DOI (10 µM, n=7, closed circle) and 2-me-5-HT (10 µM, n=7, open square) were applied into the bath throughout the experiment. The right panel shows the representative average traces in a slice marked with corresponding symbols, which were taken at the time period indicated with corresponding numbers. Scales represent 0.4 mV and 2 ms, respectively. (C) Summary plot of the effect of antagonists for 5-HT receptor subtypes. *p<0.05 and ***p<0.001 compared with the baseline. (D) Summary plot of the effect of agonists for 5-HT receptor subtypes. *p<0.05 compared with the baseline.
In another set of experiments, DOI (10 µM), a specific 5-HT2 receptor agonist, mimicked the induction of LTP facilitated by the application of 5-HT in 8-week-old rats (128.4±8.1% of the baseline FP amplitude, n=7, p<0.05), while 8-OH-DPAT (10 µM), a specific 5-HT1A receptor agonist (103.3±2.4% of the baseline FP amplitude, n=7, p=0.21), or 2-me-5-HT, a specific 5-HT3 receptor agonist (106.2±3.4% of the baseline FP amplitude, n=7, p=0.12), failed to induce LTP (Fig. 3B). These results also support the hypothesis that the activation of the 5-HT2 receptor might be involved in the 5-HT facilitation of the induction of LTP in adult rats.
Effect of specific 5-HT2 receptor antagonists on the facilitated induction of LTP
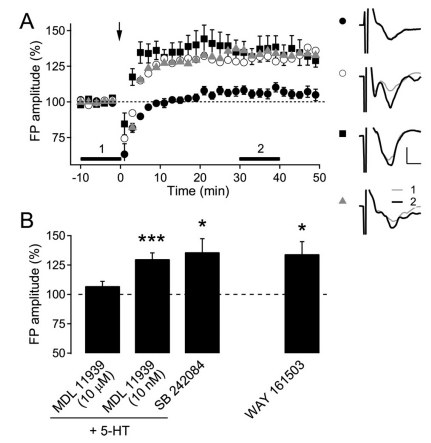
Both 5-HT2A and 5-HT2C receptors are expressed in pyramidal neurons in the neocortex of the rat [21,28]. Thus, we further investigated the involvement of these receptor subtypes in the 5-HT facilitation of LTP (Fig. 4). Co-application of the specific 5-HT2A/C receptor blocker MDL 11939 at a high concentration (10 µM) consistently abolished LTP facilitated by 5-HT (107.2±3.9% of the baseline FP amplitude, n=5, p=0.14). However, MDL 11939 as a selective 5-HT2A receptor antagonist at a low concentration (10 nM) did not inhibit the facilitation of LTP by 5-HT application (130.0±5.3% of the baseline FP amplitude, n=11, p<0.001). The application of the selective 5-HT2C receptor antagonist SB 242084 (5 µM) did not inhibit the facilitation of LTP (135.9±11.4% of the baseline FP amplitude, n=5, p<0.05). In addition, we tested the effect of another specific 5-HT2C receptor agonist, WAY 161503 (10 µM), which consistently evoked LTP in 8-week-old rats (134.3±10.6% of the baseline FP amplitude, n=8, p<0.05). These results suggest that the activation of either 5-HT2A or 5-HT2C might be sufficient for the 5-HT facilitation of LTP in adult rats.
Fig. 4.
Effect of 5-HT2 receptor antagonists and a agonist on the 5-HT facilitation of LTP induction in 8-week-old rats. (A) The effect of 5-HT2 receptor antagonists and agonists on layer 2/3 LTP induced by TBS to layer 4 (indicated with arrow) in 8-week-old rats. MDL 11939 (10 µM, n=5, closed circle), MDL 11939 (10 nM, n=11, open circle), and SB 242084 (5 µM, n=5, closed square) were co-applied into the bath with 5-HT (10 µM). WAY 161503 (10 µM, n=8, gray triangle) was applied without 5-HT into the bath. The right panel shows the representative average traces in a slice marked with corresponding symbols, which were taken at the time period indicated with corresponding numbers. Scales represent 0.4 mV and 2 ms, respectively. (B) Summary plot of the effect of antagonists and a agonist for 5-HT2 receptors. *p<0.05 and ***p< 0.001 compared with the baseline.
DISCUSSION
Here we showed that, in contrast to the inhibitory effect of 5-HT on the induction of LTP in young rats (3~5 weeks old), 5-HT facilitated the induction of NMDA receptor-dependent LTP in adult rats (8~10 weeks old). The LTP in adult rats was inhibited by the application of 5-HT2 receptor antagonists and mimicked by the application of 5-HT2 receptor agonists. Our results demonstrate a switching role of 5-HT in the induction of LTP during postnatal development after adolescence in the rat visual cortex.
