Abstract
This technical note describes a new bench-top method for producing anisotropic hydrogels composed of gradient layers of soluble factors, particles, polymer concentrations or material properties. Each gradient layer was produced by a previous gradient method in which a droplet of one precursor solution was added to a thin layer of a second solution. The ensuing rapid capillary flow along the open channel generated a gradient precursor solution, which was then crosslinked to form a gradient gel. Repeating these steps allowed a layered gel to be iteratively constructed with as many gradient layers as desired. This technique renders the synthesis of multi-layered gradient gels accessible to virtually any researcher and should help simplify the production of more biologically relevant cellular microenvironments.
Introduction
Materials incorporating gradients of chemical and biological properties have a host of applications including biomolecule delivery, drug screening and cell biology studies.1, 2 An important class of biomaterials used for gradient generation are hydrogels, which mimic the extracellular matrix (ECM).3 Hydrogels may be synthesized with tailored three-dimensional (3D) microenvironments4 by manipulating their chemistry, crosslinking density and response to environmental stimuli,2, 5 and can be selectively formed inside microfluidic devices through gelation of prepolymer solutions.6 Gradient hydrogels can mimic in vivo biological gradients or can be used to test a continuum of conditions on a single biological sample.2 Due to their utility, a vast body of literature exists on producing gradient hydrogels2, 3, 7 (see also references within refs 8 and 9).
To make gradient synthesis accessible to a broader range of researchers, we recently developed a benchtop technique powered by capillary flow and molecular diffusion to generate multi-centimetre concentration gradients incorporating microspheres, cells, chemicals and various prepolymer mixtures.8, 9 The technique required only coated glass slides that could be obtained commercially or custom made with inexpensive off-the-shelf components (glass slides, tape/vinyl and hydrophobic spray). A hydrophilic stripe was “pre-wet” with a solution, held in place by a hydrophobic boundary but no physical channel walls. A droplet of a second solution was added at one end of the fluid stripe, causing a transient ~10 cm s-1 capillary flow that spread the droplet solution along the stripe and generated a gradient in the relative concentrations of the droplet and pre-wet solutions. The gradient shape was controlled by the droplet and pre-wet volumes and the viscosity and surface tension of the solutions.
In vivo, cells are exposed to gradients of many different factors offering chemical, physical and mechanical cues.2 To produce more biologically relevant cellular microenvironments, it is advantageous to produce biomaterials incorporating multiple gradients of such factors. The classical tree-like gradient generator has been used to generate overlapping soluble gradients of complex shapes10 and immobilized protein gradients positioned orthogonally or parallel on hydrogels.11 Three soluble gradients were superposed within a circular microfluidic palette.12 Overlapping soluble and microparticle gradients have also been produced by convection within a microchannel,13 and overlapping microsphere gradients were produced by alternately pipetting different microsphere solutions on opposite ends of a fluid stripe.8 Herein we describe a simple technique for generating multi-gradient hydrogels layer by layer in an open channel. The open channel technique and device operate under the same physical principles of surface tension driven flow and convection driven gradient generation as in our previous fluid stripe technique.8, 9
