Abstract
Rationale
Individuals learn associations between alcohol’s sensory properties and intoxication, with such conditioned stimuli (CS) becoming involved in craving and relapse. However, these CS also carry idiosyncratic associations.
Objectives
Test brain responses to novel CS conditioned with alcohol intoxication.
Methods
Fourteen heavy drinkers (age= 24.9 +/− 3.2) performed a reaction time task with embedded novel geometric CS, and were told only that the task was to measure alcohol’s effect on speed. Rapid intravenous alcohol infusion (the unconditioned stimulus; UCS) began with the appearance of a CS+, using pharmacokinetic modeling to increment breath alcohol by ~18mg% in 200 sec per each of six CS-UCS pairings. Placebo-saline infusion with CS− used the same infusion parameters in same-day randomized/counterbalanced sessions. The next morning subjects, connected to inactive IV pumps, underwent fMRI of the same task with mixed brief presentations of CS+, CS−, and irrelevant CS and were told that alcohol could be infused at any time during imaging.
Results
CS− responses were significantly greater than those of CS+ in medial frontal cortex. Notably, CS+ responses were negative, suggesting reduced neural activity. Negative activity was most pronounced in early scans, extinguishing with time. As subjects were told that alcohol could be administered in fMRI, a CS+ without alcohol is similar to a negative prediction error, with associated reduced frontal activity during withheld reward.
Conclusions
Novel stimuli relatively free of demand characteristics can be classically conditioned to intermittent brain exposure of even low alcohol concentrations, permitting imaging studies of conditioned alcohol expectancies.
Introduction
Stimuli that become classically conditioned to alcohol by repeated pairing with drinking induce craving (Carter & Tiffany, 1999), shift implicit attention toward alcohol-related stimuli (Field & Duka, 2002), and promote relapse (Cooney et al., 1997; Litt et al., 2000; Cox et al., 2002). Studies have thus turned to determining the brain areas that most respond to alcohol’s sights, smell and tastes (e.g., Filbey et al., 2008b; Kareken et al., 2010a; Kareken et al., 2010b; Myrick et al., 2008; Tapert et al., 2003). Brain regions responding to such stimuli comprise reward system areas, including the ventral striatum and the medial prefrontal regions projecting to it (Chiba et al., 2001; Haber et al., 2006; Williams & Goldman-Rakic, 1998).
Such “naturally conditioned” stimuli have particular face validity, most so when the stimuli can be tailored to an individual’s habitual drinking. However, there are potential limitations to stimuli encountered in the routine course of drinking, as a naturally conditioned stimulus (CS) possesses many other associations besides intoxication— an aspect that will most certainly vary across subjects. Moreover, alcohol pictures are used extensively in advertising, resulting in frequent cue exposure in the absence of alcohol. Similarly, varied and idiosyncratic learning histories would occur between subjects, even with the taste or aroma of a preferred drink. This variation would be particularly prominent between heavier and lighter drinkers, and likely extends to the circumstances of intoxication. These additional associations can evoke behavioral changes related to alcohol, drinking history, and expectancies about alcohol’s influence on behavior (Freeman et al., 2010; Kramer & Goldman, 2003; Friedman et al., 2007). Demand characteristics that influence subjects to behave in ways they believe comply with the experimenter’s desires may also be a problem with cues of such overt significance. Finally, using a CS with a prior conditioning history precludes the ability to study early stages of reinforcement learning.
Some studies have conditioned novel cues to alcohol intoxication, producing conditioned responses such as heart rate (Staiger & White, 1988), gaze time on the CS (Field & Duka, 2002; McCaul et al., 1989), and skin conductance (Glautier et al., 1994; McCaul et al., 1989) with varying consistency. One source of variance in these studies is that alcohol’s rewarding properties could change as a function of differential alcohol pharmacokinetics across individuals (Glautier et al., 2000). This is particularly true since the timing of the unconditioned stimulus (UCS; alcohol), its rewarding properties, and even its arrival in the brain varies significantly from incontrollable absorption kinetics associated with oral ingestion. This results in uncertain delays between CS and UCS presentation, and hence varied UCS magnitude (Ramchandani et al., 2006).
In this study of heavy drinkers, our principal aim was to test a novel paradigm that classically conditions unfamiliar stimuli with alcohol intoxication. To accomplish this, we used physiologically based pharmacokinetic (PBPK) modeling (O’Connor et al., 1998; Ramchandani et al., 1999) to control UCS variability, and embedded into a reaction time (RT) task a novel graphical symbol (CS+) that briefly preceded and continued through a rapidly rising limb of intravenously (IV) infused ethanol. Differential conditioning was used to similarly pair a CS− with saline infusion during a separate session. The following day, we employed fMRI to determine if responses in the brain’s reward network differed according to CS type.
Methods
Subjects
Fifteen heavy drinking males participated, with one subject excluded for excessive motion in fMRI. None of the final 14 subjects (Table 1) had a history of treatment for alcohol use disorders, and none were seeking treatment. Three fulfilled formal DSM-IV criteria for alcohol dependence. While we did not exclude prior drug experimentation (13 of 14 subjects), a review of drug use history/frequency did not indicate current/prior dependence on illicit drugs. Three subjects tested positive for cannabinoids at a screening interview, two of whom continued to test positive on study days. None tested positive for stimulants, opioids, barbiturates, benzodiazepines, phencyclidine or tricyclic anti-depressants at either interview or during the remainder of the study. All had breath alcohol (BrAC) measurements of 0.00 on admission for study. Given the 1½ day procedure, the three participating smokers were allowed to smoke between experimental periods to avoid nicotine withdrawal.
Table 1.
