Abstract
Maintaining the physiologic integrity of paralyzed limbs may be critical for those with spinal cord injury (SCI) to be viable candidates for a future cure. No long-term intervention has been tested to attempt to prevent the severe musculoskeletal deterioration that occurs after SCI. The purposes of this study were to determine whether a long-term neuromuscular electrical stimulation training program can preserve the physiological properties of the plantar flexor muscles (peak torque, fatigue index, torque-time integral, and contractile speed) as well as influence distal tibia trabecular bone mineral density (BMD). Subjects began unilateral plantar flexion electrical stimulation training within 6 wk after SCI while the untrained leg served as a control. Mean compliance for the 2-yr training program was 83%. Mean estimated compressive loads delivered to the tibia were ~1–1.5 times body weight. The training protocol yielded significant trained versus untrained limb differences for torque (+24%), torque-time integral (+27%), fatigue index (+50%), torque rise time (+45%), and between-twitch fusion (+15%). These between-limb differences were even greater when measured at the end of a repetitive stimulation protocol (125 contractions). Peripheral quantitative computed tomography revealed 31% higher distal tibia trabecular BMD in trained limbs than in untrained limbs. The intervention used in this study was sufficient to limit many of the deleterious muscular and skeletal adaptations that normally occur after SCI. Importantly, this method of load delivery was feasible and may serve as the basis for an intervention to preserve the musculoskeletal properties of individuals with SCI.
INTRODUCTION
Chronic complete spinal cord injury (SCI) induces secondary musculoskeletal deterioration that ultimately can be life threatening (Shields and Dudley-Javoroski 2003). Without normal use, paralyzed muscle rapidly atrophies, creating a catabolic state, poor cosmesis, and increased risk for secondary complications (Shields and Cook 1992). Physiologically, paralyzed muscle gradually converts to a fast-fatigable phenotype (Crameri et al. 2000; Grimby et al. 1976; Shields 1995), limiting its usefulness for applying repetitive electrically elicited therapeutic loads to the skeletal system. Paralyzed muscle demonstrates decreased oxidative enzymes (Grimby et al. 1976), extensive fatigue (Bickel et al. 2004; Shields 1995, 2002; Slade et al. 2004; Thomas et al. 2003), and decreased cross-sectional area (Castro et al. 1999). Early losses in fatigue resistance (low-frequency fatigue) are likely due to impaired excitation-contraction coupling because of increased proportions of fibers expressing fast sarco(endo)plasmic reticulum Ca+2-ATPase (SERCA) (Talmadge et al. 2002). Long-term losses in fatigue resistance may reflect both excitation-contraction coupling compromise (SERCA) as well as decrements in oxidative capacity (succinate dehydrogenase, SDH) (Shields 1995).
Crameri and coauthors reported that 4 mo of electrical stimulation training in people with acute SCI (<4 wk) preserved the pretraining proportions of type I, IIA, and IIX quadriceps muscle fibers (Crameri et al. 2000). Subjects who did no training showed the expected shift toward predominantly type IIX fibers. In another study, people with subacute SCI (~46 wk) who trained for 8 wk showed substantial improvements in quadriceps cross-sectional area (Dudley et al. 1999). No previous studies, however, have quantified the long-term training-induced changes to the musculoskeletal system from the acute phase to the chronic phase of SCI.
Maintaining paralyzed muscle tissue may prove to be a valuable means for improving the general health and wellbeing of individuals with SCI. Without regular loading via muscular contraction, bone demineralization occurs at a rate of 2–4%/mo after SCI (Biering-Sorensen et al. 1990; Eser et al. 2004; Garland et al. 1992; Wilmet et al. 1995), eventually reaching steady state near fracture threshold (Eser et al. 2005). Bone strength is sufficiently compromised that electrical stimulation of certain muscles is contraindicated because of the high risk for fracture (Hartkopp et al. 1998). In previous studies of subjects with chronic SCI, electrical stimulation to muscles has been shown to only marginally influence bone mineral density (BMD) (Belanger et al. 2000; Bloomfield et al. 1996; Eser et al. 2003). Thus the term “neurogenic osteoporosis” emerged to describe the link between impaired neural function and altered bone metabolism leading to osteoporosis (Garland et al. 2004). Because of the extensive destruction of the trabecular lattice in long-term disuse osteoporosis (Modlesky et al. 2004; Slade et al. 2005), it appears that BMD loss after chronic paralysis is irreversible (Parfitt 1987). Few studies have attempted to intervene early after SCI to determine if bone loss can be prevented.
Several factors undermine our understanding of how long-term training paradigms affect post-SCI musculoskeletal plasticity. The magnitude of the stress applied to tissue is often not estimated, thus the dose of stress delivered to paralyzed tissue may not meet a threshold consistent with overload and/or endurance training principles (Enoka 2002). Subject compliance is also difficult to quantify, limiting the ultimate conclusions that can be made during long-term studies. Last, variability between individuals with SCI may preclude the ability to detect an important physiological effect of the long-term intervention.
The purposes of this study were to determine whether a long-term electrical stimulation training program would preserve the physiological properties of the plantar flexor muscles (peak torque, fatigue index, torque-time integral, and contractile speed) as well as influence distal tibia trabecular BMD in individuals with acute SCI. We hypothesized that limbs that underwent a timely, quantifiable dose of training would demonstrate higher torques, higher fatigue indices, and higher torque-time integrals than limbs from the same subjects that remained untrained. We also hypothesized that this dose of training would attenuate the neurogenic osteoporosis associated with SCI.
METHODS
Subjects
Seven men with SCI participated in this study (Table 1). The protocol was approved by the University of Iowa Human Subjects Institutional Review Board. All subjects provided written informed consent before participating. Inclusion criteria were complete spinal cord injury (ASIA class A) (American Spinal Injury Association 2002) above T12 as determined by neurological examination, passive ankle dorsiflexion to neutral, passive knee flexion to ≥90° in a seated position. Exclusion criteria were lower motor neuron injury below T12, lower extremity trauma, pressure ulcers, or peripheral/systemic infection.
TABLE 1.
Subject demographic data
| Subject | SCI Level |
Age at Enrollment |
Trained Limb |
Training Duration, yr |
Overall Compliance (percentage of Goal) |
|---|---|---|---|---|---|
| 1 | C5 | 30 | R | 2.31 | 84.9634 |
| 2 | C6 | 43 | L | 2.35 | 93.4005 |
| 3 | T9 | 29 | R | 3.02 | 72.3965 |
| 4 | T10 | 22 | R | 2.13 | 101.0369 |
| 5 | T4 | 37 | L | 1.87 | 52.0103 |
| 6 | T4 | 22 | R | 3.05 | 83.3083 |
| 7 | T4 | 21 | R | 2.27 | 80.1556 |
SCI, spinal cord injury.
The subjects enrolled in the study within the first 6 wk after SCI. After an initial testing session, the subjects began a unilateral plantar flexion electrical stimulation training protocol. Subjects were invited to train for ≤3 yr. All subjects completed ≥2.0 yr of training.
Test apparatus and experimental protocol
Subjects remained in their wheelchairs during the test procedure. One leg underwent training and the other served as an untrained within-subject control.
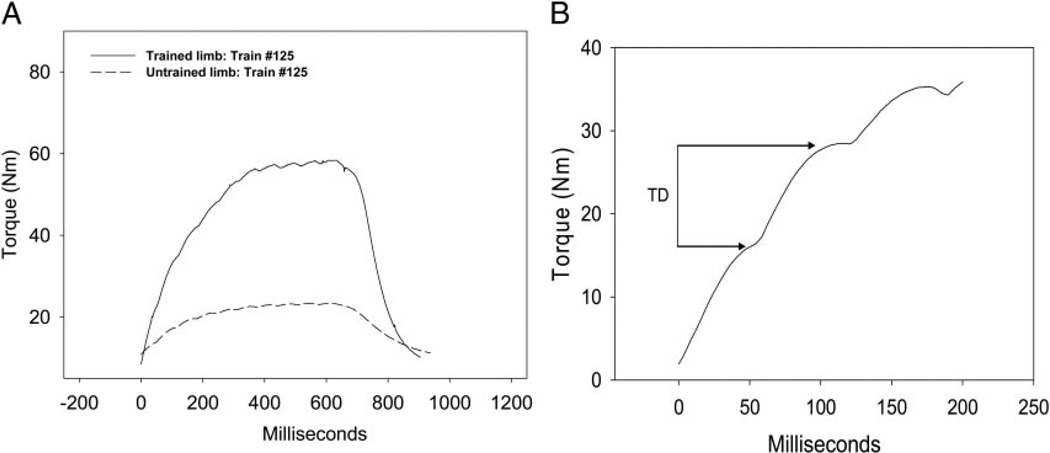
The ankle was stabilized in a system that measured isometric plantar flexion torque as described previously (Shields 1995; Shields and Chang 1997; Shields et al. 1997, 1998). The knee was positioned at 90° flexion and the ankle was in neutral joint position. A probe over the tibial nerve delivered electrical impulses to the plantar flexor muscles. The test position (knee flexed) minimized the contribution of the gastrocnemius to the plantar flexor torque (Sale et al. 1982). The constant current electrical stimulator had a range of 0–200 mA at 400 V. It was triggered by digital pulses from a data-acquisition board (Metrabyte DAS 16F, Keithley Instruments, Cleveland, OH) housed in a microcomputer under custom software control. The stimulation intensity was supra-maximal (~1.5 times the intensity required to produce a maximum twitch). The stimulator was programmed to deliver a 10-pulse train (15 Hz; train duration: 667 ms) every 2 s. A bout of exercise consisted of 125 trains (Fig. 1A). Subjects completed four bouts of exercise during each session. Each bout was separated by a 5-min rest period.
