Abstract
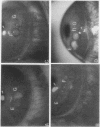
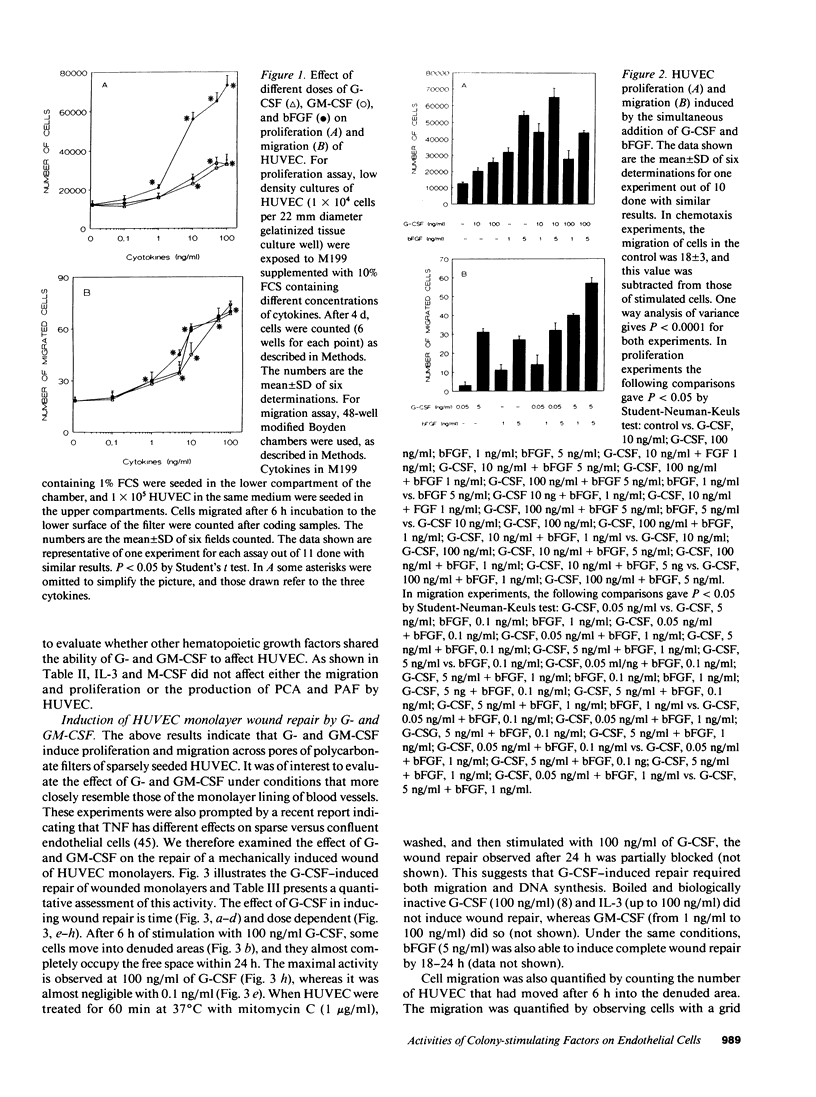
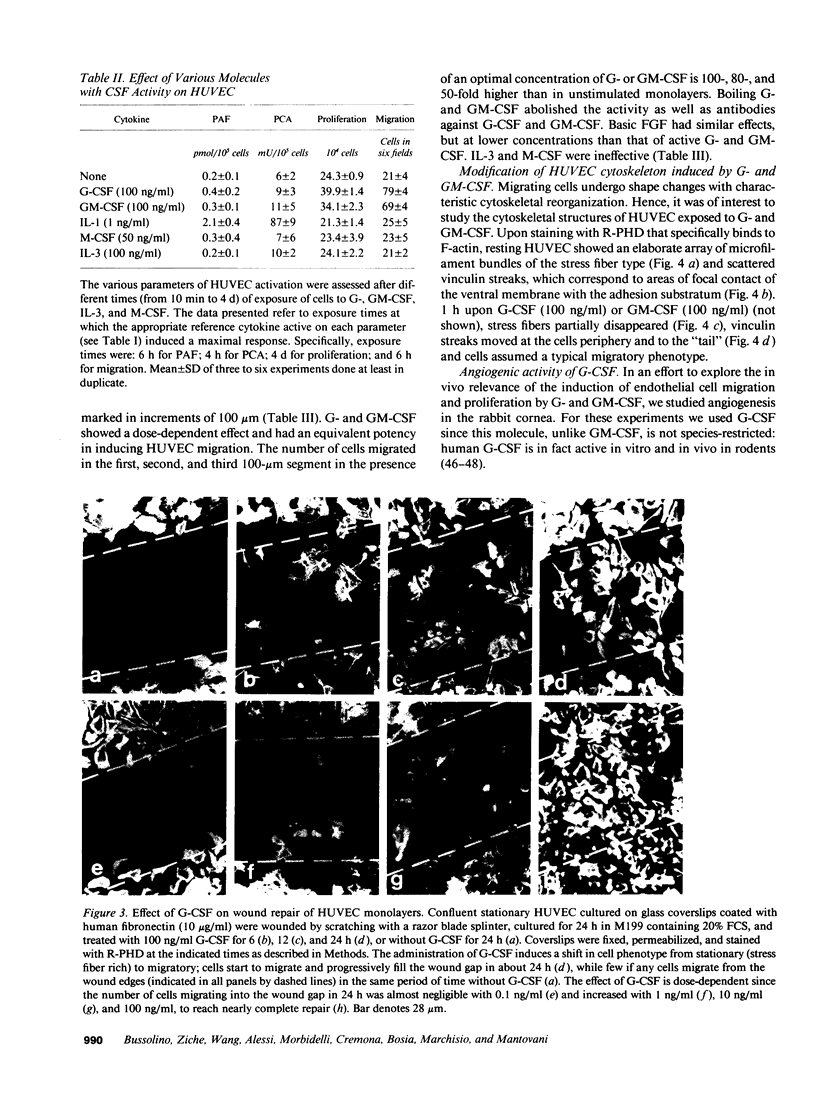
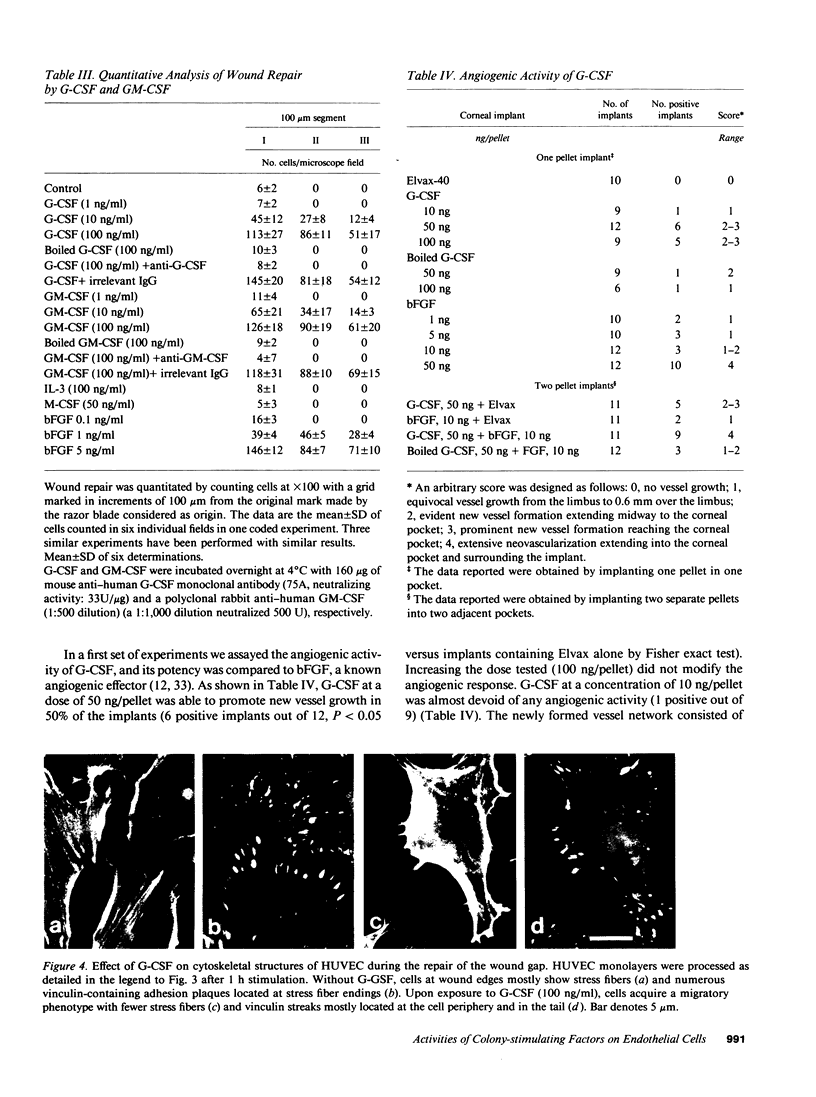
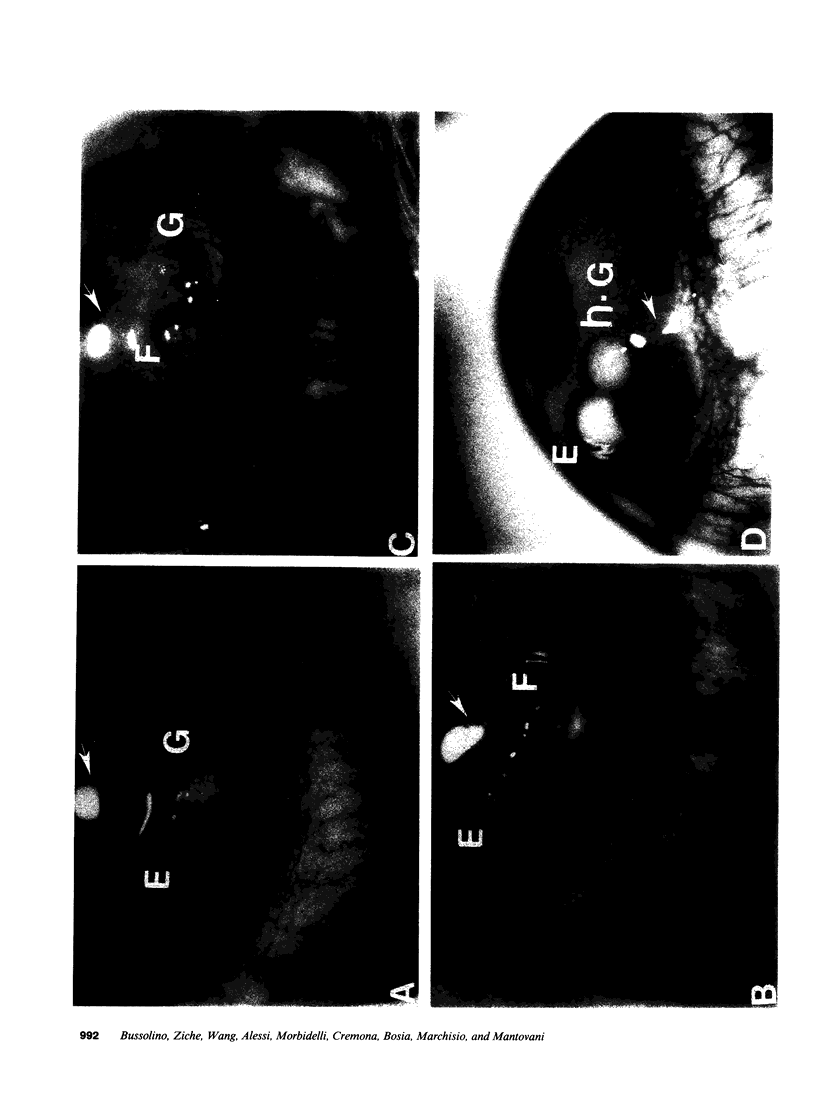
This study was designed to identify the set of functions activated in cultured endothelial cells by the hematopoietic growth factors, granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage-colony-stimulating factor (GM-CSF), and to compare them with those elicited by prototypic cytokines active on these cells. Moreover, indications as to the in vivo relevance of in vitro effects were obtained. G-CSF and GM-CSF induced endothelial cells to proliferate and migrate. In contrast, unlike appropriate reference cytokines (IL-1 and tumor necrosis factor, IFN-gamma), G-CSF and GM-CSF did not modulate endothelial cell functions related to hemostasis-thrombosis (production of procoagulant activity and of platelet activating factor), inflammation (expression of leukocyte adhesion molecule-1 and production of platelet activating factor), and accessory function (expression of class II antigens of MHC). Other colony-stimulating factors (IL-3 and macrophage-colony-stimulating factor) were inactive on all functions tested. In comparison to basic fibroblast growth factor (bFGF), G-CSF and GM-CSF induced lower maximal proliferation of endothelial cells, whereas migration was of the same order of magnitude. G-CSF and GM-CSF stimulated repair of mechanically wounded endothelial monolayers. Exposure to both cytokines induced shape changes and cytoskeletal reorganization consistent with a migratory phenotype. To explore the in vivo relevance of the in vitro effects of these cytokines on endothelium, we studied the angiogenic activity of human G-CSF in the rabbit cornea. G-CSF, but not the heat-inactivated molecule, had definite