Subject Characteristics
| Mean | SD | % (Num) | |
|---|---|---|---|
| Age | 24.9 | 3.2 | |
| Years Education | 14.2 | 1.6 | |
| Drinks/week* | 20.7 | 10.6 | |
| Drinks/Drinking day* | 6.2 | 2.0 | |
| Heavy Drinking Days/week*† | 2.1 | 1.1 | |
| Age First Drink | 15.1 | 2.2 | |
| Age Regular Drinking | 18.1 | 1.6 | |
| AUDIT | 13.9 | 6.3 | |
| Male | 100 (14) | ||
| Caucasian | 57 (8) | ||
| Smokers | 21 (3) | ||
| Family History of Alcoholism | 36 (5) | ||
Notes: n = 14.
Estimated from the Timeline Followback Interview (Sobell et al., 1986).
> 4 drinks per occasion. AUDIT = Alcohol Use Disorder Identification Test (Saunders et al., 1993).
Procedures
Subjects were invited to participate in a study of alcohol’s effect on RT, and told that they would undergo two sessions of RT testing, once during IV alcohol infusion, once during IV saline infusion (single blind infusion conditions counterbalanced across morning and afternoon sessions). After spending the night in the Indiana University General Clinical Research Center, subjects had fMRI sessions the following morning.
RT probe task
During an RT “probe task” (e.g., see Field et al., 2004), subjects saw the CS and an adjacent asterisk (randomized to the left and right visual fields), both of which disappeared to reveal a green arrow (response probe) that required a timed response (see Figure 1). Briefly, the task allowed for the investigation of any possible implicit visual attention to CS stimuli (the green arrow probe) in the lateral visual fields. Nineteen CS presentations occurred per each of six conditioning runs (see below). Subjects were instructed to watch the stimulus array (CS and asterisk) attentively, and when the masking stimuli disappeared, to find and determine the direction of the green probe arrow by pressing an ‘up’ or ‘down’ arrow as quickly as possible. Although the symbols were systematically paired with a given infusate (CS+ with alcohol, CS− with saline), subjects were never made explicitly aware of this pairing, and never told that the symbols served any function other than to hide the response probe. The side of the CS+ and CS−, the side of the response probe, and whether the probe was hidden by a CS or asterisk were all counterbalanced and matched for frequency. An irrelevant CS (CSi) was employed for task practice, with the symbols used for the CS+, CS−, and CSi randomly assigned across subjects. To assess implicit attention drawn to the CS+, median RT was analyzed as a function of CS type and the side of the response probe relative to the CS.
Figure 1. fMRI Choice Reaction Time Paradigm.
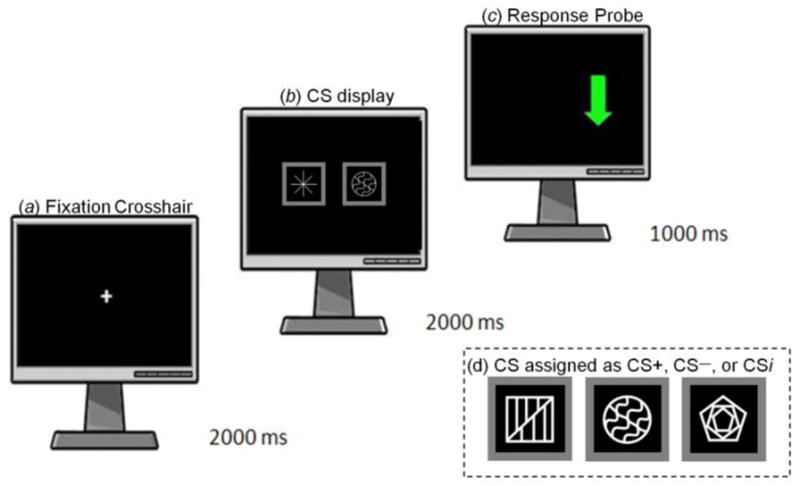
(a) Null crosshair fixation preceded a trial onset of (b) two stimuli: An asterisk adjacent to a CS+ or CS− (side randomized/counterbalanced). The stimuli then disappeared to reveal the response probe (c; ↓ or ↑), which was counterbalanced by side, when subjects pressed an ‘up’ or ‘down’ button as quickly as possible. Intertrial intervals were jittered slightly in time. (d) Three stimuli randomly assigned to the CS types. CSi were used only in task practice, and not during conditioning.
IV Infusion paradigm (Figure 2)
Figure 2.