FIG. 1.
Representative examples. A: soleus torque at the end of a 125-train fatigue bout for a single subject. B: illustration of twitch difference (TD).
Training protocol
Subjects attended laboratory-based stimulation sessions two to four times monthly, depending on factors such as work obligations and driving distance. The trained leg was tested during each visit. The untrained leg was tested approximately once every 3 mo to minimize a training effect in that limb. The subjects performed the plantar flexion stimulation protocol at home using a custom portable stimulator and stabilization system. The home-training system duplicated the limb position and motion constraint used in the laboratory. Subjects affixed reusable adhesive carbon electrodes over their plantar flexor muscles for home training. Stimulation parameters were identical to parameters used in the laboratory sessions. Subjects performed four stimulation bouts per day on 5 days each week with 5 min of rest between each bout.
Justification for training protocol
We designed the muscle stimulation training protocol according to three main design criteria: we strived to “overload” the muscle to induce hypertrophy, to repetitively stress the muscle to increase endurance, and to exceed a dose of compressive load hypothesized to be osteogenic for the distal tibia. The protocol also needed to be feasible for subjects to conveniently perform over multiple years to assist with subject compliance. In a previous study of the torque-frequency relationship of acutely and chronically paralyzed muscle, we determined that muscular overload (~60% of maximal torque) can be generated via 15 -z supra-maximal stimulation (Shields and Chang 1997). We also previously determined that eliciting muscle contractions with a 1 on:2 off work-rest cycle (Burke like protocol) with a 15-Hz frequency induced significant low-frequency fatigue without compromising neuromuscular transmission. Thus the muscle itself (excitation-contraction coupling) would be stressed repetitively, meeting our endurance training criterion (Chang and Shields 2002; Shields 1995, 2002; Shields et al. 1998). Next, we resolved the plantar flexion peak torque (Nm) into its shear and compressive force vectors using a biomechanical model (Shields et al. 2006) [6 cm moment arm for plantar flexors (Maganaris et al. 1998, 2000)]. We determined that using the stimulation parameters described in the preceding text, the compressive load on the tibia was ~1–1.5 times body weight (BW). This dose of load is consistent with our design criterion for a likely osteogenic load (Frost 2003). The entire protocol took 35 min to complete.
Training protocol compliance
The electrical stimulation system was programmable by laboratory personnel but only had a start or stop button at the subject interface. The stimulators were designed to operate when the subject’s skin impedance was recognized (based on a range previously established in the laboratory). The stimulators did not engage unless the electrodes were in place and the skin impedance matched that of the subject. Once activated, the preprogrammed stimulus frequency, intensity, repetitions, and duration were delivered unless the subject aborted the bout. A microprocessor and memory chip within the stimulator recorded the date, time, and number of stimulus pulses received by the subject. These data were downloaded during a laboratory session. In this way, we were able to precisely quantify each subject’s compliance with the training protocol. The training protocol specified that 10,000 electrically stimulated contractions be completed each month (4 bouts of 125 contractions/day × 5 day/wk × 4 wk = 10,000 contractions). Compliance was calculated as the percent of the recommended number of contractions a subject completed in each month.
Dependent variables
We identified five muscle physiology variables of interest. Torque was defined as the peak torque achieved from the torque-time curve for any given contraction. Torque-time integral was defined as the integrated area under each torque-time curve. The value of the integral depends not only on peak torque but also on the duration of the contraction and the degree of fusion between the individual summated twitch responses (contractile speed properties). Fatigue index (FI) was computed according to the following formula: 100 * (minimum torque in a bout/maximum torque in a bout). Higher values indicate greater resistance to fatigue.
In each torque train, torque rise time was defined as the elapsed time in milliseconds between 20 and 80% of peak torque for that train. Rise time is influenced by the overall torque of each contraction and by the rate of torque development, which is known to vary according to muscle fiber type. We desired an estimate of contractile fusion within the 10-pulse torque train, another factor known to vary among fiber types. By visually inspecting the torque records, we concluded that the region of greatest change in torque (i.e., no plateau) was in the vicinity of pulse 2. We therefore subtracted the torque of the trough after pulse 1 from the maximum torque generated by pulse 2, yielding a difference value that reflects the degree of twitch fusion within the summated torque response (Fig. 1B). A higher twitch difference indicates less fusion between twitches.
Data processing
Subjects attended as many as 170 laboratory-based test sessions during their participation in the study. This longitudinal investigation therefore generated a considerable volume of data. To reduce the volume of data and to allow for time-based aggregation of group data, we have identified seven time bins for training sessions (Table 2). We selected one session per subject for each time bin. Only sessions that included testing of the trained and untrained limb were considered for selection. Eligible sessions were then selected on the basis of temporal proximity to the time criterion of each bin. All subjects trained until at least bin 5 (2 yr). Due to hospitalization or scheduling conflicts, subjects 5, 2, and 1 each missed one session in bins 2, 4, and 5, respectively. Because only two subjects appear in the final bin (3 yr), data from bin 7 are not presented in results and are not statistically analyzed.
TABLE 2.
Time bins used for group aggregation
| Bin | Bin Time, yr | Time of Session, yr | Subjects/Bin |
|---|---|---|---|
| 1 | 0.00 | 0.06 (0.01) | 7 |
| 2 | 0.50 | 0.66 (0.03) | 6 |
| 3 | 1.00 | 0.99 (0.03) | 7 |
| 4 | 1.50 | 1.62 (0.04) | 6 |
| 5 | 2.00 | 1.95 (0.04) | 6 |
| 6 | 2.50 | 2.24 (0.04) | 6 |
| 7 | 3.00 | 3.04 (0.01) | 2 |
Values are means ± SE for time of session. For each bin, one session from each subject was selected that most closely approximated the time criterion in column 2. Occasionally, subjects lacked a session in a given bin due to hospitalization or scheduling conflicts (bins 2, 4, and 5). One subject ceased training after bin 5. Two subjects continued training beyond bin 6.
