Coronary ostial stenosis or atresia (COSA, a new acronym) is a spectrum of rare developmental conditions with different pathophysiologic mechanisms and clinical implications. Together, the various forms of COSA should be considered a subclass of congenital coronary artery anomalies (CAAs). Although COSA includes various entities, these entities have two features in common. First, the defect is congenital, although it may progress during pre-natal and postnatal life. Second, the developmental defect causes ostial or proximal coronary obstruction (that is, stenosis or atresia). A fundamental distinction must be made between COSA and a related condition that involves the ectopic origin of a coronary artery from the opposite coronary artery (which is frequently, although erroneously, called “single coronary artery”). Here, I present an in-depth discussion of COSA (Table I) to aid the clinical understanding and management of individual cases.
TABLE I. Defining Features of COSA
By definition, COSA is congenital1–6; however, in some clinical adult cases, it can be difficult to determine whether a given defect has been present since birth.7–9 Also, total or partial-obstruction COSA might not be recognized until late after birth, by which time mild or nonexistent functional stenosis at birth might have become severe or totally occlusive (atretic).
In adult patients with coronary ostial or proximal coronary stenosis, it can be difficult to rule out acquired causes, such as arteritis, which can produce conditions morphologically similar to COSA; for example, atherosclerotic, syphilitic, Kawasaki, and Takayasu aortitis have all been cited as causes of acquired ostial stenosis or occlusion.7–9 The absence of significant risk factors for atherosclerosis is relevant to the diagnosis of COSA but, by itself, is inconclusive.
Coronary ostial stenosis or atresia affects the left coronary artery (L-COSA) more frequently than it does the right coronary artery (R-COSA). The aortic ostium in a case of COSA can be in the normal location or at an ectopic site. When the left ostium is located at a sinus of Valsalva that is abnormal for the distal coronary distribution, the case is one of anomalous coronary artery origin from the opposite sinus with intramural course (ACAOS),10 in which the proximal “crossing” trunk is stenotic because it is located intramurally (passing to the correct side of the heart, taking a tangential direction off the ostium, and lying within the aortic wall).
Other features present in some cases of COSA include an ostial dimple (a rudiment of the coronary ostium that is usually followed more distally by proximal coronary stem atresia11) and a blind distal left main trunk, which is best appreciated upon computed tomographic angiography.
In COSA, the presence of only 1 or 2 full-diameter connecting collateral vessels (without narrowing at the transition between the providing and the receiving vessel, as shown in Fig. 1) is suggestive of congenital origin in any given case of ostial stenosis or atresia. In contrast, in cases of postnatally acquired collateral vessels, one should expect a rich network of channels that typically involves all of the septal, infundibular, and atrial branches. In addition, such acquired collateral vessels enlarge progressively after birth, and they are typically smaller than the distal recipient vessels; this “step-up phenomenon” is typically observed only in postnatally acquired collateral vessels. Collateral vessels with similar features occur in atherosclerotic occlusion of coronary vessels, as well as in anomalous origin of the left or the right coronary artery from the pulmonary artery.12
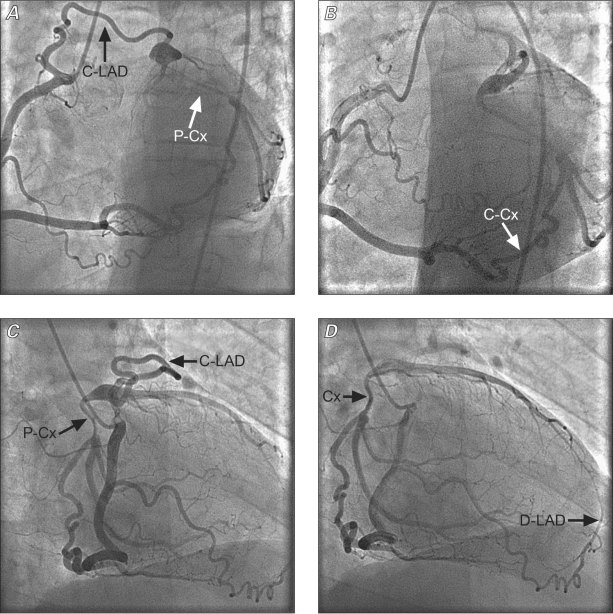
Fig. 1 Four selective coronary angiographic still images of the right coronary artery, obtained in a 52-year-old patient with chronic, atypical chest pain. The images were obtained in left anterior oblique (caudal and cranial: A and B) views, and 30- and 45-degree right anterior oblique (C and D) views. The only 2 collateral vessels off the right coronary artery are clearly shown (full-size diameters of the 2 connecting branches to the mid circumflex coronary artery, or C-Cx, and the proximal left anterior descending coronary artery, or C-LAD). Note that the proximal LAD is relatively short, while an anterior right ventricular branch provides for the distal anterior, interventricular branch (D-LAD in panel D). Also, the proximal circumflex artery (P-Cx) is diminutive probably because of the preferential flow from the distal collateral vessel (C-Cx), off the right coronary artery. C-Cx = collateral to the circumflex coronary artery; C-LAD = collateral to the left anterior descending coronary artery; D-LAD = distal left anterior descending coronary artery; P-Cx = proximal circumflex coronary artery
The absence of clinical angina (or ischemia at stress testing) tends to confirm the diagnosis of COSA, rather than acquired ostial stenosis or atresia. Similarly, myocardial scarring, which implies an event of acute ischemic myocardial damage, is rare in patients with COSA. As a corollary, only rarely is a surgical bypass intervention required to treat congenital coronary ostial atresia: the collateral vasculature is usually adequate.