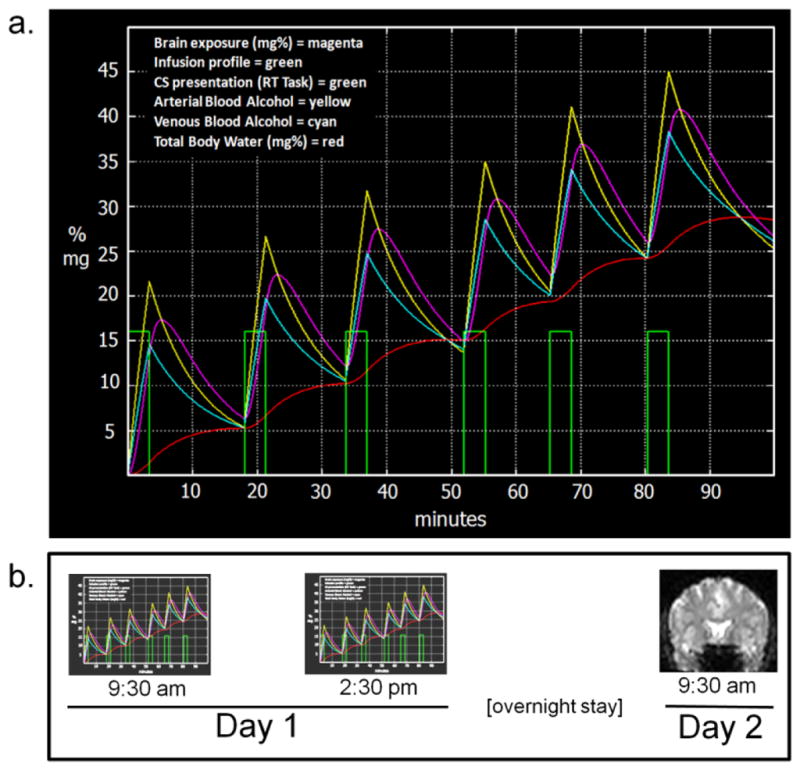
a. PBPK modeling of brain, arterial, venous, and body water alcohol levels during the single alcohol conditioning session. Conditioning sessions included 6 infusions (duration 200 sec each) followed by a 15-minute resting period over the course of 90 minutes. The RT task (and embedded CS) started coincidentally with the onset of six runs of the infusion pumps, and continued for the same duration as the infusion pumps. This resulted in a CS onset just prior to ethanol’s rising limb in the brain (magenta curve, simulated by PBPK modeling) and short delay conditioning procedure. The saline session ran using the same timing, pump rates, and task, with only the CS changed (saline infusions were predicted by the CS− instead of the CS+). To avoid frequent intrusion from BrAC measurements (which could not occur in imaging given the magnetic field), BrAC was obtained only once, immediately after completing the final (6th) ascending limb. b. Approximate experimental timeline. Morning and afternoon infusion sessions were randomized/counterbalanced between alcohol and placebo.
Alcohol and saline infusion employed PBPK modeling (O’Connor et al., 1998; Ramchandani et al., 1999), using transformations of height, weight, and gender to calculate a scalloped infusion profile comprising six rapidly ascending limbs, each incrementing BrAC by approximately 18 mg% over 200 sec, and each followed by a sharp drop (driven primarily by the distribution of infused alcohol into body water). The intent was six unconditioned stimulus presentations throughout the 90-min infusion session. The rate of ascent was intended to be as rapid as possible, resulting in a large “peak-to-trough” contrast in BrAC, and a final BrAC (45 mg%) that allowed a decline to 0 mg% by afternoon in the case of morning alcohol (Figure 2 for detail and model estimates of alcohol concentration in total body water, venous and arterial blood, and brain). Infusions were conducted with the subject alone in a sound-proof booth while seated in a recliner; all communication with the subject used a microphone and speaker. Jittered 15 minute resting periods occurred between pairings, during which the subject could read. Subjects were alerted two minutes in advance of the beginning of a new infusion trial/task. Breath alcohol measurements were obtained using a Dräger Alcotest® 7410 breath alcohol meter.
Subjective responses
At the peak of each of the six ascending limbs, subjects rated subjective reactions, with items displayed on the same monitor used for the RT task. Subjects used the same response pad as for the RT task to position a cursor on a continuous line (range 0 – 100 units, without the unit numbers displayed) to rate “How intoxicated do I feel right now?”, “How anxious do I feel right now?”, “How tired do I feel right now?”, “How relaxed do I feel right now?”, and “How much am I enjoying the effects of alcohol right now?” Subjects also rated the number of drinks they had felt as if they were experiencing, (“Right now, I feel as if I’ve had ____ drinks.”), with responses incremented in 0.5 drinks. During imaging, these questions were again displayed, with subjects responding similarly on the MRI-compatible fiber-optic response box (Current Designs, Philadelphia, PA) used for the probe task during imaging.
fMRI of the probe task
Subjects performed the same probe task during fMRI as employed during conditioning, except that the task was performed as a mixed-event paradigm of 6:19 min in which all three CS were presented (16 events per each of the CS types, jittered in time with delays between 3000 and 13000 ms using OptSeq v. 2.0; http://surfer.nmr.mgh.harvard.edu/optseq/). Stimuli were back-projected onto a screen at the rear of the scanner bore, which subjects viewed with the head coil mirror. Intertrial interval parameters remained the same as during conditioning. To keep the same demand characteristics as during conditioning, subjects retained their IV cannulae for imaging and were told that they could again receive either alcohol or saline. However, no infusion was performed during the imaging, with the IV pumps remaining off.
Four blood oxygenation level dependent (BOLD) scans were conducted on a Siemens 3T Magnetom Trio-Tim scanner equipped with a 12-channel head coil array using a BOLD echo planar imaging pulse sequence (193 image volumes, gradient echo, repetition time 2000 ms, echo time 29 ms, flip angle 76°, 35 interleaved axial slices with 3 mm thickness and no gap, acquisition matrix 88×88, 2.5×2.5×3.0 mm3 voxels, GRAPPA acceleration factor 2) with a 3D prospective acquisition correction algorithm adjusting for real time head movement (Thesen et al., 2000). A T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence was utilized to acquire whole-brain high resolution anatomical images (1.0×1.0×1.2 mm3 voxels) for co-registration and normalization to the Montreal Neurological Institute (MNI) coordinate system.
Data analysis
Image analysis used SPM5 (Wellcome Department of Imaging Neuroscience, University College, London, UK). Functional volumes were corrected for slice acquisition timing and rigid-body realigned to account for residual movement after prospective motion correction. Each subject’s anatomic MPRAGE was co-registered to the reference functional volume, segmented into tissue classes, with nonlinear spatial transformation parameters from this segmentation used to transform functional volumes into MNI coordinate space. Normalized functional images were interpolated to cubic (2 mm per side) voxels and smoothed by a 6 mm full-width at half-maximum isotropic Gaussian kernel.