Device fabrication and operation
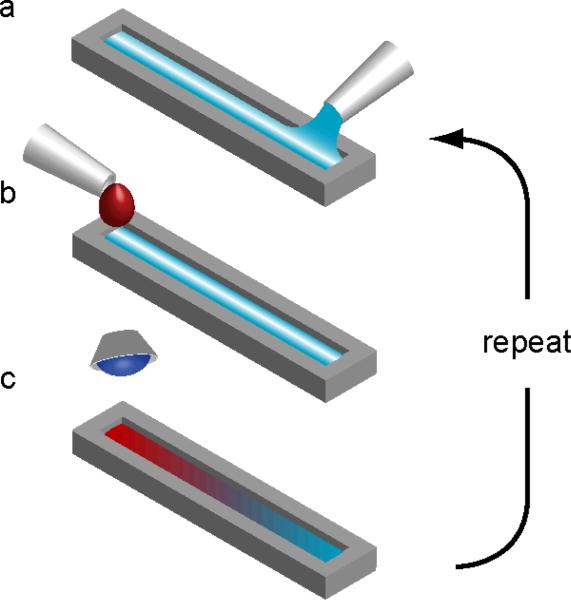
Our open channel device was built by stacking rectangular cutouts made from vinyl (8300 series, MACtac, Stow, OH) cut with a commercial cutting-plotter (CE5000-60, Graphtec America Inc., Santa Ana, CA) (Fig. S1). The channel depth was controlled by both the thickness and number of the vinyl sheets used. To prevent spillage, the top surface of the open channel was coated with hydrophobic spray (WX2100, Cytonix Corp., Beltsville, MD) and air dried for 2 days (Fig. S1). Dye experiments confirmed there was no leakage between layers or between the bottom layer and the glass. Precursor concentration gradients were produced in the open channel device in a similar manner to those in a previous coated slide device.8, 9 A “pre-wet” volume of precursor solution was pipetted into the open channel (Figs. 1a and S2). A drop of a second precursor solution was secreted and suspended from a pipette tip approximately 1 mm above the fluid at one end of the channel, and then with a quick gentle motion the drop was dislodged from the pipette so that it fell and coalesced with the pre-wet fluid in the channel (Figs. 1b and S2, ESI Video S1). The resulting gradient precursor solution was allowed to stand for 1 min and was then photocrosslinked by 60 s exposure to UV light (wavelength 360-480 nm, power 6.9 mW cm2) (Fig. 1c). The process was repeated to build up the multi-gradient gel layer by layer. Complete details of the supporting methods and materials used in this paper, such as cell culturing and imaging, are similar to those used previously8, 9 and are outlined in ESI Methods I.
Figure 1.
Protocol for synthesizing layered gradients in hydrogels within an open channel. (a) Channel is prewet with a precursor solution. (b) Droplet of a second precursor solution is added to prewet fluid stripe, generating a rapid capillary flow that spreads the droplet solution over the prewet solution. (c) The solution is left at rest to achieve desired vertical and lateral uniformity. The gradient precursor solution is then crosslinked, for example by ultraviolet (UV) light. The process a-c is then repeated for subsequent layers.
Results and discussion
The open channel device and corresponding gradient technique were used to generate multi-gradient hydrogels layer by layer. The physics of the capillary flow and gradient generation along the open channel were essentially the same as those on fluid stripes held on coated slides, and the flow speeds and gradient profiles were comparable (Fig. S3).8, 9 Adding a droplet to one end of the pre-wet open channel caused a local increase in the capillary pressure, which drove the flow and spread the droplet solution along the open channel (Fig. 1a,b and ESI Video S1). Each layer was left to stand for 1 minute before photocrosslinking.
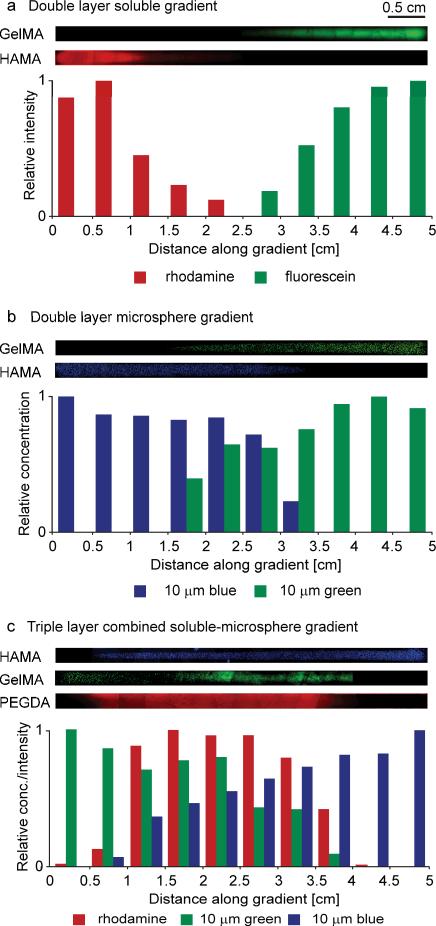
Various multi-layer hydrogels were synthesized with gradients in soluble factors, microparticles and material properties. The hydrogels used were 5% gelatin methacrylate (GelMA), 1% hyaluronic acid methacrylate (HAMA) and 20% polyethylene glycol-diacrylate (PEGDA) 2000. For each layer, a 7.5 μL droplet of precursor solution was added to 15 μL of a second precursor solution. For visualization and to mimic transported soluble factors or particles, the droplet solutions contained either fluorescent dyes (fluorescein, rhodamine) or 10 μm fluorescent microspheres (diluted 20X from stock solution). Three examples of multi-layer multi-gradient hydrogels are shown in