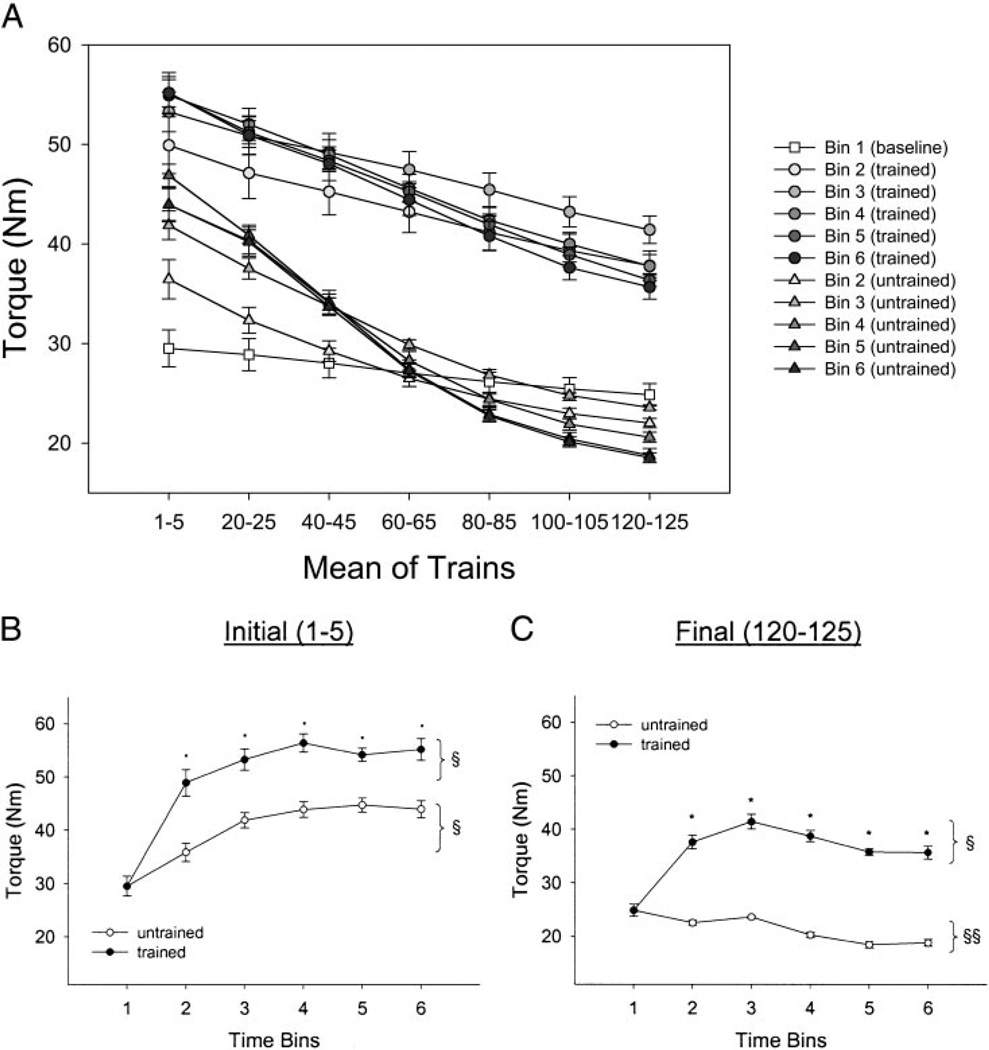
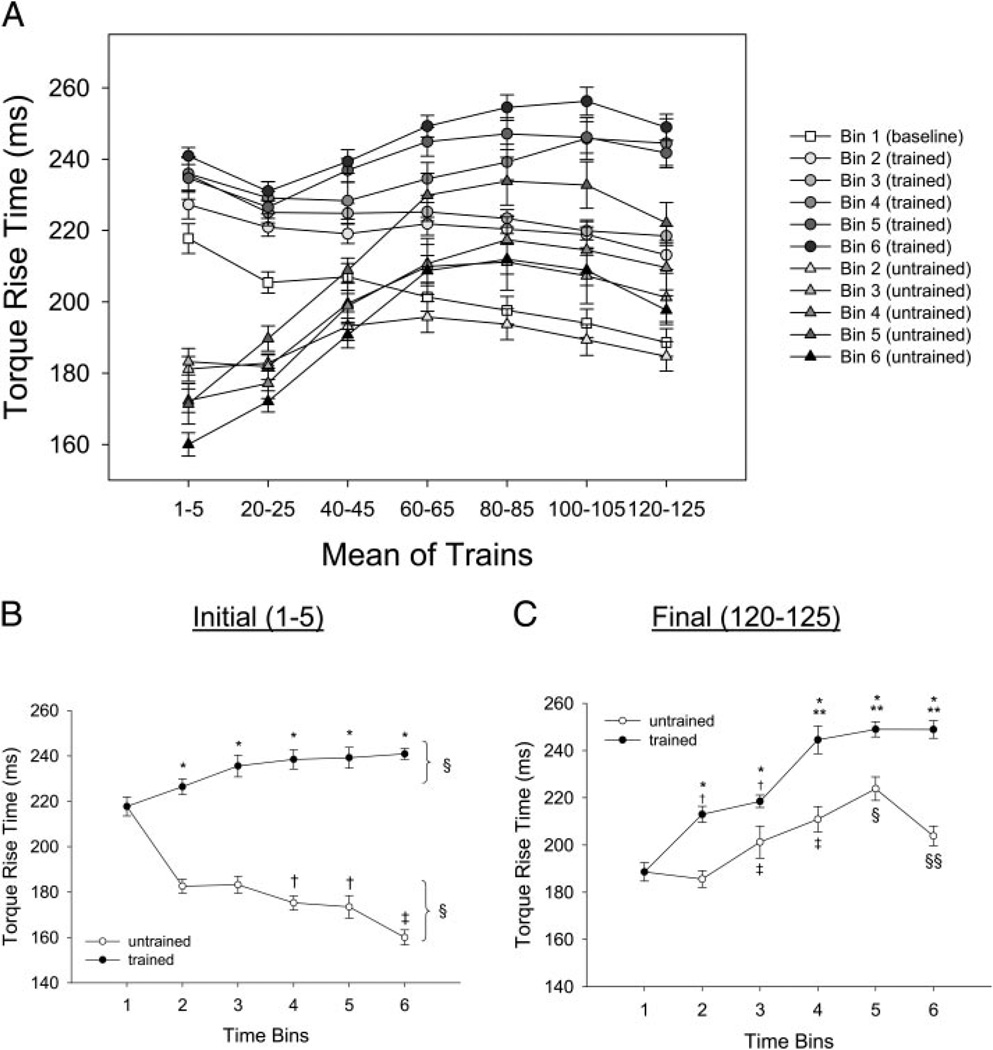
To display and analyze the data, we pooled evenly spaced samples from the 125 stimulus trains delivered at each session. Rather than depicting all 125 trains, the x axes of Figs. 2A and 5A show seven evenly spaced points, each one representing the mean of five consecutive stimulus trains. We present all data for illustrative purposes for the peak torque (Fig. 2A) and torque rise time (Fig. 5A). To examine a more sensitive view of the training effects, we plotted the first (contractions 1–5) and the last (contractions 120–125) trains by time for all dependent variables (Figs. 2, B and C, 3, A and B, 5, B and C, and 6, A and B).
FIG. 2.
Training and temporal effects for torque. Statistical significance is set at P < 0.05. A: mean ± SE soleus torque during the 125-train fatigue bout. Mean values for 5 consecutive trains at 7 points during the bout are depicted. B: mean ± SE soleus torque for trains 1–5 only. *, greater than untrained limb. §, greater than bin 1. C: mean ± SE soleus torque for trains 120–125 only. *, greater than untrained limb. §, greater than bin 1. §§, less than bin 1.
FIG. 5.
Training and temporal effects for torque rise time. Statistical significance is set at P < 0.05. A: mean ± SE soleus torque rise time during the 125-train fatigue bout. Mean values for 5 consecutive trains at 7 points during the bout are depicted. B: mean ± SE torque rise time for trains 1–5 only. *, greater than untrained limb. §, different from bin 1. †, less than bins 2 and 3. ‡, less than bins 2–5. C: mean ± SE soleus torque rise time for trains 120–125 only. *, greater than untrained limb. †, greater than bin 1. **, greater than bins 2 and 3. ‡, greater than bins 1 and 2. §, greater than bins 1–4. §§, greater than bins 1 and 2 and less than bins 4 and 5.
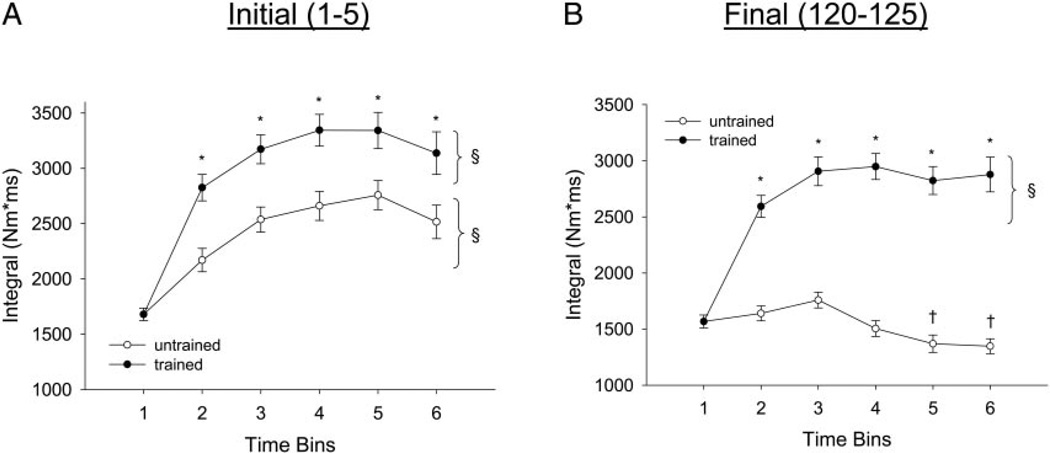
FIG. 3.
