Abstract
WEB SITE FEATURE
A 54-year-old woman was admitted with a 3-day history of dyspnea. Eight years earlier, she had undergone aortic valve replacement with a 25-mm Carbomedics mechanical aortic valve (Sorin S.p.A.; Milan, Italy) because of aortic regurgitation. Three years later, she had required emergency surgery for a type A aortic dissection. A Dacron Vascutek® graft (Terumo Cardiovascular Systems, part of Terumo Corporation; Ann Arbor, Mich) had been used, and the noncoronary sinus of Valsalva had been repaired with a bovine pericardial patch. At the current admission, neither her family history nor any findings on examination suggested Marfan syndrome or another collagen vascular disorder. Examination revealed raised venous pressure, a continuous murmur across the precordium, and bibasal pulmonary crackles. Inflammatory markers were mildly elevated (white cell count, 13.2 ×109/L and C-reactive protein, 66 mg/L). Two-dimensional transesophageal echocardiography (TEE) revealed a large pseudoaneurysm of the noncoronary sinus (Figs. 1A and 1B)—the previously inserted pericardial patch—and color-flow Doppler imaging indicated continuous flow between the aorta and the left atrium (Figs. 1C and 1D). A full-volume 3-dimensional (3D) TEE confirmed a large fistula between the aortic root and the origin of the pseudoaneurysm, with subsequent flow into the left atrium (Fig. 2). The size of the defect on 3D TEE suggested complete failure of the bovine pericardial patch (as opposed to a partial tear in the patch or a new defect in the aortic wall); this finding was confirmed intraoperatively.
Fig. 1 Transesophageal echocardiograms in A) short-axis and B) long-axis midesophageal views show the aortic valve with a large pseudoaneurysm arising from the noncoronary sinus of Valsalva. Color-flow Doppler transesophageal echocardiograms show C) abnormal color flow from the aorta posteriorly through a fistula into the left atrium (midesophageal aortic short-axis view) and D) abnormal flow from the aortic root into the left atrium through the fistula created by the rupture of a previously inserted pericardial patch (midesophageal aortic long-axis view). AoV = aortic valve; LA = left atrium; LV = left ventricle; PA = pseudoaneurysm; TV = tricuspid valve
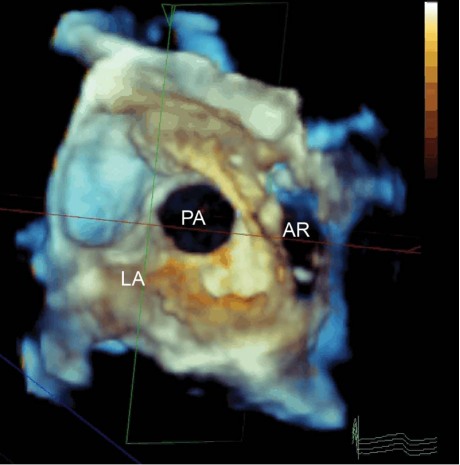
Fig. 2 Multiplanar reconstruction of a 3-dimensional full-volume transesophageal echocardiogram shows a large entry orifice of the pseudoaneurysm (PA)—as viewed from the left atrium (LA)—communicating with the aortic root (AR). The subsequent fistulous connection into the LA itself is not shown. Real-time motion image is available at www.texasheart.org/journal.
The fistula was repaired surgically with a new pericardial patch. There was no evidence of infection at surgery, and subsequent microbiological analysis showed nothing unusual.
Comment
Aorto–atrial fistula is seen infrequently; it is usually a complication of aortic root abscess formation that is caused by aortic valve endocarditis.1 Clinical diagnosis of the fistula in the absence of endocarditis is often challenging, because classic signs (for example, a continuous murmur) might be absent. Transthoracic echocardiography can be used to identify turbulent flow, but TEE is recognized as the best method for the diagnosis of aorta–atrial fistula.2 Many patients who develop aorta–atrial fistulae have had previous cardiac surgery, particularly aortic valve and aortic root surgery.3 However, in patients without a history of cardiac infection or surgery, a ruptured sinus of Valsalva aneurysm is the most common cause of aorta–atrial fistula. Published case series4,5 have recommended early surgical repair of sinus of Valsalva aneurysms—whether ruptured or unruptured—to prevent severe sequelae or death.
Supplementary Material
Footnotes
Address for reprints: Benoy Nalin Shah, MBBS, MRCP, Cardiothoracic Administration, Level E North Wing, Southampton General Hospital, Tremona Rd., Southampton SO16 6YD, UK
E-mail: benoy@doctors.org.uk
Section Editor: Raymond F. Stainback, MD, Department of Adult Cardiology, Texas Heart Institute at St. Luke's Episcopal Hospital, 6624 Fannin St., Suite 2480, Houston, TX 77030
References
- 1.Behnam R. Aortico-left atrial fistula in aortic valve endocarditis. Chest 1992;102(4):1271–3. [DOI] [PubMed]
- 2.Thomas MR, Monaghan MJ, Michalis LK, Jewitt DE. Aortoatrial fistulae diagnosed by transthoracic and transesophageal echocardiography: advantages of the transesophageal approach. J Am Soc Echocardiogr 1993;6(1):21–9. [DOI] [PubMed]
- 3.Ananthasubramaniam K. Clinical and echocardiographic features of aorto-atrial fistulas. Cardiovasc Ultrasound 2005;3:1. [DOI] [PMC free article] [PubMed]
- 4.Takach TJ, Reul GJ, Duncan JM, Cooley DA, Livesay JJ, Ott DA, Frazier OH. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg 1999;68(5):1573–7. [DOI] [PubMed]
- 5.Vural KM, Sener E, Tasdemir O, Bayazit K. Approach to sinus of Valsalva aneurysms: a review of 53 cases. Eur J Cardiothorac Surg 2001;20(1):71–6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.