Figure 2; precise drop and pre-wet contents for each layer and gradient are listed in Table S1. In Fig. 2a, a double layer hydrogel is shown with a rhodamine gradient in 1% HAMA in the bottom layer and a fluorescein gradient in 5% GelMA in the top layer. The drops were added at opposite ends of the channel to position the gradients in each layer in opposite directions. In Fig. 2b, a similar double layer gradient hydrogel using the same polymers was synthesized with 10 μm blue and green fluorescent microspheres in the bottom and top layers, respectively. The triple layer gradient hydrogel shown in Fig. 2c consisted of a rhodamine gradient in 20% PEGDA in the bottom layer, a 10 μm green fluorescent microsphere gradient in 5% GelMA in the middle layer and a 10 μm blue fluorescent microsphere gradient in 1% HAMA in the top layer. The drops used to make the gradients were positioned at opposite ends or the middle of the channel to control the position of the gradients in each layer. By changing the location of drop addition, the gradients may be repositioned (Fig. S4).
Figure 2.
Multi-layer multi-gradient hydrogels. (a) Double layer hydrogel with a rhodamine gradient in HAMA in the bottom layer and a fluorescein gradient in GelMA in the top layer. (b) Double layer hydrogel with a blue fluorescent microsphere gradient in HAMA in the bottom layer and a green fluorescent microsphere gradient in GelMA in the top layer. (c) Triple layer hydrogel with a rhodamine gradient in PEGDA in the bottom layer, a green fluorescent microsphere gradient in GelMA in the middle layer and a blue fluorescent microsphere gradient in HAMA in the top layer. Intensity levels of displayed microscope images have been increased to visualize particles. Periodic patterns in stitched microscope images of soluble gradients were due to nonuniform lighting across each subimage.
The open channel device allows larger fluid volumes to be used in each single layer than our previous coated slide device. To demonstrate this, we produced a single layer hydrogel with a microparticle concentration gradient from volumes double that used in our coated slide device. In particular, a 20 μL droplet of 1% HAMA solution containing 0.1% 10 μm microspheres was added to 40 μL pre-wet solution of 1% HAMA (Fig. S5).
The open channel device offers similar utility to our coated slide device for making biomaterial gradients with encapsulated cells. A concentration gradient of NIH-3T3 cells encapsulated in GelMA was formed by adding a droplet of 5% GelMA prepolymer solution containing cells to an open channel pre-wet with 5% GelMA (Fig. S6). Upon photocrosslinking, the polymer formed and encapsulated the cells. The 3D cell positioning was evident in microscope images, which showed cells in and out of focus in each focal plane. A subsequent Live/Dead® staining indicated that 94.4 ± 3.1% of the cells were still viable. A second biological gradient was generated in the single layer open channel device by adding a droplet of 5% GelMA containing NIH-3T3 cells to a 1% HAMA pre-wet solution. Since the cells were in the droplet, a cell concentration gradient was immediately generated along the open channel (Fig. S7a). The resulting gradient prepolymer solution was photocrosslinked, encapsulating the cells in 3D. Following 3 days of culture by a previous protocol,9 a gradient in cell spreading was observed (Fig. S7b). Cells spread well in the GelMA-rich regions and less so near the HAMA-rich region at the tip of the gradient. A host of other single layer hydrogel examples were reported and future applications discussed for our previous coated slide device,8, 9 and apply equally to the current open channel device.