Training and temporal effects for torque-time integral. Statistical significance is set at P < 0.05. A: mean ± SE soleus integral for trains 1–5 only. *, greater than untrained limb. §, greater than bin 1. B: mean ± SE soleus integral for trains 120–125 only. *, greater than untrained limb. §, greater than bin 1. †, less than bins 1–3.
FIG. 6.
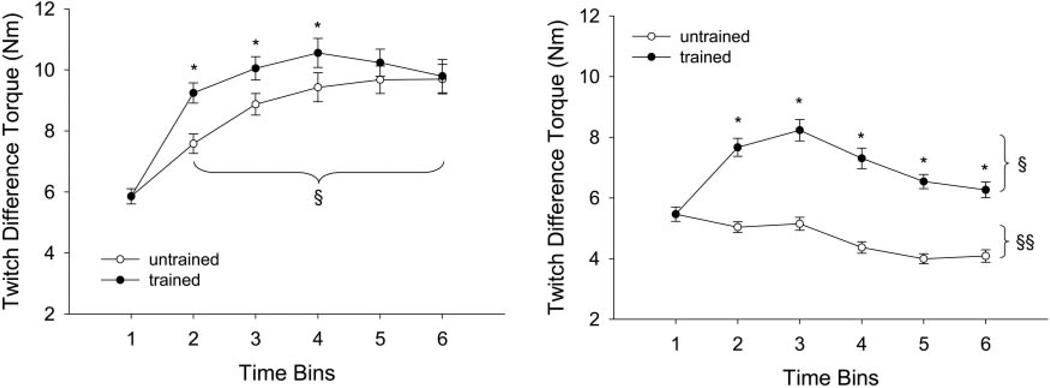
Mean ± SE twitch difference for pulse 2. Within each train of 10 pulses, the minimum torque after pulse 1 was subtracted from the maximum torque generated by pulse 2, yielding a difference value that reflects the degree of torque fusion (see Fig. 1B). Statistical significance is set at P < 0.05. A: mean ± SE twitch difference for trains 1–5 only. *, greater than untrained limb. §, greater than bin 1. B: mean ± SE soleus twitch difference for trains 120–125 only. *, greater than untrained limb. §, greater than bin 1. §§, less than bin 1.
BMD measurement
At the end of participation in the study, four subjects underwent peripheral quantitative computed tomography (pQCT) scans of bilateral distal tibiae. pQCT is a three-dimensional imaging technology that measures volumetric bone density and cross-sectional bone architecture characteristics. PQCT measurements were performed with a Stratec XCT-2000 (Stratec Medical, Pforzheim, Germany). Accuracy of this device is 2% (to the COMAC phantom); precision is ±3 mg/cm3 for trabecular bone and ±9 mg/cm3 for cortical bone (Norland Medical Systems 2000). This device is calibrated with respect to fat (fat density = 0 mg/cm3).
Each subject sat in their wheelchair and placed one leg in a “figure-4” position (the lateral side of 1 foot resting on the contralateral knee). An investigator marked the distal tip of the medial malleolus and the proximal rim of the medial tibial plateau with a permanent marker. The investigator measured tibia length between these marks, calculated 66% of the tibia length (from the distal end), and marked this site.
FIG. 4.
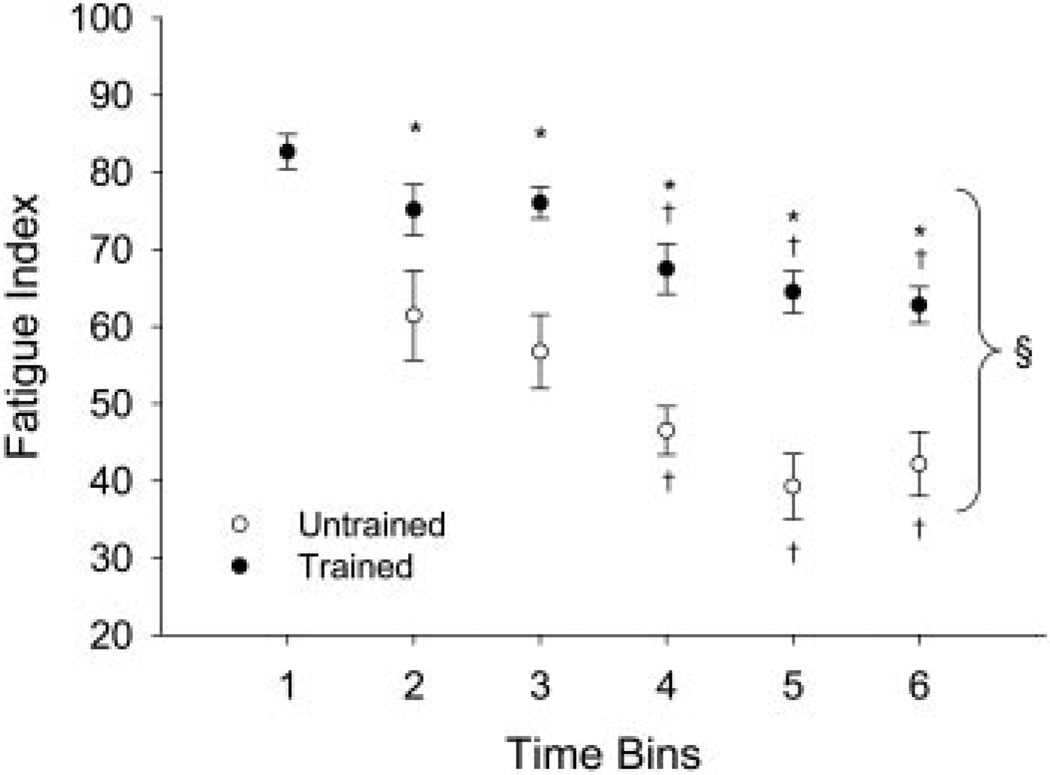
Temporal change in fatigue index. Statistical significance is set at P < 0.05. Mean ± SE fatigue index = 100 * minimum torque/maximum torque in a fatigue bout. *, greater than untrained limb. †, less than bins 1–3. §, less than bin 1.
The investigators passed one of the subject’s limbs through the pQCT gantry and then secured the subject’s foot onto a footplate. Padding was placed under the subject’s knee to distribute pressure between the popliteal fossa and the rigid knee rest. A custom-made laser that emitted a thin beam of light was mounted on top of the pQCT gantry. This was used to align the subject’s tibial tuberosity and ankle mortise to standardize limb rotation. Using an inclinometer placed just distal to the tibial tuberosity, the investigators adjusted the vertical height of the footplate to bring the pitch of the shank to horizontal. After positioning the limb, the investigators taped the knee securely into the knee rest, to minimize limb movement should spasms occur.
A radiology technician performed a scout view (rapid scan) of the talocrural joint and placed a reference line at the tibial endplate. Using this reference line, the scanner obtained images at 4 and 38% of tibia length. Subjects were repositioned (as in the preceding text) for the scan of the 66% site. Voxel size was 0.4 mm3, scanner speed was 25 mm/s, and slice thickness was 2.2 mm. We previously measured BMD variations of <5 mg/cm3 when the 4% scan site varied by as much as 6 mm in subjects with SCI.
At the 4% site, an iterative contour search algorithm was used to define the periosteal surface of the bone, based on a threshold density of 200 mg/cm3. Within the region defined by the search algorithm, voxels with density >400 mg/cm3 were defined as cortical/subcortical bone. Values lower than these thresholds were defined as trabecular bone. A 3 × 3 voxel filter then reexamined the cortical/subcortical bone regions to detect pockets of trabecular values based on neighboring voxel values. Voxels that were substantially lower than their neighbors were reassigned as trabecular bone. Thus any voxels corresponding to fat, skin, and muscle densities were eliminated.
At the 38 and 66% sites, cortical bone was predominant, necessitating a different voxel allocation strategy. All voxels with a density <711 mg/cm3 were excluded, which eliminated trabecular bone from the scan. Within the remaining cortical shell, a 3 × 3 voxel filter proofed the remaining voxels in the manner described in the preceding text, eliminating values indicative of trabecular bone. We therefore report only cortical BMD at the 38 and 66% sites.
