Abstract
Early clinical trials of eptifibatide did not show a significant association between eptifibatide and the development of thrombocytopenia, thrombosis, or disseminated intravascular coagulation. However, more recent literature has suggested a significant association between eptifibatide and the development of thrombocytopenia and thrombosis. Although the true incidence and the pathophysiology of these associations are unknown, the development of these events can be life-threatening. Herein, we describe the case of a patient who experienced acute onset of profound thrombocytopenia, developing thrombosis, pulmonary emboli, and disseminated intravascular coagulation. This paper adds to the few previous reports of cases that suggested an association between thrombocytopenia, thrombosis, and the administration of eptifibatide. To the best of our knowledge, this is the first case report in the medical literature that associates the new onset of thrombocytopenia, thrombosis, and disseminated intravascular coagulation with the administration of eptifibatide. We also provide a subject review.
Key words: Disseminated intravascular coagulation/diagnosis/etiology/therapy, eptifibatide, percutaneous coronary angioplasty, platelet aggregation inhibitors/adverse effects, platelet glycoprotein GPIIb-IIIa complex/antagonists & inhibitors, thrombocytopenia/chemically induced/prevention & control/therapy, thrombosis/chemically induced
Glycoprotein (GP) IIb/IIIa antagonists have been shown to improve cardiovascular outcomes when used in conjunction with percutaneous transluminal coronary angioplasty (PCI) and in patients who present with acute coronary syndromes (ACS).1–4 However, glycoprotein IIb/IIIa antagonists have also been associated with increased bleeding complications and thrombocytopenia.5–11 Glycoprotein IIb/IIIa antagonist-associated thrombocytopenia has been reported chiefly with abciximab, although cases involving eptifibatide have also been reported.12
We present the case of a 73-year-old man who underwent elective, protected left main coronary artery PCI with the adjunctive administration of heparin, eptifibatide, aspirin, and clopidogrel.
Case Report
In April 2010, a 73-year-old man with chest pain and shortness of breath was admitted for elective coronary angiography. His medical history was notable for coronary artery disease, 3-vessel coronary artery bypass grafting (CABG) in 2001, left-lower-extremity deep-vein thrombosis and pulmonary embolism in 2007, hypertension, and hypercholesterolemia. Thrombophilia evaluation was unremarkable, including test results for age-appropriate cancer, protein C or S deficiency, lupus anticoagulant levels, anticardiolipin antibodies, antithrombin III levels, and factor V Leiden gene mutations. Coumadin had been discontinued after 6 months of therapy, because of a presumed idiopathic event.
During the preceding 6 months, the patient had noted worsening shortness of breath, fatigue, and chest pain with activity, all of which was reminiscent of his prior angina. A pharmacologic nuclear stress test revealed moderate perfusion defects in the lateral, inferolateral, and anterior territories. Coronary angiography showed an ostial, discrete 60% lesion in the left main coronary artery with a minimal area of vascular lumen (4.2 mm2 by intravascular ultrasound), which confirmed significant stenosis. The left internal mammary artery graft to the left anterior descending coronary artery and the saphenous vein graft to the 1st diagonal branch were both patent. The saphenous vein graft to the 1st obtuse marginal branch was occluded.
The patient was given aspirin (325 mg), clopidogrel (600 mg), intravenous unfractionated heparin (5,000 U), and eptifibatide (17-mg bolus, followed by a 2 mg/kg/min continuous infusion). A 3.5 × 12-mm PROMUS® (everolimus-eluting) stent (Boston Scientific Corporation; Natick, Mass) was deployed in the left main coronary artery in order to supply the left circumflex territory that was not revascularized, and this was post-dilated with a 4.5 × 8-mm Quantum™ Maverick® balloon (Boston Scientific). Post-PCI intravascular ultrasound confirmed excellent stent expansion and apposition.