Single Coronary Artery
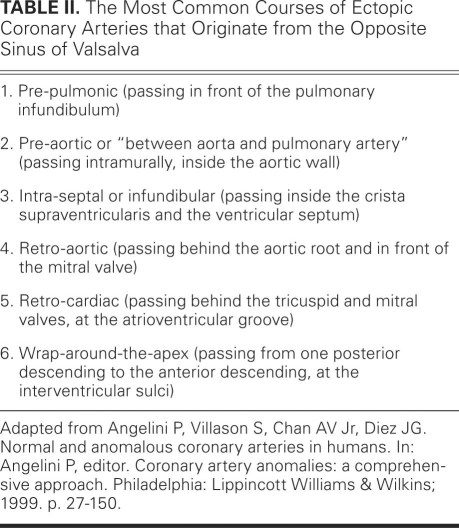
“Single coronary artery” is a misnomer: because all of the normal coronary arteries are present (that is, right, left anterior descending, and circumflex), this entity is not defined by the presence of a single coronary “artery” but by the fact that all these arteries originate from a single ostium.12 This term is clinical jargon (convenient but incorrect) used in some of the literature to refer to cases of CAA that feature a single coronary ostium, which provides flow to the whole coronary tree without interruption of continuity and without evidence of collateral vessels. Table II lists subtypes of this condition.12 In essence, these anomalies consist of the ectopic origin of the right or left coronary artery from the opposite sinus, which artery shares a proximal common trunk with the normally originating coronary artery. Less frequently, the ectopic artery originates from a distal segment of the contralateral artery, such as when the left coronary artery originates from the right coronary artery at the crux and takes a posterior course at the atrioventricular groove. The proximal trunk is a common, mixed-nature trunk that generally provides normal blood flow to both coronary arteries. Coronary stenosis or atresia is missing in this condition (hence it is not a type of COSA) unless the ectopic vessel has the above-mentioned proximal stenosis, caused by an intramural pre-aortic course. This latter course is also called “between aorta and pulmonary artery”10,12 (and it results in a form of COSA, when significant stenosis occurs), but this label is inaccurate, because the artery's proximal, ectopic coronary segment lies inside the aortic wall, and not in the space between the 2 great arteries.
TABLE II. The Most Common Courses of Ectopic Coronary Arteries that Originate from the Opposite Sinus of Valsalva
It has become clear, mainly from recent experience with intravascular ultrasonographic imaging,10 that the pre-aortic course always features intramural location of the proximal coronary trunk inside the wall of the aorta. The name recently proposed for such anomalies is ACAOS,10 and this condition is indeed the most common form of COSA. The severity of the resulting intramural stenosis in cases of ACAOS is variable, ranging from minimal stenosis to atresia, and it depends both on the degree of hypoplasia of the intramural segment and on the amount of lateral compression. The possibility that the extreme degrees of stenosis associated with ACAOS could lead to total or functional atresia, preventing all prograde flow from the ectopic ostium, must be considered in any given case of coronary ostial atresia (whether anatomic or functional). An exceptional example of ACAOS with COSA is shown in Figures 1 and 2.