Statistical analysis
A two-way repeated-measures ANOVA was used to determine if the dependent variables (muscle properties) changed across time in the trained and untrained limbs. A significant interaction between time and training conditions directed us to perform simple effects comparisons (Tukey) at each time bin. A one-way repeated-measures ANOVA was used to determine if the cortical and trabecular bone density measures were different between the trained and untrained limbs at the conclusion of the study. We rejected the null hypothesis if the statistical tests supported that the probability of detected differences was <5 of 100 times (P < 0.05).
RESULTS
Compliance and estimated tibia stress
Mean compliance for the seven time periods was 82.7% of goal (~8,270 contractions/mo). Overall cumulative compliance values for each subject for the duration of the study appear in Table 1. One subject (number 5, Table 1) was included only ≤2.0 yr because his compliance fell <70% after that time.
The estimated compressive load on the tibia of the trained side was 76.2 ± 11.1% BW in time bin 1 and rose to 127.8 ± 17.1% BW. Compressive loads in bins 5 and 6 exceeded 140% BW. Bins 2–6 were all significantly greater than bin 1 (P < 0.05) and met our intended threshold of compressive load to the tibia. Because the triceps-surae muscles’ line of action is nearly parallel to the tibia, the muscle forces closely approximate the compressive loads exerted on the tibia.
Peak torque
An example of the torque time curves for the trained and untrained limbs after 125 contractions appears in Fig. 1A. Figure 2A shows in detail the different levels of torque generated across all 125 contractions in the trained and untrained limbs as a function of time. For ease of comparison, the first five (1–5) and the last five (120–125) contractions are plotted for both the trained and untrained limbs across time in Fig. 2, B and C, respectively. A significant interaction (F = 497.0; P < 0.05) supported that the trained and untrained limbs responded differently across time. The trained limbs generated significantly higher peak torques, on average, than the untrained limbs at the start (trains 1–5) of the activation protocol (~24% greater) and at the end (trains 120–125) of the activation protocol (~75% greater; Fig. 2, B and C, respectively). These significant between-limb differences in peak torque were observed at each time bin (2–6). The largest average increase in peak torque occurred within the first 6 mo of training (~66%; bin 2) reaching a peak (~82% increase) by 1.5 yr (bin 4). The untrained limb showed, on average, an increase (P < 0.05) in peak torque for trains 1–5 at all time bins (2–6, ~32%) but showed a decrease (P < 0.05) in peak torque for trains 120–125 for all times (bins 2–6, ~20%). Accordingly, the trained limb at 2 yr (bin 6) generated nearly 100% more torque at the 125th contraction of the stimulation protocol than the untrained limb.
Torque-time integral
The trained limbs generated significantly higher torque-time integrals, on average, than the untrained limbs at the start (trains 1–5) of the activation protocol (~27% greater) and at the end (trains 120–125) of the activation protocol (~90% greater; Fig. 3, A and B). The largest increase in torque-time integral for contractions 1–5 occurred within the first 6 mo of training (~66%; bin 2). The untrained limb showed, on average, an increase (P < 0.05) in torque-time integral for trains 1–5 (~45%) but showed, on average, a decrease (P < 0.05) in torque-time integral for trains 120–125 (~10%). Thus the untrained limb showed a decreased ability to sustain work (torque-time integral) during the 125 contraction protocol as a function of time post-SCI.
Fatigue index
Figure 4 shows the FI for the trained and untrained limbs across time. A significant interaction (F = 48.0; P < 0.05) supported that the FI for the trained and untrained limbs responded differently across time. The trained and untrained limb FI at all time bins were significantly lower than the FI of the baseline condition (bin 1; P < 0.05). The trained limb FI was, on average, significantly higher than untrained FI at all times after baseline (~50%). The trained limb FI at bins 5 and 6, during which these subjects would be classified as having “chronic” SCI (>2 yrs), was >62% higher than the corresponding untrained limbs.
Torque rise time
Figure 5A shows the torque rise time generated across all 125 contractions in the trained and untrained limbs as a function of time. A significant interaction (F = 153.3; P < 0.05) supported that the rise time for the trained and untrained limbs responded differently across time. The trained limbs generated significantly longer torque rise times, on average, than the untrained limbs at the start (trains 1–5) of the activation protocol (~45% increase) and at the end (trains 120–125) of the activation protocol (~20% increase; Fig. 5, B and C). These significant between-limb differences in torque rise time were observed at each time bin (2–6). An increase in rise time for the trained leg during contractions 1–5 occurred progressively over the duration of the study, becoming significant by bin 3 (P < 0.05). The untrained limb showed, on average, a decrease (P < 0.05) in the rise time for trains 1–5 (~20%) over bins 2–6. These results indicate that the trained leg, during the first five contractions, retained and slightly increased the contraction durations while the untrained limb became faster. Both limbs showed, on average, an increase in rise time for trains 120–125 (~25 and 8%) with a progressive increase in rise time from bins 1–5.
The increased rise time by the final contractions of the train (120–125) in the trained limb was highly correlated (r = 0.95) with the overall slowing that the trained muscle demonstrated across time bins (as can be seen in contractions 1–5). Conversely, the untrained limb became faster (decreased rise times) as a function of time for contractions 1–5 and showed a significant increase in rise time by the 120–125th contractions. Thus the untrained limb developed a faster contraction (decreased rise time) in the fresh state, but showed increased contractile slowing by the 120–125th contractions.
In the fresh state (contractions 1–5), torque rise time was strongly, positively correlated with torque in the trained limb (r = 0.93). However, on the untrained side, a strong, negative correlation emerged between rise time and torque (r = 0.90). Therefore the torque gains apparent in the untrained limb during bins 2–6 were accompanied by decreased not increased rise time. Decreased rise time (with continued generation of high torque) implicates faster contractile speed in the untrained limb.
Twitch difference
The trained limbs generated significantly higher twitch differences, on average, than the untrained limbs at the start (trains 1–5) of the activation protocol (~15% increase; bins 2–4) and at the end (trains 120–125) of the activation protocol (~60% increase; Fig. 6, A and B). The twitch difference increased for the trained leg during contractions 1–51 with the greatest increase occurring by bin 2 (P < 0.05).
The untrained limb showed, on average, an increase (P < 0.05) in twitch difference for trains 1–5 (~45%) but showed, on average, a decrease (P < 0.05) in twitch difference for trains 120–125 (~10%). The decrease in twitch difference for contractions 120–125 support that significant slowing of the muscle occurred during repetitive activation of the untrained limb.
BMD
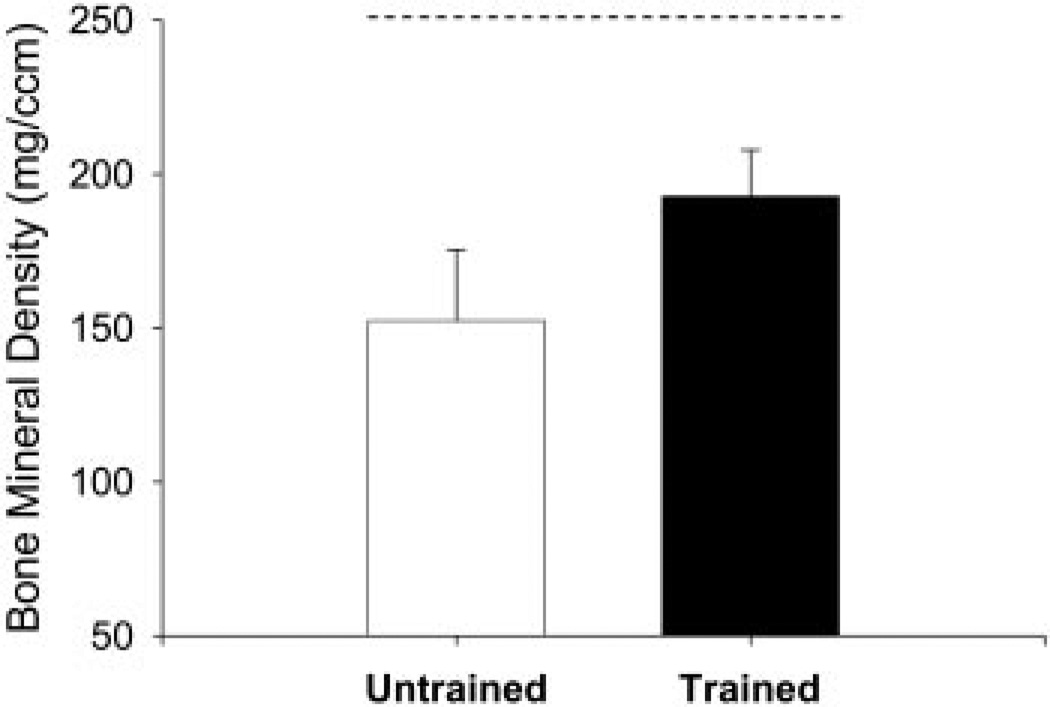
The extent of trabecular bone loss in the untrained limb as compared with the trained limb is depicted in Fig. 7. Trabecular BMD in the trained limb was, on average, 31% higher than the untrained limb BMD at the 4% site at the conclusion of the training program (Fig. 8). The BMD was, on average, 40 mg/cm3 higher in the trained limb than in the untrained limb (P < 0.05). At the 38 and 66% sites, there was no significant difference in cortical bone density between the trained and untrained limbs.