Conclusions
We have reported a simple technique for generating multi-gradient hydrogels layer by layer within an open channel. Each gradient layer was produced by generating a concentration gradient in precursor solution by capillarity driven flow convection and then photocrosslinking the gradient precursor solution before repeating the procedure for subsequent layers. We demonstrated our technique by creating two and three layer hydrogels incorporating multiple soluble and microparticle gradients, mimicking simple systems for studying co-cultures. We also created single layer gradient hydrogels with particle, soluble and material gradients similar to our previous single layer fluid stripe technique. Since the device is open, there is no restriction on the number of layers and the intermediate and final gradient hydrogels may be directly accessed for visualization and analysis. Future work could include generating multi-gradient co-cultures incorporating different types of material and soluble gradients. We hope the open channel device and corresponding bench-top fabrication and operation protocols will enable and inspire more research utilizing multi-gradient biomaterials.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (HL092836, DE019024, EB012597, AR057837, DE021468, HL099073, EB008392), the National Science Foundation (DMR0847287), the Institute for Soldier Nanotechnology, the Office of Naval Research and the US Army Corps of Engineers. F. Piraino was partially supported by a Progetto Rocca Visiting PhD Student Fellowship. We thank Dr. Fumiki Yanagawa for technical help. FP, GCU, MJH and AK planned the research. FP, GCU and MJH contributed equally as lead authors, developed device and protocols, performed experiments, analyzed data and wrote the paper. AK supervised the research. FP, GCU, MJH and AK revised the manuscript. All authors agreed on the final contents of the manuscript.
Footnotes
† Electronic Supplementary Information (ESI) available. See DOI: 10.1039/b000000x/
References
- 1.Lee PI, Kim C-J. J. Controlled Release. 1991;16:229–236. [Google Scholar]; Peppas NA, Khare AR. Adv. Drug Deliver. Rev. 1993;11:1–35. [Google Scholar]; Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sant S, Hancock MJ, Donnelly JP, Iyer D, Khademhosseini A. Can. J. Chem. Eng. 2010;88:899–911. doi: 10.1002/cjce.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 4.Burdick JA, Khademhosseini A, Langer R. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]; DeLong SA, Moon JJ, West JL. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]; Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kloxin AM, Tibbitt MW, Anseth KS. Nat. Protocols. 2010;5:1867–1887. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]; Marklein RA, Burdick JA. Soft Matter. 2010;6:136–143. [Google Scholar]; Shamloo A, Heilshorn SC. Lab Chip. 2010;10:3061–3068. doi: 10.1039/c005069e. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen KT, West JL. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 7.Genzer J, Bhat RR. Langmuir. 2008;24:2294–2317. doi: 10.1021/la7033164. [DOI] [PubMed] [Google Scholar]; Keenan TM, Folch A. Lab Chip. 2008;8:34–57. doi: 10.1039/b711887b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kim S, Kim HJ, Jeon NL. Integr. Biol. 2010;2:584–603. doi: 10.1039/c0ib00055h. [DOI] [PubMed] [Google Scholar]
- 8.Hancock MJ, He J, Mano JF, Khademhosseini A. Small. 2011;7:892–901. doi: 10.1002/smll.201002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock MJ, Piraino F, Camci-Unal G, Rasponi M, Khademhosseini A. Biomaterials. 2011;32:6493–6504. doi: 10.1016/j.biomaterials.2011.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dertinger SKW, Chiu DT, Jeon NL, Whitesides GM. Anal. Chem. 2001;73:1240–1246. [Google Scholar]
- 11.Cosson S, Kobel SA, Lutolf MP. Adv. Func. Mater. 2009;19:3411–3419. [Google Scholar]; Allazetta S, Cosson S, Lutolf MP. Chem. Commun. 2011;47:191–193. doi: 10.1039/c0cc02377a. [DOI] [PubMed] [Google Scholar]
- 12.Atencia J, Morrow J, Locascio LE. Lab Chip. 2009;9:2707–2714. doi: 10.1039/b902113b. [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Hancock MJ, He J, Villa-Uribe JL, Wang B, Cropek DM, Khademhosseini A. Biomaterials. 2010;31:2686–2694. doi: 10.1016/j.biomaterials.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.