angiogenic activity, without any sign of inflammatory reactions. G-CSF was less active than bFGF. However, the combination of a nonangiogenic dose of bFGF with G-CSF resulted in an angiogenic response higher than that elicited by either individual cytokines. Thus, G-CSF and GM-CSF induce endothelial cells to express an activation/differentiation program (including proliferation and migration) related to angiogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. C., Gasson J. C., Kaufman S. E., Quan S. G., Williams R. E., Avalos B. R., Gazdar A. F., Golde D. W., DiPersio J. F. Nonhematopoietic tumor cells express functional GM-CSF receptors. Blood. 1989 Mar;73(4):1033–1037. [PubMed] [Google Scholar]
- Berdel W. E., Danhauser-Riedl S., Steinhauser G., Winton E. F. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989 Jan;73(1):80–83. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Breviario F., Tetta C., Aglietta M., Mantovani A., Dejana E. Interleukin 1 stimulates platelet-activating factor production in cultured human endothelial cells. J Clin Invest. 1986 Jun;77(6):2027–2033. doi: 10.1172/JCI112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Camussi G., Aglietta M., Braquet P., Bosia A., Pescarmona G., Sanavio F., D'Urso N., Marchisio P. C. Human endothelial cells are target for platelet-activating factor. I. Platelet-activating factor induces changes in cytoskeleton structures. J Immunol. 1987 Oct 1;139(7):2439–2446. [PubMed] [Google Scholar]
- Bussolino F., Gremo F., Tetta C., Pescarmona G. P., Camussi G. Production of platelet-activating factor by chick retina. J Biol Chem. 1986 Dec 15;261(35):16502–16508. [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Defilippi P., Turrini F., Sanavio F., Edgell C. J., Aglietta M., Arese P., Mantovani A. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989 Feb 2;337(6206):471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Turrini F., Alessi D., Ghigo D., Costamagna C., Pescarmona G., Mantovani A., Bosia A. Stimulation of the Na+/H+ exchanger in human endothelial cells activated by granulocyte- and granulocyte-macrophage-colony-stimulating factor. Evidence for a role in proliferation and migration. J Biol Chem. 1989 Nov 5;264(31):18284–18287. [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Salvidio G., Baglioni C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med. 1987 Nov 1;166(5):1390–1404. doi: 10.1084/jem.166.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Zsebo K. M., Inoue H., Hines D., Boone T. C., Chazin V. R., Tsai L., Ritch T., Souza L. M. In vivo stimulation of granulopoiesis by recombinant human granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2484–2488. doi: 10.1073/pnas.84.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S. American Association of Pathologists president's address. New roles for the endothelium in inflammation and immunity. Am J Pathol. 1987 Dec;129(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Dedhar S., Gaboury L., Galloway P., Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Breviario F., Erroi A., Bussolino F., Mussoni L., Gramse M., Pintucci G., Casali B., Dinarello C. A., Van Damme J. Modulation of endothelial cell functions by different molecular species of interleukin 1. Blood. 1987 Feb;69(2):695–699. [PubMed] [Google Scholar]
- Dejana E., Languino L. R., Polentarutti N., Balconi G., Ryckewaert J. J., Larrieu M. J., Donati M. B., Mantovani A., Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985 Jan;75(1):11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach H., Lieberman H., Bach R., Godman G., Brett J., Stern D. Enhanced responsiveness of endothelium in the growing/motile state to tumor necrosis factor/cachectin. J Exp Med. 1989 Sep 1;170(3):913–931. doi: 10.1084/jem.170.