Within-subject, fixed-effects of the BOLD response to CS+, CS−, and CSi were estimated using SPM’s canonical hemodynamic response function. The response probe events (pooled over all CS) were also modeled to account for the effects of their appearance, resulting in four conditions per task-run. In within-subject SPM models, event onsets were specified to coincide with the appearance of each CS and response probe. Six movement parameters from realignment were included as regressors to account for residual motion. A high-pass filter with a cut-off of 1/128 Hz was applied to each voxel’s time series to remove low-frequency noise, while an autoregressive AR(1) model accounted for serial correlations. Given our primary hypotheses and the nature of the conditioning, second-stage random effects analyses focused only on CS+ and CS− in a 2(stimulus presentation) × 4(scan) SPM5 factorial model, where both stimulus and scan were allowed to correlate. To account for possible extinction from non-reinforcement (no alcohol) during imaging, BOLD responses were examined as averages across all four functional scans, and from individual scans. A corrected (family wise error; FWE) cluster statistic at height threshold p< 0.001 (uncorrected) tested for significance, focusing on the insula, striatum, and medial and orbital frontal (striatal projection) cortex (reward regions). An exploratory threshold of p< 0.001 (uncorrected; extent threshold k > 10 voxels) was also used. Where appropriate, BOLD effects were extracted from functional regions of interest using MarsBar (Brett et al., 2002).
Behavioral data (subjective responses to alcohol, RT to the response probe) as well as extracted mean BOLD contrast values in functional regions of interest were analyzed in SPSS (Release 18, IBM Corp, Somers, NY).
Results
Pavlovian Conditioning
Ethanol infusion, subjective perceptions
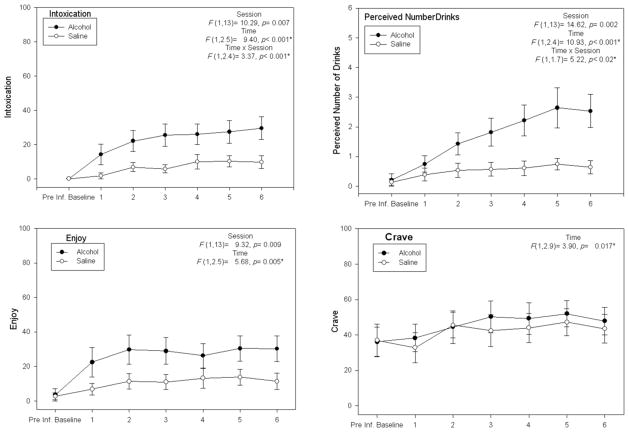
Average BrAC across all subjects at the end of ethanol conditioning was 45.1 mg% (SD= 7.0): nearly the exact intended target. Subjective effects were analyzed in Session (2; alcohol v. saline) × Time Point (6) general linear models (Figure 3), which showed significant Session main effects for “Intoxicated,” “Number of Drinks,” and “Enjoy,” as well as Session × Time interactions for “Intoxicated” and “Number of drinks.” A Time main effect existed for “Craving,” but without a main effect for session or an interaction. No other subjective effects showed main effects or interactions. Thus, subjects appeared to subjectively differentiate between the effects of the two infusates during conditioning outside the scanner.
Figure 3. Subjective responses.
Plots for subjective responses in which there were significant main effects or interactions. Ratings obtained at peak estimated ethanol concentration following each of the six conditioning runs (see Figure 2). *p-value adjusted with Greenhouse-Geisser correction for inhomogeneity of variance.
Motor performance
Analyzed in a mixed effects linear model, task accuracy in judging response probe direction was high across both alcohol and saline sessions (grand mean= 93% accuracy across all 6 runs, SD = 1.9%), and did not differ according to infusion. RT (grand mean= 572 ms, SD= 129) was unaffected by infusion (ps > 0.3). Accuracy and RT were similarly analyzed only at the last trial (6), at peak ethanol concentration, and also showed no effects for accuracy (grand mean= 18.6, SD= 0.6) or RT (grand mean= 567 ms, SD= 140) (ps > 0.5).
Neuroimaging
Subjective perceptions
Only two of 14 subjects reported any intoxication during imaging. The first reported intoxication after the third (rating= 20/100) and fourth (rating= 41/100) functional scan, when this subject reported feeling 0.5 and 1.0 drinks, respectively. The second subject reported feeling 0.5 drinks only after the second scan, when he rated intoxication as only 1/100. This subject’s ratings then returned to baseline. The 12 remaining subjects reported no intoxication and no sense of having had alcohol during imaging. General linear models showed no other effects on the remaining ratings across the four measured time points (ps > 0.35), with the exception “Relaxed” (F[3,39]= 3.89, p=0.02) where ratings prior to the last functional scan (mean 26.0; SD=20.4) were significantly lower than before the first functional scan (mean 40.8; SD=22.6).
Reaction time to CS
Accuracy in determining response probe orientation was high across all scans (median = 98%, range 92% – 100%). RT to decide probe direction (each subject’s median RT of correct responses only to avoid outliers) was analyzed as a function of CS (CS+, CS−) and side of probe presentation (same as or opposite the CS). If implicit attention is drawn to the CS+, RT should be slower in judging probe direction when the probe appears opposite the CS+ (i.e., a CS × Side interaction).