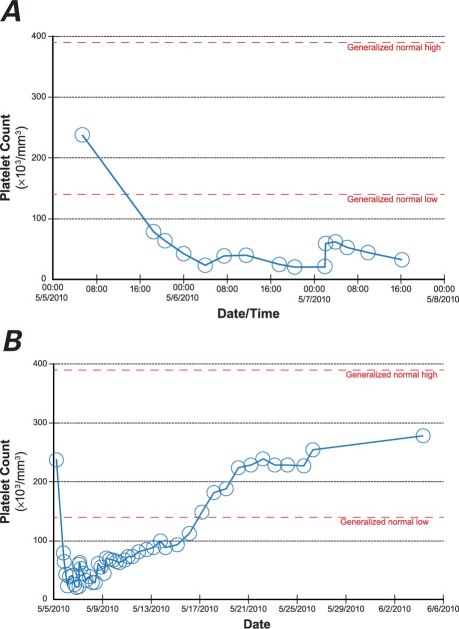
A complete blood count (CBC) 3 hours after cardiac catheterization revealed significant thrombocytopenia: a platelet count of 78 × 103/mm3 (baseline, 238 × 103/mm3) (Fig. 1A). Eptifibatide was immediately discontinued (aspirin and clopidogrel were continued). A CBC repeated 1 hour later in a non-EDTA collection tube revealed a platelet count of 64 ×103/mm3. Six hours after his PCI, the patient became lightheaded while having a bowel movement. His systolic blood pressure was 80 mmHg, and his heart rate was 50 beats/min. His pre-syncopal symptoms and blood pressure improved after the administration of 1.5 L of normal saline solution.
Fig. 1 Graphs show eptifibatide-induced thrombocytopenia A) immediately after percutaneous coronary intervention, and B) during hospitalization.
A bedside, limited transthoracic echocardiogram (TTE) ruled out the presence of pericardial effusion. A noncontrast computed tomographic scan of the chest, abdomen, and pelvis showed no evidence of retroperitoneal bleeding, hematoma, or pericardial effusion. At that time, the electrocardiogram, serum creatinine, hemoglobin, hematocrit, prothrombin time, troponin, and partial thromboplastin levels all remained within normal limits. During the course of the evening, the patient became hypoxemic and hypotensive, requiring 4 L of nasal cannula oxygen and manifesting systolic blood pressures of 80 to 110 mmHg. A subsequent measurement of his troponin level was elevated at 2.69 ng/mL, a repeat electrocardiogram showed a new right bundle branch block without ischemic changes, and a repeat TTE study revealed right ventricular enlargement and strain. A lower-extremity Doppler ultrasonogram confirmed acute bilateral lower-extremity deep-vein thrombosis, and a ventilation-perfusion scan indicated the presence of multiple filling defects, which was consistent with a high probability of acute pulmonary embolism.
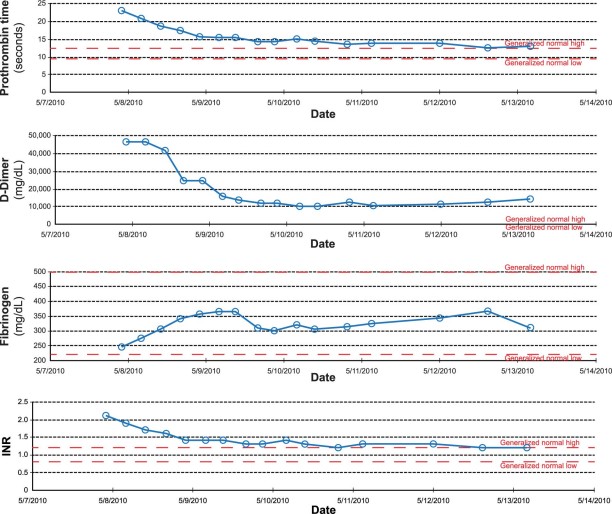
Subsequent laboratory testing established the development of severe thrombocytopenia (Fig. 1), acute renal failure (creatinine, 3.57 mg/dL; and glomerular filtration rate, 17), liver dysfunction (aspartate aminotransferase, 2,328 U/L; and alanine aminotransferase, 2,976 U/L) and disseminated intravascular coagulation (DIC) (Fig. 2). Direct review of the peripheral blood smear did not reveal the presence of a hemolytic process. Argatroban was initiated until 2 heparin platelet factor 4A (PF4A) tests were negative, at which point heparin infusion therapy was reinstituted. The patient's course in the intensive care unit was notable for intubation, hemodialysis, and infusion of packed red blood cells, platelets, fresh frozen plasma, and cryoprecipitate. The platelet count gradually recovered (Fig. 1B) during continued therapy with aspirin, heparin infusion, and clopidogrel. With supportive therapy, renal and liver function gradually recovered as well, and there was no evidence of sepsis or its sequela, DIC. After making a successful transition to warfarin, the patient was discharged in good clinical condition to a subacute rehabilitation facility, 19 days after admission. He returned as an outpatient 8 months after discharge without any complaints and reported that he had returned to all his previous activities without restrictions.