Heightened neuronal plasticity during a certain period of postnatal development as critical period has received much attention because long-term synaptic plasticity is involved in sensory cortical development, which is shaped by sensory experience [6,29]. Because the critical period is a very complex phenomenon, many mechanisms are suggested for its closure during postnatal development. Among them, age-dependent decline in the induction of long-term synaptic plasticity [7] and late maturation of inhibitory circuits [30,31] have been extensively studied. In the present study, layer-2/3 LTP evoked by the stimulation of layer 4 declined to the baseline level at the ages of 8 and 10 weeks. However, it is known that layer-2/3 LTP evoked by the stimulation of layer 4 is less affected by age, in contrast to the age-dependent decline of layer 2/3 LTP evoked by the stimulation of white matter [23,32]. However, LTP and LTD decreased during postnatal development in the pathway from layer 4 to layer 2/3 in our previous studies [11,22], which might have been a result of the recruitment of inhibitory circuits using a short-duration (0.1 ms), high-intensity stimulation pulse [22]. In the present study, we used stimulation with a longer-duration (0.2 ms), low-intensity stimulation pulse to decrease the inhibitory components in FP, by which LTP in the pathway from layer 4 to layer 2/3 was induced in 5-week-old rats, consistent with our previous study [22]. In the present study, this LTP also declined to the baseline at 8 weeks of age (Fig. 1). The discrepancy might have resulted from the inclusion of large inhibitory components in our experiments, as shown in our previous study [22]. Because the proportion of inhibitory components in the FP was critical in the induction of LTP and LTD, late maturation of intracortical inhibitory circuits might inhibit the induction of LTP in this pathway in aged rats [24].
The induction of LTP and LTD in the visual cortex is modulated by various neuromodulators including acetylcholine (ACh), norepinephrine (NE) and 5-HT [10,33-36], which convey information on the behavioral state of the animal. These neuromodulators appear to act as enabling factors for long-term synaptic plasticity [36,37] and ocular dominance plasticity [5,38,39]. However, in contrast to the consistent effect of ACh and NE, 5-HT exhibits a dissimilar effect on the induction of LTP/LTD depending on the species (cats vs. rats) and the age of animals (young vs. adult). Furthermore, 5-HT facilitates the induction of either LTP or LTD in cat visual cortex depending on the expression of 5-HT2 receptors [4]. Whereas increasing the content of 5-HT in the visual cortex inhibits the induction of LTP and LTD during critical period in rats [11-13], 5-HT facilitates the induction of LTP and reinstates ocular dominance plasticity in adult rats [19,20]. In the present study, we found that acute treatment of 5-HT facilitated the induction of NMDA receptor-dependent LTP in slices from adult rats at the ages of 8 and 10 weeks, which contrasted with the 5-HT inhibition in the induction of LTP in young rats. The results of experiments with 5-HT-depleted slices and the increase in endogenous 5-HT by citalopram have consistently supported these results. This switching role of 5-HT between young and adult rats might be explained by the expression of 5-HT receptor changes with postnatal development in the neocortex [4,21]. Levels of expression and localization of 5-HT receptor subtypes are critical in the induction of LTP [4] and in modulation of cellular excitability [28,40]. Thus, cellular expression of 5-HT receptor subtypes in excitatory and inhibitory neurons during postnatal development remains to be studied.
In addition, 5-HT exerts a trophic function by increasing BDNF levels in the adult brain [41]. Chronic administration of the SSRI fluoxetine or local infusion of 5-HT restores susceptibility to monocular deprivation in adulthood and increases the expression of BDNF [19,20]. In the present study, we demonstrated that the acute administration of 5-HT also facilitated the induction of LTP. Whereas 5-HT1A receptors were involved in the chronic effect of 5-HT and increased expression of BDNF [20], the acute facilitating effect was mediated by the activation of 5-HT2 receptors in the present study. Phospholipase C is coupled to 5-HT2 receptors, which might increase the intracellular calcium level by the release from IP3-sensitive stores [40,42] and could exert additive effects on the activation of NMDA receptors for LTP induction. In contrast, in young rats, enhanced GABAAergic synaptic currents by 5-HT2 receptor activation in layer 2/3 pyramidal neurons might inhibit the induction of LTP [40]. Since balance between excitation and inhibition (E/I) is critical in the induction of long-term synaptic plasticity [22,43], serotonergic fine tuning of the E/I balance [25] might also play a critical role in the induction of LTP.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the financial support of the Catholic Medical Center Research Foundation, in the program year 2009.