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Hancock G. E., Kaplan G., Cohn Z. A. Keratinocyte growth regulation by the products of immune cells. J Exp Med. 1988 Oct 1;168(4):1395–1402. doi: 10.1084/jem.168.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Twardzik D. R., Schwartz S. M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science. 1986 Sep 5;233(4768):1078–1080. doi: 10.1126/science.3461562. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Ishibashi S., Ogawa H., Murase T., Takaku F., Shibata S. Cachectin/TNF as well as interleukin-1 induces prostacyclin synthesis in cultured vascular endothelial cells. Biochem Biophys Res Commun. 1986 Dec 15;141(2):482–487. doi: 10.1016/s0006-291x(86)80198-x. [DOI] [PubMed] [Google Scholar]
- Kojima S., Tadenuma H., Inada Y., Saito Y. Enhancement of plasminogen activator activity in cultured endothelial cells by granulocyte colony-stimulating factor. J Cell Physiol. 1989 Jan;138(1):192–196. doi: 10.1002/jcp.1041380125. [DOI] [PubMed] [Google Scholar]
- Malavasi F., Tetta C., Funaro A., Bellone G., Ferrero E., Franzone A. C., Dellabona P., Rusci R., Matera L., Camussi G. Fc receptor triggering induces expression of surface activation antigens and release of platelet-activating factor in large granular lymphocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2443–2447. doi: 10.1073/pnas.83.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleef R. R., Bevilacqua M. P., Sawdey M., Gimbrone M. A., Jr, Loskutoff D. J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263(12):5797–5803. [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Ziche M., Alessandri G., Gullino P. M. Gangliosides promote the angiogenic response. Lab Invest. 1989 Dec;61(6):629–634. [PubMed] [Google Scholar]