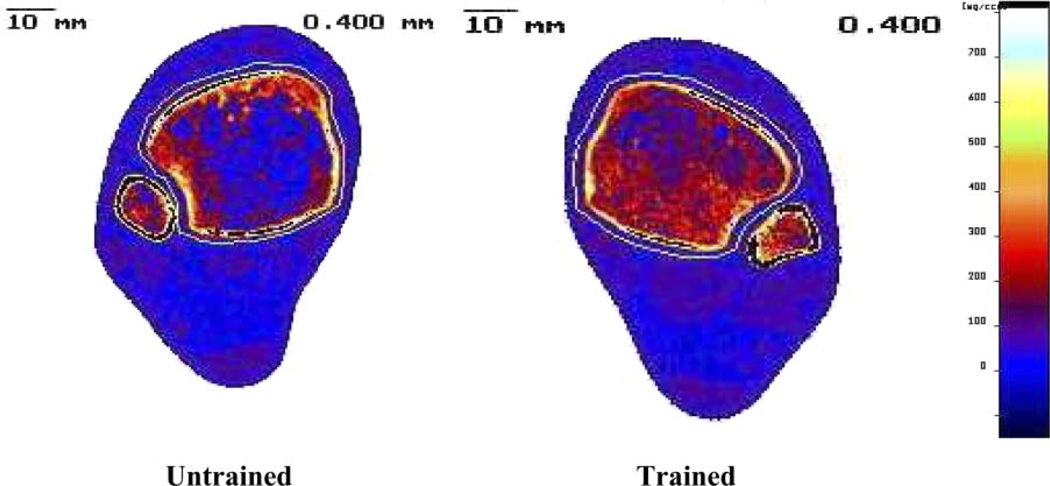
FIG. 7.
Representative example of peripheral quantitative computed tomography (pQCT) scan image at the 4% site (distal tibial epiphysis) of the trained (right) and untrained (left) limbs at 2.5 yr post injury: subject 6 (Table 1).
FIG. 8.
Mean ± SE bone mineral density at the distal tibia (4% site) for 4 subjects who underwent pQCT analysis. - - -, typical able-bodied bone density at this site (Eser et al. 2004).
DISCUSSION
The protocol used in this study yielded significant training effects on the muscle torque, torque-time integral, fatigue index, and contractile speed properties of the paralyzed plantar flexor muscles. This training effect persisted for >2 yr. A combination of higher torque-producing capacity and reduced fatigability in the trained limb enabled it to perform a greater magnitude of contractile work (represented as torque-time integral) during repetitive activation when compared with the contralateral untrained limb. The stress induced by this protocol not only caused adaptations in the muscle but also led to a significant difference in trabecular bone density. Collectively, these physiological findings support that a 35-min/d protocol designed to stress the musculoskeletal system can be highly effective in preventing some of the extensive musculoskeletal changes associated with chronic paralysis.
Torque
Plantar flexion peak torque and torque-time integral rapidly increased in response to the training protocol and remained elevated for the duration of the study. The time frame for the increase in peak torque and torque-time integral in this study is consistent with able-bodied training studies that also provided sufficient overload to muscle that was atrophied (Moritani and deVries 1980). In SCI, profound muscle atrophy is known to occur within the first weeks after injury. In the present study, baseline measures (<6 wk post-SCI) are indicative of significant atrophy.
Previous reports have demonstrated that electrically induced exercise of paralyzed muscle readily yields hypertrophy, both of muscle fiber cross-sectional area (Chilibeck et al. 1999; Crameri et al. 2000, 2002, 2004) and of whole-muscle area (Dudley et al. 1999; Hjeltnes et al. 1997; Scremin et al. 1999). In two previous studies, muscle fiber cross-sectional area increased the most when the resistance to exercise was the highest (Crameri et al. 2004; Dudley et al. 1999). The present study was designed to generate 60% of the total muscle force (Shields and Chang 1997) in an effort to “overload” the muscle and induce hypertrophy. This required that the limb be constrained because isometric contractions induce higher forces than concentric contractions (Crameri et al. 2004). Few studies attempting to adapt paralyzed muscle tissue have constrained the limb or have selected a stimulation frequency with a particular target force in mind (Stein et al. 1992).
A novel finding in this study was that the untrained limb showed an increase in torque over time after SCI. As early as bin 3, torque in the untrained limb exceeded the baseline measures. Several factors likely contribute to the untrained muscle showing an increase in torque from the acute to the chronic condition. First, passive and/or active tissue adaptations may alter force transmission in the plantar flexors (Maganaris et al. 2005; McDonald et al. 2005) from the acute to the chronically paralyzed condition. Proliferation of inelastic tissue may have augmented the transmission of muscular forces in the untrained limb. Second, within 6 wk after SCI, when baseline measures were taken, reflex responses are not yet hyperactive after SCI (Schindler-Ivens and Shields 2000). The advent of reflex contractions after 6 mo to 1 yr appears to coincide with the transformation of fiber type. Third, gradual transformation of paralyzed muscle to a fast fatigable phenotype (Shields 1995; Talmadge 2000; Talmadge et al. 2002), as supported by the increased fatigability and slowing of contractile speeds with fatigue, could also contribute to increased torque via a higher specific tension (Morse et al. 2005). Last, the contralateral untrained limb may have benefited from a cross-educational effect (Hortobagyi et al. 1999; Moritani and deVries 1979). Because all of these subjects showed clinically complete lesions, this would support a segmental contribution to cross-education with training (Aagaard et al. 2002; Enoka 1997). However, individuals with chronic SCI who have not participated in any muscle training have been reported to generate extremely high torques in the plantar flexor muscles (Shields 1995). Therefore the increased torque in the untrained limb was likely attributable to adaptations in connective tissue and muscle fiber properties.
Fatigue index
Although the trained limb FI fell after bin 1, it remained higher than the untrained limb FI at all time bins. The trained limb FI throughout 2.5 yr of training was slightly higher than our previously published values for untrained paralyzed soleus muscle (Shields 1995, 2002; Shields and Chang 1997). The untrained limb FI was also higher than our previously reported findings for chronically paralyzed subjects (FI ~30), even at bins 5 and 6 (FI = 39 and 42, respectively). However, our previous studies used a 20-Hz stimulation protocol to activate the muscle every second for 333 ms. The current study, to optimize endurance and strength training, used a 15-Hz protocol that activated the muscle every 2 s with a 667-ms train. Additionally, as previously discussed, we cannot overlook the possibility that cross-education influenced the contralateral untrained limb motor neurons.
The trained limb likely increased its ability to perform work because of both oxidative changes (Chilibeck et al. 1999; Mohr et al. 1997) and excitation-contraction coupling adaptations. Exercise, consistent with the protocol used in this study, has been shown to stress the sarcoplasmic reticulum Ca2+[r] release (Leppik et al. 2004). The protocol therefore appears likely to have induced an adaptation of SERCA involved with excitation-contraction coupling. Adaptations to SERCA enzymes and excitation-contraction coupling may occur before changes in oxidative enzymes and myosin type (Talmadge et al. 2002).