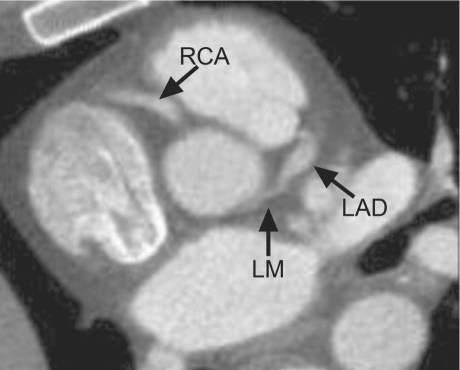
Fig. 2 Computed tomographic angiogram of the same patient shows a proximal left main trunk, the presence of which was not suggested by the selective coronary angiograms (Fig. 1). Indeed, the proximal left main trunk must be severely stenotic as a consequence of lateral compression at the intramural course. In this case, intravascular ultrasonographic confirmation is pending. LAD = left anterior descending coronary artery; LM = left main trunk; RCA = right coronary artery
Exceptional Types of COSA
Ectopic Ostium Located in the Left or Right Ventricle
Anomalous origin of a coronary artery from the right or left ventricle leads to functional impairment (coronary steal), usually not because of obstruction, but because of torrential blood flow into the same ventricle.13–15 Such flow occurs in both systole and diastole in cases of right ventricular origin, and it occurs only in diastole in cases of left ventricular origin. In both types, there is collateral flow that originates from the contralateral coronary artery. In such cases, the ventricular location of the ectopic coronary ostium cannot be established by direct catheter angiography but can be visualized by coronary computed axial tomography, nuclear magnetic imaging, echocardiography (especially if aided by Doppler interrogation), or contralateral coronary selective angiography. The ectopic left ventricular ostium has been reported in some of these cases to be partially or totally occluded, usually by an ostial membrane of unclear nature (such as a dysplastic aortic leaflet). Ischemia is most frequent in cases without such ostial obstruction.
Most cases of right ventricular origin of a coronary artery occur within the context of “pulmonary atresia with intact intraventricular septum.”16 In such cases, one or both coronary arteries have an ectopic origin from the right ventricle. In this circumstance, the supra-systemic right ventricular systolic pressure enables systolic flow of coronary nutrients into the myocardium, as well as into the aorta (that is, coronary steal). Because the diastolic blood flow is quite disturbed, clinical ischemia is still frequent in this context.12–14
Anomalous Origin of a Coronary Artery from the Pulmonary Artery
Cases of anomalous origin of a coronary artery from the pulmonary artery most commonly involve the left coronary artery.12,16 This type of CAA should probably be considered a type of COSA because the drainage of the ectopic coronary artery into the pulmonary implies leakage of coronary flow into the pulmonary artery, away from the myocardium (another example of coronary steal). Functionally, then, these cases are similar to typical cases of coronary atresia without ectopic coronary ostium: only 1 ostium is apparent at the aortic root, and collateral circulation is the only source of flow to the left coronary artery. Myocardial ischemia is especially likely to occur in the neonatal period (at maturation of pulmonary resistance) and when the runoff into the pulmonary artery is greater than the myocardial nutrient flow. In those cases, reimplantation surgery or bypass with proximal ligation is indicated.16 In addition, similar cases can become complicated by the development of ostial stenosis of the ectopic coronary artery (wall disease in association with turbulent flow), but this evolution actually improves coronary nutrient flow by decreasing arterial steal.
The Cases Reported in This Issue
My thoughts on COSA, set forth above, inform my interpretations of the 2 cases reported in this issue of the Journal. 17,18
Case Report by Shen and Colleagues17
This case of coronary anomaly is clearly a mixed-nature example of COSA. The basic anomaly was ectopic origin of the left coronary artery from the left ventricular outflow tract (located at the interleaflet triangle, between the noncoronary and left sinuses). This anomalous but well-formed left coronary artery ostium had been sealed by an adherent membrane, at some point in the patient's lifetime that cannot be established. In some such cases, displacement of an aortic cusp is the causal mechanism.2,3,6,13 The presence of ischemia indicates the need for surgical repair, and bypass grafting is usually the ideal procedure.
Case Report by Ghosh and Chauhan18
This report describes a case of single coronary ostium, with no evidence of a dimple of the aortic root or of the distal left main trunk, in a case that was interpreted as ostial atresia of the left coronary artery. Indeed, Figure 1 of Ghosh and Chauhan seems to show angiographically, in a right anterior oblique projection, that the left anterior descending and circumflex coronary arteries split in the presence of an acute angle, as is expected in a patient with a blind, occluded left main trunk. However, a computed tomographic angiographic image (Fig. 2 of that report) suggests that the junction between the left anterior descending and circumflex arteries has a wide angle, and the image reveals no evidence of a blind left main pouch, which favors the opposite interpretation from that of the authors—that this is a case of COSA with a congenital origin. The left coronary in this interpretation would congenitally and primarily originate from the right coronary, with a wrap-around-the-apex left main pattern. Indeed, the posterior-to-anterior descending connection features a single channel with a full-size diameter along its entire length (that is, there is no step-up phenomenon). This is not suggestive of an acquired collateral vessel. Moreover, the absence of additional collateral connections between the right and left coronary arteries is a significant impediment to calling this case one of left main acquired atresia of the proximal left coronary artery.