RT grand mean was 483 ms (SD= 89). A CS(2) × Side(2) mixed linear model, showed no significant main effects of CS or Side, and the CS × Side interaction was insignificant (ps > 0.4). Analyzed for the first scan only, there was a marginal main effect of Side (p= 0.053), wherein RT was slightly faster when the probe appeared on the side of any CS (468 ms, SD= 81) compared to the probe’s appearance opposite the CS (484 ms, SD= 89). There was, however, no CS × Side interaction (p > 0.6).
Brain responses
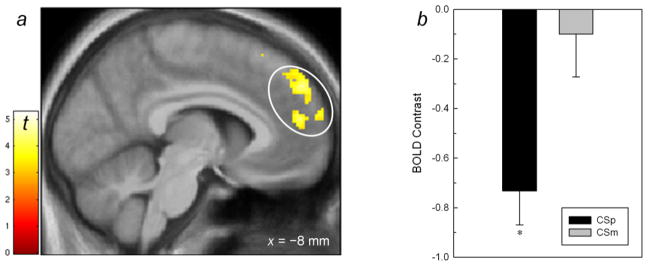
Relative to CS−, CS+ produced no significant BOLD activation ([CS+ > CS−] contrast). However, the reverse comparison [CS− > CS+] showed a significantly large 496 voxel cluster (p < 0.001, corrected cluster statistic) where the peak voxel was significant after correcting for the whole brain volume (p < 0.05 FWE corrected; Figure 4a, Table 2). Analyzing extracted mean BOLD activity from this significantly large cluster in a mixed linear model, there was no significant BOLD response to the CS− itself (p > 0.45), either with reference to the CS+ or the implicit baseline. Rather, for the CS+ as contrasted against the implicit baseline, there was a significant negative response (p < 0.001; Figure 4b).
Figure 4.
a. BOLD response from the [CS− > CS+] comparison showing a single, confluent 496 voxel cluster (white oval, p< 0.05, FWE corrected cluster statistic) in the medial superior frontal gyrus and anterior cingulate. Display threshold, p< 0.001, k>10 voxels. b. Plot of extracted activity from the delineated cluster in (a). *Significantly less than the implicit baseline (zero), p< 0.001.
Table 2.
Activation Foci
| Region | ||||
|---|---|---|---|---|
| Z | k | p-value | MNI Coordinates (x, y, z) | |
| L-Medial Prefrontal (superior frontal gyrus) | 4.96 | 496* | < 0.001† | −8, 54, 36 |
| L-Medial Prefrontal (superior frontal gyrus) | 4.68 | < 0.001 | −10, 46, 36 | |
| L-Anterior Cingulate/Medial Prefrontal | 4.12 | < 0.001 | −6, 52, 16 | |
| L-Superior Frontal Gyrus | 4.93 | 51 | < 0.001 | −12, 20, 60 |
| L-Superior Frontal Gyrus | 3.38 | < 0.001 | −18, 24, 56 | |
| R-Middle Frontal Gyrus (deep) | 4.28 | 52 | < 0.001 | 22, 52, 12 |
| R-Paracentral Lobule | 4.06 | 105* | < 0.001 | 4, −34, 64 |
| R-Paracentral Lobule | 3.79 | < 0.001 | 4, −26, 62 | |
| R-Paracentral Lobule | 3.12 | 0.001 | −2, −24, 56 | |
| L-Middle Frontal Gyrus (deep) | 3.79 | 12 | < 0.001 | −28, 22, 34 |
| R-Cingulate | 3.78 | 11 | < 0.001 | 8, 26, 18 |
| L-Orbitofrontal (medial) | 3.77 | 15 | < 0.001 | −12, 50, −14 |
| L-Dorsolateral Prefrontal (medial frontal gyrus) | 3.76 | 12 | < 0.001 | −44, 26, 40 |
| L-Inferior Frontal Gryus/Lateral Orbitofrontal | 3.75 | 30 | < 0.001 | −36, 48, −6 |
| L-Inferior Frontal Gyrus | 3.75 | 23 | < 0.001 | −48, 32, −4 |
| R-Polar Medial Prefrontal | 3.75 | 14 | < 0.001 | 10, 64, 10 |
| R-Superior Temporal Gyrus (posterior) | 3.74 | 13 | < 0.001 | 60, −36, 6 |
| L-Lateral Orbitofrontal | 3.69 | 33 | < 0.001 | −36, 32, −12 |
| L-Lateral Orbitofrontal | 3.13 | 0.001 | −44, 34, −18 | |
| R-Cingulate/Medial Prefrontal | 3.56 | 14 | < 0.001 | 4, 48, 22 |
| L-Superior Frontal Gyrus | 3.52 | 14 | < 0.001 | −10, 32, 58 |
| L-Superior Frontal Gyrus/Medial Prefrontal | 3.47 | 11 | < 0.001 | −2, 38, 50 |
Notes. MNI= Montreal Neurological Institute;
p< 0.05 cluster statistic corrected for whole-brain family-wise error;
p < 0.05 peak voxel, corrected for whole-brain family-wise error. L= Left. R=Right.
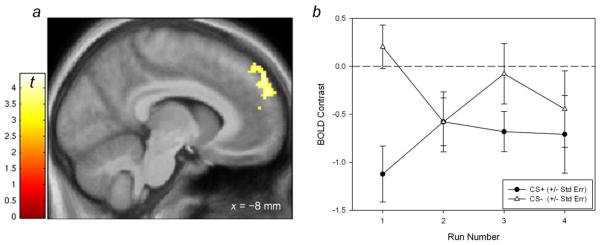
Extinction might be expected given the lack of alcohol reinforcement, with a diminishing response to the CS+ over time. To test this, the [CS− > CS+] comparison was used to define the region of response at the first scan only, and then analyzed this area as a function of scan number. At scan 1, the [CS− > CS+] contrast again resulted in a significantly large (364 voxel, FWE-corrected cluster statistic p < 0.001) cluster of activation in the superior frontal gyrus (Figure 5a; compared to Figure 4a). Activity in this cluster was extracted with MarsBar and analyzed in a CS(2) × Scan(4) mixed linear model, which showed a significant main effect for CS (p= 0.013), as well as a significant CS × Scan interaction (p = 0.003) that reflected a changing CS effect over time (Figure 5b). Consistent with what should be an exponential course of extinction (Rescorla & Wagner, 1972), the CS+ BOLD response was significantly less than that for CS− during scan 1 (p < 0.001). The two CS types were insignificantly different during scans 2 and 4 (ps > 0.3), although they were significantly different at scan 3 (p = 0.03).