Myosin type is also highly adaptable to training. Upregulation of mRNA for MHC-I and downregulation of mRNA for MHC-IIX have been observed after only 2 wk of training (Harridge et al. 2002). In the quadriceps of individuals with SCI, two previous studies demonstrated that 1 yr of cycle training led to a predominance of MHC IIA (Andersen et al. 1996; Mohr et al. 1997). People with SCI who began quadriceps training very soon after SCI (<4 wk) experienced significantly lower declines in vastus lateralis MHC I than subjects who did not train (Crameri et al. 2000). Importantly, quadriceps training with an isometric load yielded greater expression of MHC I than did training without a load (Crameri et al. 2004). Thus we believe the dose of stress applied to the muscle in this study was/is an important consideration when designing exercise interventions for individuals with SCI. It should be noted that baseline torque recordings (bin 1) revealed significant atrophy within 6 wk without concomitant increases in muscle fatigue. The preservation of fatigue properties early during “reduced use” has been described previously by others (Nordstrom et al. 1995; Robinson et al. 1991).
Contractile speed
Contractile speed slowing (decrease in the rate of calcium transport or impairment of cross-bridge attachment and detachment rates) yields greater within-train torque summation by limiting the amount of relaxation that can occur before the arrival of each subsequent stimulus pulse. In the context of the present study, contractile slowing would be reflected by an increase in torque rise time or by a decrease in the single twitch difference (more fused) (Shields et al. 1997). In the pretraining condition (bin 1), the soleus muscle has high fatigue resistance and thus would not be expected to undergo substantial contractile slowing during the 125 stimulus trains. Training, however, yielded rapid differentiation between the trained and untrained limbs (Fig. 5, B and C).
Further evidence for an association between contractile slowing and fatigue can be seen in the results for the twitch difference (Fig. 6, A and B). The twitch difference is expected to be low in slow muscles (which exhibit highly summated torque responses) and high in muscles that function as a composite of fast fibers As expected, the recently paralyzed soleus (bin 1) demonstrated low twitch differences that remain steady during the fatigue bout. Both limbs demonstrated a slight increase in the twitch difference in contractions 1–5 across time (Fig. 6A; likely due to increased peak torque in both) but demonstrated clearly different responses by the 125th contraction (all time bins; Fig. 6B). These findings support that training maintained much of the force generating capacity as well as the muscle speed properties >2 yr after SCI. We are unable to state that the training kept the muscle “normal,” as our baseline measures were taken within 6 wk of SCI.
Neurogenic osteoporosis
A quantifiable dose of compressive loading (~140% BW) yielded a 31% difference in distal tibia BMD when compared with limbs that did not train. Because the subjects began training soon after SCI, it seems likely that the training stimulus served to attenuate the normally occurring loss of BMD after SCI. The present study suggests that trabecular bone (predominant the 4% tibia site) readily responds to stresses induced by isometric loading even after significant neurological compromise. The trained limb bone mineral density (~200 mg/cm3), while significantly greater than the untrained limbs (~150 mg/cm3), was not normal (~240 mg/cm3). As we predicted, cortical bone, which predominates at the 38 and 66% sites, was not affected by the training protocol.
Studies of muscle-bone loading in the able-bodied model have likewise shown different adaptive strategies for trabecular and cortical bone. Female weight lifters demonstrated significantly higher trabecular BMD values at the distal femur but minimal changes in cortical BMD after training (Heinonen et al. 2002). Eser and coauthors reported cortical BMD of the femur and tibia in individuals with SCI differed little from normative values (Eser et al. 2004). Trabecular BMD, on the other hand, was significantly reduced at both sites. The present study is the first to demonstrate that the distal tibia, an area at high risk for fracture after SCI (Garland and Adkins 2001), can maintain >30% of its trabecular bone mineral density with a timely dose of load. If this level of increased bone density translates into increased bone strength (Jiang et al. 1998; Lang et al. 1997; Wachter et al. 2002), then this protocol could have an important role in preventing ankle fractures after SCI. However, to date, the predictability of bone fracture based on bone density measures have not been clearly established in individuals with SCI.
Evidence that neural and skeletal systems closely interact provides the basis for the term “neurogenic osteoporosis” (Maimoun et al. 2005). Neural ligands such as calcitonin-gene related peptide (CGRP) are expressed in finely myelinated and unmyelinated A-delta and C nerve fibers that innervate trabecular bone (Imai and Matsusue 2002). Nerve fibers that stain for CGRP have been shown to increase during fracture healing (Hukkanen et al. 1993). Accordingly, it is conceivable that bone loss after SCI is the result of impaired neural function. The findings from this study support that electrical stimulation that induces “high loads” is a potent stimulus to influence BMD in the presence of neurological impairment (SCI). The extent to which compressive load on bone triggers activity in sensory neurons that ultimately influence bone metabolism is not known and warrants future investigation.
Summary and conclusions
A quantifiable dose of physiological muscle stress delivered 5 day/wk for 2–3 yr showed a mean compliance level of ~83%. Accordingly, this intervention, which involved ~35 min of activity per day, limited many of the physiological muscular and skeletal changes that normally occur after SCI (Shields 2002). Home-based stimulation systems that allow subjects to integrate frequent training into their daily lives appear important as we translate knowledge about post-SCI musculoskeletal plasticity. This study verified that neurogenic osteoporosis as a result of SCI can be influenced by compressive loads approaching 1.5 times BW. Future studies need to establish the optimal “therapeutic dose” of stress needed to maintain the musculoskeletal properties of the entire paralyzed extremities of those with SCI.
ACKNOWLEDGMENTS
The authors thank D. Frei, A. Miller, D. Walker, D. Gerleman, and D. Schiferl for technical expertise. S. Schindler-Ivens, L. Frey Law, Y.-J. Chang, P. Deshpande, A. Littman, and S. Madhavan facilitated the successful execution of this lengthy project. The authors especially thank the subjects of this study, each of whom gave hundreds of hours of their time to this research endeavor.
GRANTS
This work was supported by an award (R01-HD 39445) from the National Center for Medical Rehabilitation Research to R. K. Shields and by the Christopher Reeve and Sam Schmidt Paralysis Foundations to R. K. Shields. S. Dudley-Javoroski received a scholarship from the Foundation for Physical Therapy.
REFERENCES
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol. 2002;92:2309–2318. doi: 10.1152/japplphysiol.01185.2001. [DOI] [PubMed] [Google Scholar]
- American Spinal Injury Association. International Standards for Neurological Classification of SCI. Atlanta, GA: American Spinal Injury Association; 2002. [Google Scholar]
- Andersen JL, Mohr T, Biering-Sorensen F, Galbo H, Kjaer M. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES) Pflugers Arch Eur J Physiol. 1996;431:513–518. doi: 10.1007/BF02191897. [DOI] [PubMed] [Google Scholar]
- Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–1098. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- Bickel CS, Slade JM, VanHiel LR, Gordon WL, Dudley GA. Variable-frequency-train stimulation of skeletal muscle after spinal cord injury. J Rehabil Res Dev. 2004;41:33–40. doi: 10.1682/jrrd.2004.01.0033. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19:61–68. doi: 10.1016/8756-3282(96)00109-3. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Shields RK. Within-train neuromuscular propagation varies with torque in paralyzed human muscle. Muscle Nerve. 2002;26:673–680. doi: 10.1002/mus.10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37:264–268. doi: 10.1038/sj.sc.3100785. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29:104–111. doi: 10.1002/mus.10522. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Weston A, Climstein M, Davis GM, Sutton JR. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports. 2002;12:316–322. doi: 10.1034/j.1600-0838.2002.20106.x. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Weston AR, Rutkowski S, Middleton JW, Davis GM, Sutton JR. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: a pilot study. Eur J Appl Physiol. 2000;83:409–415. doi: 10.1007/s004210000263. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Castro MJ, Rogers S, Apple DF., Jr A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol. 1999;80:394–396. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Neural adaptations with chronic physical activity. J Biomech. 1997;30:447–455. doi: 10.1016/s0021-9290(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Neuromechanics of Human Movement. Champaign, IL: Human Kinetics; 2002. [Google Scholar]
- Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33:412–419. doi: 10.1046/j.1365-2362.2003.01156.x. [DOI] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86:498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec. 2003;275A:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Spinal Cord Injury Rehabil. 2001;6:37–46. [Google Scholar]
- Garland DE, Adkins RH, Kushwaha V, Stewart C. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–206. doi: 10.1080/10790268.2004.11753748. [DOI] [PubMed] [Google Scholar]
- Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Grimby G, Broberg C, Krotkiewska I, Krotkiewski M. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med. 1976;8:37–42. [PubMed] [Google Scholar]
- Harridge SD, Andersen JL, Hartkopp A, Zhou S, Biering-Sorensen F, Sandri C, Kjaer M. Training by low-frequency stimulation of tibialis anterior in spinal cord-injured men. Muscle Nerve. 2002;25:685–694. doi: 10.1002/mus.10021. [DOI] [PubMed] [Google Scholar]
- Hartkopp A, Murphy RJ, Mohr T, Kjaer M, Biering-Sorensen F. Bone fracture during electrical stimulation of the quadriceps in a spinal cord injured subject. Arch Phys Med Rehabil. 1998;79:1133–1136. doi: 10.1016/s0003-9993(98)90184-8. [DOI] [PubMed] [Google Scholar]
- Heinonen A, Sievanen H, Kannus P, Oja P, Vuori I. Site-specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int. 2002;70:469–474. doi: 10.1007/s00223-001-1019-9. [DOI] [PubMed] [Google Scholar]
- Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol Regulatory Integrative Comp Physiol. 1997;273:R1072, R1079. doi: 10.1152/ajpregu.1997.273.3.R1072. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Scott K, Lambert J, Hamilton G, Tracy J. Cross-education of muscle strength is greater with stimulated than voluntary contractions. Mot Control. 1999;3:205–219. doi: 10.1123/mcj.3.2.205. [DOI] [PubMed] [Google Scholar]
- Hukkanen M, Konttinen YT, Santavirta S, Paavolainen P, Gu XH, Terenghi G, Polak JM. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience. 1993;54:969–979. doi: 10.1016/0306-4522(93)90588-7. [DOI] [PubMed] [Google Scholar]
- Imai S, Matsusue Y. Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc Res Tech. 2002;58:61–69. doi: 10.1002/jemt.10119. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao J, Augat P, Ouyang X, Lu Y, Majumdar S, Genant HK. Trabecular bone mineral and calculated structure of human bone specimens scanned by peripheral quantitative computed tomography: relation to biomechanical properties. J Bone Miner Res. 1998;13:1783–1790. doi: 10.1359/jbmr.1998.13.11.1783. [DOI] [PubMed] [Google Scholar]
- Lang TF, Keyak JH, Heitz MW, Augat P, Lu Y, Mathur A, Genant HK. Volumetric quantitative computed tomography of the proximal femur: precision and relation to bone strength. Bone. 1997;21:101–108. doi: 10.1016/s8756-3282(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, Mc-Kenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+release, and Ca2+uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Changes in Achilles tendon moment arm from rest to maximum isometric plantarflexion: in vivo observations in man. J Physiol. 1998;510:977–985. doi: 10.1111/j.1469-7793.1998.977bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. In vivo measurement-based estimations of the human Achilles tendon moment arm. Eur J Appl Physiol. 2000;83:363–369. doi: 10.1007/s004210000247. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve. 2006;33:85–92. doi: 10.1002/mus.20441. [DOI] [PubMed] [Google Scholar]
- Maimoun L, Fattal C, Micallef JP, Peruchon E, Rabischong P. Bone loss in spinal cord-injured patients: from physiopathology to therapy. Spinal Cord. 2005 Sep 13; doi: 10.1038/sj.sc.3101832. [EPub] [DOI] [PubMed] [Google Scholar]
- McDonald MF, Garrison MK, Schmit BD. Length-tension properties of ankle muscles in chronic human spinal cord injury. J Biomech. 2005;38:2344–2353. doi: 10.1016/j.jbiomech.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19:48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- Mohr T, Andersen JL, Biering-Sorensen F, Galbo H, Bangsbo J, Wagner A, Kjaer M. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35:1–16. doi: 10.1038/sj.sc.3100343. [erratum appears in Spinal Cord 35: 262, 1997] [DOI] [PubMed] [Google Scholar]
- Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115–130. [PubMed] [Google Scholar]
- Moritani T, deVries HA. Potential for gross muscle hypertrophy in older men. J Gerontol. 1980;35:672–682. doi: 10.1093/geronj/35.5.672. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiolgical cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol. 2005;99:1050–1055. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- Nordstrom MA, Enoka RM, Reinking RM, Callister RC, Stuart DG. Reduced motor unit activation of muscle spindles and tendon organs in the immobilized cat hindlimb. J Appl Physiol. 1995;78:901–913. doi: 10.1152/jappl.1995.78.3.901. [DOI] [PubMed] [Google Scholar]
- Norland Medical Systems. XCT 2000 Technical Reference. White Plains, NY: Norland Medical Systems; 2000. [Google Scholar]
- Parfitt AM. Trabecular bone architecture in the pathogenesis and prevention of fracture. Am J Med. 1987;82:68–72. doi: 10.1016/0002-9343(87)90274-9. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Enoka RM, Stuart DG. Immobilization-induced changes in motor unit force and fatigability in the cat. Muscle Nerve. 1991;14:563–573. doi: 10.1002/mus.880140611. [DOI] [PubMed] [Google Scholar]
- Sale D, Quinlan J, Marsh E, McComas AJ, Belanger AY. Influence of joint position on ankle plantarflexion in humans. J Appl Physiol. 1982;52:1636–1642. doi: 10.1152/jappl.1982.52.6.1636. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S, Shields RK. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp Brain Res. 2000;133:233–241. doi: 10.1007/s002210000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, Scremin OU. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80:1531–1536. doi: 10.1016/s0003-9993(99)90326-x. [DOI] [PubMed] [Google Scholar]
- Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. doi: 10.1152/jn.1995.73.6.2195. [DOI] [PubMed] [Google Scholar]
- Shields RK. Muscular, skeletal, and neural adaptations following spinal cord injury. J Orthop Sports Phys Ther. 2002;32:65–74. doi: 10.2519/jospt.2002.32.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Chang Y-J. The effects of fatigue on the torque-frequency curve of the human paralyzed soleus muscle. J Electromyogr Kinesiol. 1997;7:3–13. doi: 10.1016/s1050-6411(96)00015-6. [DOI] [PubMed] [Google Scholar]
- Shields RK, Chang YJ, Ross M. Neuromuscular propagation after fatiguing contractions of the paralyzed soleus muscle in humans. Muscle Nerve. 1998;21:776–787. doi: 10.1002/(sici)1097-4598(199806)21:6<776::aid-mus10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Shields RK, Cook TM. Lumbar support thickness: effect on seated buttock pressure in individuals with and without spinal cord injury. Phys Ther. 1992;72:218–226. doi: 10.1093/ptj/72.3.218. [DOI] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S. Musculoskeletal deterioration and hemicorporectomy after spinal cord injury. Phys Ther. 2003;83:263–275. [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Dudley-Javoroski S, Frey Law L. Electrically induced muscle contractions influence bone density decline after spinal cord injury. Spine. doi: 10.1097/01.brs.0000201303.49308.a8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RK, Law LF, Reiling B, Sass K, Wilwert J. Effects of electrically induced fatigue on the twitch and tetanus of paralyzed soleus muscle in humans. J Appl Physiol. 1997;82:1499–1507. doi: 10.1152/jappl.1997.82.5.1499. [DOI] [PubMed] [Google Scholar]
- Slade JM, Bickel CS, Dudley GA. The effect of a repeat bout of exercise on muscle injury in persons with spinal cord injury. Eur J Appl Physiol. 2004;92:363–366. doi: 10.1007/s00421-004-1103-8. [DOI] [PubMed] [Google Scholar]
- Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA. Trabecular bone is more deteriorated in spinal cord injured versus estrogenfree postmenopausal women. Osteoporos Int. 2005;16:263–272. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- Stein RB, Gordon T, Jefferson J, Sharfenberger A, Yang JF, de Zepetnek JT, Belanger M. Optimal stimulation of paralyzed muscle after human spinal cord injury. J Appl Physiol. 1992;72:1393–1400. doi: 10.1152/jappl.1992.72.4.1393. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000;23:661–679. doi: 10.1002/(sici)1097-4598(200005)23:5<661::aid-mus3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Castro MJ, Apple DF, Jr, Dudley GA. Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol. 2002;92:147–154. doi: 10.1152/japplphysiol.000247.2001. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Griffin L, Godfrey S, Ribot-Ciscar E, Butler JE. Fatigue of paralyzed and control thenar muscles induced by variable or constant frequency stimulation. J Neurophysiol. 2003;89:2055–2064. doi: 10.1152/jn.01002.2002. [DOI] [PubMed] [Google Scholar]
- Wachter NJ, Krischak GD, Mentzel M, Sarkar MR, Ebinger T, Kinzl L, Claes L, Augat P. Correlation of bone mineral density with strength and microstructural parameters of cortical bone in vitro. Bone. 2002;31:90–95. doi: 10.1016/s8756-3282(02)00779-2. [DOI] [PubMed] [Google Scholar]
- Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]