From my perspective, the case is one of congenital ectopic origin of the left coronary artery from the right-sided vessel, which should be renamed “mixed common trunk,” because it is more than a dominant right coronary artery—it provides all of the patient's coronary circulation. The aggressive form of the observed, well documented, associated coronary atherosclerotic disease is neither explained nor predicted by increased coronary flow in the common trunk; rather, it is the product of a malignant, unmitigated case of early atherosclerosis.
Footnotes
Address for reprints: Paolo Angelini, MD, P.O. Box 20206, Houston, TX 77225
E-mail: PAngelini@leachmancardiology.com
References
- 1.Tuna IC, Bessinger FB, Ophoven JP, Edwards JE. Acute angular origin of left coronary artery from aorta: an unusual cause of left ventricular failure in infancy. Pediatr Cardiol 1989;10(1):39–43. [DOI] [PubMed]
- 2.Waxman MB, Kong Y, Behar VS, Sabiston DC Jr, Morris JJ Jr. Fusion of the left aortic cusp to the aoic [sic] wall with occlusion of the left coronary ostium, and aortic stenosis and insufficiency. Circulation 1970;41(5):849–57. [DOI] [PubMed]
- 3.Kurosawa H, Wagenaar SS, Becker AE. Sudden death in a youth. A case of quadricuspid aortic valve with isolation of origin of left coronary artery. Br Heart J 1981;46(2):211–5. [DOI] [PMC free article] [PubMed]
- 4.Karadag B, Ayan F, Ismailoglu Z, Goksedef D, Ataev Y, Vural VA. Extraordinary cause of ischemic chest pain in a young man: congenital ostial atresia of the right coronary artery. J Cardiol 2009;54(2):335–8. [DOI] [PubMed]
- 5.Bansal M, Golden AB, Siwik E. Images in cardiovascular medicine. Anomalous origin of the right coronary artery from pulmonary artery with ostial stenosis. Circulation 2009;120 (23):e282. [DOI] [PubMed]
- 6.Josa M, Danielson GK, Weidman WH, Edwards WD. Congenital ostial membrane of left main coronary artery. J Thorac Cardiovasc Surg 1981;81(3):338–46. [PubMed]
- 7.Kumar GV, Agarwal NB, Javali S, Patwardhan AM. Takayasu's arteritis with ostial and left main coronary artery stenosis. Tex Heart Inst J 2007;34(4):470–4. [PMC free article] [PubMed]
- 8.Suzuki A, Kamiya T, Ono Y, Takahashi N, Naito Y, Kou Y. Indication of aortocoronary by-pass for coronary arterial obstruction due to Kawasaki disease. Heart Vessels 1985;1(2): 94–100. [DOI] [PubMed]
- 9.Aizawa H, Hasegawa A, Arai M, Naganuma F, Hatori M, Kanda T, et al. Bilateral coronary ostial stenosis and aortic regurgitation due to syphilitic aortitis. Intern Med 1998;37(1): 56–9. [DOI] [PubMed]
- 10.Angelini P, Flamm SD. Newer concepts for imaging anomalous aortic origin of the coronary arteries in adults. Catheter Cardiovasc Interv 2007;69(7):942–54. [DOI] [PubMed]
- 11.Angelini P. The case of a fascinating dimple. Am J Cardiol 1993;72(1):102–3. [DOI] [PubMed]
- 12.Angelini P, Villason S, Chan AV Jr, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 27–150.
- 13.Pirelli L, Yu PJ, Srichai MB, Khvilivitzky K, Angelini P, Grau JB. Ectopic origin of left coronary ostium from left ventricle, with occlusive membrane: a previously unreported anomaly, with an embryologic interpretation. Tex Heart Inst J 2008;35(2):162–5. [PMC free article] [PubMed]
- 14.Musiani A, Cernigliaro C, Sansa M, Maselli D, De Gasperis C. Left main coronary artery atresia: literature review and therapeutical considerations. Eur J Cardiothorac Surg 1997;11(3):505–14. [DOI] [PubMed]
- 15.Culbertson C, De Campli W, Williams R, Helton G, Young N, Hardy C. Congenital valvar aortic stenosis and abnormal origin of the right coronary artery: rare combination with important clinical implications. Pediatr Cardiol 1995;16(2):73–5. [DOI] [PubMed]
- 16.Angelini P. Functionally significant versus intriguingly different coronary artery anatomy: anatomo-clinical correlations in coronary anomalies. G Ital Cardiol 1999;29(6):607–15. [PubMed]
- 17.Shen TC, Chou HH, Tsai KT. Ectopic and atretic ostium of left main coronary artery from interleaflet triangle between left and noncoronary cusps. Tex Heart Inst J 2012;39(1):60–2. [PMC free article] [PubMed]
- 18.Ghosh P, Chauhan A. Rare variant of anomalous left coronary artery: single right coronary artery continuing distally. Tex Heart Inst J 2012;39(1):63–4. [PMC free article] [PubMed]