Figure 5.
a. BOLD contrast from the [CS− > CS+] comparison as defined by run one only, showing a significantly large (364 voxel cluster; p< 0.05, FWE corrected cluster statistic) in the left superior frontal gyrus. Display threshold, p< 0.001, k>10 voxels. b. Plot of extracted activity from run 1 in panel (a), with progression of habituation.
Within our reward-related anatomic areas of interest, smaller effects (p < 0.001, k > 10 voxels) were present in lateral and medial orbitofrontal cortex (Table 2). Of these three areas, only the left lateral response at [−36, 32, −12] showed a clear directional response from one of the stimulus types. As with the significant medial frontal cluster, the BOLD response to the CS+ was significantly below zero (p = 0.002), while the response to the CS− was not (p > 0.5). Unlike the medial frontal cluster, this lateral orbital cluster did not vary across time.
Discussion
This study examined the brain’s response to stimuli experimentally paired with a rapid infusion of intravenous alcohol and placebo saline. As reflected by subjective ratings, subjects distinguished between the infusates. In fMRI the morning following this Pavlovian conditioning (where subjects neither perceived nor received alcohol), there emerged a significant medial frontal BOLD response to CS+ associated with alcohol infusion in medial prefrontal regions we reported to be activated by alcoholic drink odors in heavy drinkers (Kareken et al., 2010a). In the current sample, however, the BOLD responses to the CS+ were significantly negative (below implicit baseline), whereas the CS− response remained at the implicit baseline. Negative BOLD responses are accompanied by declines in both regional cerebral blood flow and the cerebral metabolic rate of oxygen, implicating reduced neuronal activity (Shmuel et al., 2002).
Medial prefrontal cortex— a region to which the ventral striatum projects (Haber et al., 2006)— responds positively to “natural” CSs, such as the sight, smell, or tastes of alcoholic drinks (e.g. Bragulat et al., 2008; Filbey et al., 2008a; Kareken et al., 2010a; Kareken et al., 2010b; Myrick et al., 2008), or to monetary rewards (Schott et al., 2008; McClure et al., 2007). Importantly, medial frontal activity reflects the subjective value of food (Hare et al., 2008; Hare et al., 2009) and money (Kable & Glimcher, 2007). If such studies show that medial prefrontal cortex activity rises in response to stimuli associated with desired rewards, why would responses to our experimental CS+be significantly negative?
The answer likely lies in the nature of conditioning and reward availability during fMRI. Most brain imaging studies of drug cues do not pair reward (drug) receipt with cue exposure in any contingent way or even make the drug available during imaging. More particularly, we are unaware of imaging studies in which a displayed alcohol cue implies the arrival of alcohol, and subjects in previously published studies would have no expectation (implicit or explicit) of this occurring. In our design, subjects were repeatedly exposed to novel stimuli just prior to a rapidly rising concentration of alcohol in the brain. During imaging, subjects remained connected to the IV infusion pumps when viewing the CS, but without alcohol infusion. This can be viewed as analogous to a “negative prediction error,” wherein a reward predicted by a CS+ fails to arrive. Moreover, differences between the CS+ and CS− were strongest during the first BOLD scan, with a general attenuation over time, as might be expected with persistent non-reinforcement of the CS+. Thus extinction followed a roughly asymptotic course, albeit with some variability (stimulus differences emerging at the third scan, and again becoming insignificant by the fourth).
As initially demonstrated by Schultz and colleagues (Mirenowicz & Schultz, 1996; Schultz et al., 1997), negative prediction errors result in a phasic decrease of midbrain dopamine cell activity, and in a graded manner that reflects the probability of reward delivery (Fiorillo et al., 2003). Using fMRI, a similar response to prediction errors has been demonstrated in humans, where BOLD responses in the ventral striatum (a target of the dopaminergic midbrain) become negative with the absence of expected reward, such as juice or money (e.g., Abler et al., 2006; Bray & O’Doherty, 2007; Knutson et al., 2001; McClure et al., 2003; O’Doherty et al., 2003). Some studies, however, also find increased BOLD responses to omitted reward or negative feedback (e.g. Pagnoni et al., 2002; Rodriguez et al., 2006). While striatal responses to reward prediction have received the most attention, decreased BOLD responses to cued yet withheld reward have been observed in medial and orbital frontal areas (O’Doherty et al., 2003; Ramnani et al., 2004; Knutson et al., 2001; Knutson et al., 2003; Kim et al., 2006), which might be expected given the connectivity between medial- and orbito-frontal areas and the striatum. We did observe a smaller negative BOLD response to the CS+ in left lateral orbital cortex, although this area did not appear to extinguish with time.