ABBREVIATIONS
- LTP
long-term potentiation
- LTD
long-term depression
- 5-HT
5-hydroxytryptamine
- SSRI
selective serotonin reuptake inhibitor
- TBS
theta-burst stimulation
- NMDA
N-methyl-D-aspartate
- ACSF
artificial cerebrospinal fluid
- BDNF
brain-derived neurotrophic factor
- D-AP5
D-aminopentanoate
- FP
field potential
- GABA
gamma-aminobutyric acid
- PCA
para-chloroamphetamine
References
- 1.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 2.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Dori I, Dinopoulos A, Blue ME, Parnavelas JG. Regional differences in the ontogeny of the serotonergic projection to the cerebral cortex. Exp Neurol. 1996;138:1–14. doi: 10.1006/exnr.1996.0041. [DOI] [PubMed] [Google Scholar]
- 4.Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci USA. 2000;97:1841–1844. doi: 10.1073/pnas.97.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci USA. 2000;97:1951–1952. doi: 10.1073/pnas.070044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 7.Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, Hensch TK. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proc Natl Acad Sci USA. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 10.Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Jang HJ, Cho KH, Hahn SJ, Kim MJ, Yoon SH, Jo YH, Kim MS, Rhie DJ. Serotonin inhibits the induction of NMDA receptor-dependent long-term potentiation in the rat primary visual cortex. Brain Res. 2006;1103:49–55. doi: 10.1016/j.brainres.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 12.Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Effects of serotonin on the induction of long-term depression in the rat visual cortex. Korean J Physiol Pharmacol. 2010;14:337–343. doi: 10.4196/kjpp.2010.14.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001;21:1532–1537. doi: 10.1523/JNEUROSCI.21-05-01532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edagawa Y, Saito H, Abe K. The serotonin 5-HT2 receptor-phospholipase C system inhibits the induction of long-term potentiation in the rat visual cortex. Eur J Neurosci. 2000;12:1391–1396. doi: 10.1046/j.1460-9568.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 15.Edagawa Y, Saito H, Abe K. 5-HT1A receptor-mediated inhibition of long-term potentiation in rat visual cortex. Eur J Pharmacol. 1998;349:221–224. doi: 10.1016/s0014-2999(98)00286-6. [DOI] [PubMed] [Google Scholar]
- 16.Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994;643:10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 17.Pollandt S, Drephal C, Albrecht D. 8-OH-DPAT suppresses the induction of LTP in brain slices of the rat lateral amygdala. Neuroreport. 2003;14:895–897. doi: 10.1097/00001756-200305060-00025. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi S, Matsumoto M, Togashi H, Ueno K, Yoshioka M. The serotonergic modulation of synaptic plasticity in the rat hippocampo-medial prefrontal cortex pathway. Neurosci Lett. 2003;342:179–182. doi: 10.1016/s0304-3940(03)00293-3. [DOI] [PubMed] [Google Scholar]
- 19.Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castrén E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 20.Maya Vetencourt JF, Tiraboschi E, Spolidoro M, Castrén E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- 21.Li QH, Nakadate K, Tanaka-Nakadate S, Nakatsuka D, Cui Y, Watanabe Y. Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J Comp Neurol. 2004;469:128–140. doi: 10.1002/cne.11004. [DOI] [PubMed] [Google Scholar]
- 22.Jang HJ, Cho KH, Kim HS, Hahn SJ, Kim MS, Rhie DJ. Age-dependent decline in supragranular long-term synaptic plasticity by increased inhibition during the critical period in the rat primary visual cortex. J Neurophysiol. 2009;101:269–275. doi: 10.1152/jn.90900.2008. [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc Natl Acad Sci USA. 1997;94:3380–3383. doi: 10.1073/pnas.94.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. The development of phasic and tonic inhibition in the rat visual cortex. Korean J Physiol Pharmacol. 2010;14:399–405. doi: 10.4196/kjpp.2010.14.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau AW, Amar M, Le Roux N, Morel N, Fossier P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb Cortex. 2010;20:456–467. doi: 10.1093/cercor/bhp114. [DOI] [PubMed] [Google Scholar]
- 26.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABAA receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 30.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 31.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci. 1999;19:1599–1609. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 35.Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 36.Bröcher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- 37.Markram H, Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 1990;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Gu Q, Cynader MS. Blockade of serotonin-2C receptors by mesulergine reduces ocular dominance plasticity in kitten visual cortex. Exp Brain Res. 1997;114:321–328. doi: 10.1007/pl00005640. [DOI] [PubMed] [Google Scholar]
- 39.Gordon B, Mitchell B, Mohtadi K, Roth E, Tseng Y, Turk F. Lesions of nonvisual inputs affect plasticity, norepinephrine content, and acetylcholine content of visual cortex. J Neurophysiol. 1990;64:1851–1860. doi: 10.1152/jn.1990.64.6.1851. [DOI] [PubMed] [Google Scholar]
- 40.Jang HJ, Cho KH, Park SW, Kim MJ, Yoon SH, Rhie DJ. Layer-specific serotonergic facilitation of IPSC in layer 2/3 pyramidal neurons of the visual cortex. J Neurophysiol. 2012;107:407–416. doi: 10.1152/jn.00535.2011. [DOI] [PubMed] [Google Scholar]
- 41.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 2000;20:8365–8376. doi: 10.1523/JNEUROSCI.20-22-08365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005;50:126–133. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]