Lack of striatal responses may seem surprising since their role in prediction errors is well documented. However, the design of this experiment could preclude observing this phenomenon, at least efficiently. Since alcohol’s slow kinetics do not lend themselves to a binary, on-off administration (as with other rewards such as juice or money that are often used in such studies), temporally precise striatal responses may be harder to detect, especially since midbrain responses are sensitive to temporal delays between a CS and the reward it predicts (Hollerman & Schultz, 1998). And it is in this sense that we cannot precisely capture the “temporal difference” aspect of prediction errors, in which a discrete reward outcome is assessed at a particular time. While we can better calculate and control alcohol’s time course with PBPK modeling than with oral ingestion, the precise “time of reward” is not discrete (as with the juice or money delivery in many such experiments), but is more distributed over a longer time. Thus, our paradigm is not a direct translation of most prediction error experiments that measure temporal difference parameters, and responses to our stimuli occur averaged over a protracted period of non-reward. On the other hand, we have previously used positron emission tomography— an imaging technique with considerably less temporal resolution than fMRI— to find depressed ventral striatal dopaminergic transmission when a cued alcohol infusion failed to occur (Yoder et al., 2009)— a finding in accordance with prediction error theory. Therefore, rather than relying on a primary sensory reward (e.g. juice) or a secondary reinforcer (money), our paradigm may more validly reflect brain responses to cues predictive of a longer-acting pharmacological reward.
Failure to obtain a predicted reward may play an important role in addiction. Before a given stimulus has acquired associative properties linked to the drug experience, the unexpected arrival of a rewarding sensation would be “better than predicted;” by contrast, absence of predicted reward would be “worse than predicted,” and a reflection of disappointment (Redish et al., 2007). Thus, we speculate that the depression in prefrontal activity observed here is a manifestation of that disappointment, and one potentially related to the drive to seek alcohol. One example of this might be tolerance, when a given “dose” of alcohol fails to achieve the previously experienced degree of reward (see Lapish et al., 2006). Similarly, if alcohol is more valued by heavy drinkers, the medial prefrontal depression to an unreinforced CS+ might be a measurable neural reflection of that subjective value and disappointment (Hare et al., 2009; Kim et al., 2006).
The artificial stimuli used here, as well as the non-oral route of rapid alcohol administration might limit the study’s relevance to the more common pairing of visual and oral compound cues with ethanol, as it would normally be ingested. This may be a valid criticism, and we are unaware of experiments conducted where neural responses are measured during presentation of conditioned stimuli that have been paired with orally administered ethanol (certainly MRI and positron emission tomography are inhospitable settings for such a design). Nonetheless, the procedure in this study has several distinct advantages over those with more naturalistic stimuli, where conditioning is implied rather than actually conducted. First, the neural responses here should be free of semantic priming. For example, many studies show that alcohol cues (absent alcohol’s actual presence), activate semantic networks, with marked effects on behavior and how other semantic stimuli are interpreted (Freeman et al., 2010; Friedman et al., 2007; Goldman, 2002). While such priming is important and thought to play a causal role in alcoholism (Roehrich & Goldman, 1995; Stacy, 1997), it may or may not have anything to do with the implicit learning implicated in classical conditioning, and more explicitly measured here. Thus, previous research using naturalistic stimuli may be assessing classical conditioning, semantic learning/activation, or (more likely) some combination of the two. This may also be part of the reason that the neural responses in the present study differ in sign from those of previous studies using such stimuli. In fact, our novel stimuli, after conditioning, did not differentially affect RT as a function of CS and probe side, as has been observed in studies employing alcohol photographs (e.g., see Field et al., 2004; Townshend & Duka, 2001). It may be that stimuli with greater, more extensive, and complex conditioning histories are required for such effects.
A particular constraint merits comment: Our study design easily lends itself to examining extinction and negative prediction errors. However, even if intravenously infused, the time course of alcohol, may make it difficult to examine positive prediction errors (unexpected reward), or even correctly signaled reward. That is, discrete delivery of alcohol in an on-off, binary fashion cannot be achieved in a realistic time scale that enables adequate statistical power in fMRI. Finally, the sole evidence of conditioning is that of the BOLD response itself, and the measurement of a behavioral conditioned response that mirrors the time course of the BOLD contrast could add confidence to the results.
In summary, the brain response to a CS embedded in a reaction time test differed in these heavy drinkers according to the experimentally induced CS association. CS+ that predicted the rapid rising limb of alcohol concentration induced a highly significant depression in BOLD response compared to CS− stimuli predictive of the same rate of delivery of saline placebo. This suggests that medial prefrontal cortex may play a role in reflecting the absence of predicted rewarding outcomes. The data also suggest that brain responses can be measured to novel stimuli that are experimentally paired with alcohol intoxication—a method that could be useful in the study of alcoholism and its risk factors.
Acknowledgments
Supported by R21 AA018020 (DAK), R01 AA017661 (DAK), the Indiana Alcohol Research Center P60 AA007611, T32 AA007462, and the Indiana University Clinical Research Center (RR 025761). We gratefully acknowledge Kyle Windisch (Department of Psychiatry) for paradigm programming, and Michele Beal and Courtney Robbins for assistance with imaging (Department of Radiology).
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuro Image. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA. Alcohol Sensitizes Cerebral Responses to the Odors of Alcoholic Drinks: An fMRI Study. Alcoholism: Clinical and Experimental Research. 2008;32:1124–1134. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S, O’Doherty J. Neural Coding of Reward-Prediction Error Signals During Classical Conditioning With Attractive Faces. Journal of Neurophysiology. 2007;97:3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [Abstract] Neuro Image. 2002;16:2. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Research. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. Journal of Abnormal Psychology. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug and Alcohol Dependence. 2002;68:237–243. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Zetteler J, Bradley BP. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology. 2004;176:88–93. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Cues paired with a low dose of alcohol acquire conditioned incentive properties in social drinkers. Psychopharmacology. 2002;159:325–334. doi: 10.1007/s00213-001-0923-z. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcoholism: Clinical and Experimental Research. 2008a;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the Taste of Alcohol Elicits Activation of the Mesocorticolimbic Neurocircuitry. Neuropsychopharmacology. 2008b;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete Coding of Reward Probability and Uncertainty by Dopamine Neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Freeman N, Friedman RS, Bartholow BD, Wulfert E. Effects of alcohol priming on social disinhibition. Experimental and Clinical Psychopharmacology. 2010;18:135–144. doi: 10.1037/a0018871. [DOI] [PubMed] [Google Scholar]
- Friedman RS, McCarthy DM, Bartholow BD, Hicks JA. Interactive effects of alcohol outcome expectancies and alcohol cues on nonconsumptive behavior. Experimental and Clinical Psychopharmacology. 2007;15:102–114. doi: 10.1037/1064-1297.15.1.102. [DOI] [PubMed] [Google Scholar]
- Glautier S, Bankart J, Williams A. Flavour conditioning and alcohol: a multilevel model of individual differences. Biological Psychology. 2000;52:17–36. doi: 10.1016/s0301-0511(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Glautier S, Drummond C, Remington B. Alcohol as an unconditioned stimulus in human classical conditioning. Psychopharmacology. 1994;116:360–368. doi: 10.1007/BF02245341. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Expectancy and Risk for Alcoholism: The Unfortunate Exploitation of a Fundamental Characteristic of Neurobehavioral Adaptation. Alcoholism: Clinical and Experimental Research. 2002;26:737–746. [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-Related Cortical Inputs Define a Large Striatal Region in Primates That Interface with Associative Cortical Connections, Providing a Substrate for Incentive-Based Learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the Role of the Orbitofrontal Cortex and the Striatum in the Computation of Goal Values and Prediction Errors. Journal of Neuroscience. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuro Image. 2010a;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Liang T, Wetherill L, Dzemidzic M, Bragulat V, Cox C, Talavage T, O’Connor SJ, Foroud T. A Polymorphism in GABRA2 Is Associated With the Medial Frontal Response to Alcohol Cues in an fMRI Study. Alcoholism: Clinical and Experimental Research. 2010b;34:2169–2178. doi: 10.1111/j.1530-0277.2010.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Is Avoiding an Aversive Outcome Rewarding? Neural Substrates of Avoidance Learning in the Human Brain. PLoS Biol. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuro Report. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuro Image. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kramer DA, Goldman MS. Using a modified Stroop task to implicitly discern the cognitive organization of alcohol expectancies. Journal of Abnormal Psychology. 2003;112:171–175. [PubMed] [Google Scholar]
- Lapish CC, Seamans JK, Judson CL. Glutamate-Dopamine Cotransmission and Reward Processing in Addiction. Alcoholism: Clinical and Experimental Research. 2006;30:1451–1465. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Stitzer ML. Conditioned Opponent Responses: Effects of Placebo Challenge in Alcoholic Subjects. Alcoholism: Clinical and Experimental Research. 1989;13:631–635. doi: 10.1111/j.1530-0277.1989.tb00395.x. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal Prediction Errors in a Passive Learning Task Activate Human Striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time Discounting for Primary Rewards. Journal of Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of Naltrexone and Ondansetron on Alcohol Cue-Induced Activation of the Ventral Striatum in Alcohol-Dependent People. Archives of General Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcoholism: Clinical and Experimental Research. 1998;22:202–210. [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal Difference Models and Reward-Related Learning in the Human Brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5:97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism: Clinical and Experimental Research. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Neumark Y, Zimmermann US, Morzorati SL, Wit H. The Alcohol Clamp: Applications, Challenges, and New Directions - An RSA 2004 Symposium Summary. Alcoholism: Clinical and Experimental Research. 2006;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuro Image. 2004;23:777–786. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A, Kurth-Nelson Z. Reconciling reinforcement learning models with behavioral extinction and renewal: Implications for addiction, relapse, and problem gambling. Psychological Review. 2007;114:784–805. doi: 10.1037/0033-295X.114.3.784. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Rodriguez PF, Aron AR, Poldrack RA. Ventral striatal/nucleus accumbens sensitivity to prediction errors during classification learning. Human Brain Mapping. 2006;27:306–313. doi: 10.1002/hbm.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrich L, Goldman MS. Implicit priming of alcohol expectancy memory processes and subsequent drinking behavior. Experimental and Clinical Psychopharmacology. 1995;3:402–410. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic Functional Magnetic Resonance Imaging Activations during Reward Anticipation Correlate with Reward-Related Ventral Striatal Dopamine Release. Journal of Neuroscience. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele P-F, Adriany G, Hu X, Ugurbil K. Sustained Negative BOLD, Blood Flow and Oxygen Consumption Response and Its Coupling to the Positive Response in the Human Brain. Neuron. 2002;36:1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stacy AW. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. Journal of Abnormal Psychology. 1997;106:61–73. doi: 10.1037//0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- Staiger PK, White JM. Conditioned alcohol-like and alcohol-opposite responses in humans. Psychopharmacology. 1988;95:87–91. doi: 10.1007/BF00212773. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective Acquisition Correction for Head Motion With Image-Based Tracking for Real-Time fMRI. Magnetic Resonance in Medicine. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology. 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, Kareken DA. When What You See Isn’t What You Get: Alcohol Cues, Alcohol Administration, Prediction Error, and Human Striatal Dopamine. Alcoholism: Clinical and Experimental Research. 2009;33:139–149. doi: 10.1111/j.1530-0277.2008.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]