Fig. 2 Graphs show the laboratory trends of disseminated intravascular coagulation during the patient's hospitalization (shown by date). INR = international normalized ratio
Discussion
Early clinical trials of eptifibatide did not reveal a significant association between eptifibatide and the development of thrombocytopenia, thrombosis, or DIC. However, more recent literature has suggested a significant association between eptifibatide and the development of thrombocytopenia and thrombosis. These events can be life-threatening, yet their true incidence and the causal associations are unknown.
Heparin, eptifibatide, and clopidogrel are often administered at the same time during PCI and for the treatment of acute coronary syndromes. Each agent has been associated, independently, with thrombocytopenia.11 The co-administration of these agents can make it difficult to discern causation in drug-induced thrombocytopenia.
Early in the diagnostic evaluation, we eliminated pseudothrombocytopenia as a potential cause for our patient's presentation. Pseudothrombocytopenia is a relatively common, benign, and artificial laboratory process that occurs when blood samples are collected in EDTA tubes and automated CBCs are performed. Platelets adhere to leukocytes, resulting in an apparent thrombocytopenic condition. The propensity for platelet clumping, and therefore the incidence of pseudothrombocytopenia, increases in the presence of GP IIb/IIIa inhibitors.13 In our patient, pseudothrombocytopenia was ruled out by confirmation of thrombocytopenia in an EDTA-free tube and by direct review of the peripheral blood smear.
Heparin-induced thrombocytopenia (HIT) has been well described14–17 and was included in the differential diagnosis. Two types of HIT have been classified in accordance with the timing of thrombocytopenia in relation to heparin exposure. Heparin-induced thrombocytopenia type I (rapid-onset), manifests itself within the first 24 to 48 hours after heparin re-exposure in patients who were exposed to heparin products within the preceding 100 days. Type I is a consequence of residual heparin antibodies formed during the initial heparin exposure to the heparin PF4A complex. Heparin-induced thrombocytopenia type 2 (typical-onset) is the result of the initial antibody-mediated response to the heparin-PF4A complex. Typical-onset HIT occurs 5 to 10 days after the initial heparin exposure.15 The platelet nadir associated with both HIT subtypes is rarely less than 60 × 103/mm3.14
Our patient had been exposed to heparin previously, in 2001 for a CABG procedure and again in 2007 for the treatment of pulmonary embolism. Heparin-induced thrombocytopenia can result in a paradoxical prothrombotic state through the activation of the coagulation cascade via platelet-integrin complexes that become exposed during the antibody-mediated destruction of platelets.18 Therefore, HIT with thrombosis (HITT) seemed a plausible explanation for the combination of thrombocytopenia and thrombosis found in our patient. Although uncommon, rapid-onset HITT occurring less than 24 hours after heparin re-exposure (such as in our patient) has been described.14 However, 3 negative heparin-PF4A antibody tests and a nadir platelet count of 23 × 103/mm3 significantly decreased the likelihood that heparin was responsible for this presentation. In addition, the subsequent administration of heparin, after the resolution of thrombocytopenia and a negative serologic evaluation, did not result in a 2nd episode of thrombocytopenia, as would be expected had this been a HITT reaction.
Although relatively uncommon, Thrombotic Thrombocytopenic Purpura–Hemolytic Uremic Syndrome (TTP-HUS) after the administration of clopidogrel has been reported (128 case reports).19 The constellation of microangiopathic hemolytic anemia, thrombocytopenia, fever, neurologic dysfunction, and renal insufficiency as seen in TTP-HUS typically occurs in less than 2 weeks after the initial drug exposure. In our case, both the absence of hemolytic anemia and the patient's prior exposure to clopidogrel after his CABG in 2001 (with no clinical manifestation of TTP-HUS) made clopidogrel an unlikely precipitant for thrombocytopenia.
On the basis of multiple factors—clinical presentation, acuity of onset, severity of thrombocytopenia, review of the peripheral blood smear, multiple negative heparin-PF4A tests, and the subsequent re-exposure to heparin and clopidogrel without the recurrence of thrombocytopenia or thrombosis—it is likely that eptifibatide was responsible for the combination of severe thrombocytopenia, thrombosis, and DIC in this case presented here. By inhibiting the final pathway of platelet activation, GP IIb/IIIa antagonists provide a significant death and re-infarction benefit in the treatment of ACS, and as adjunctive therapy during PCI.2,20,21 This clinical benefit, however, must be weighed against the risk of adverse events, including bleeding and thrombocytopenia.22 Rates of GP IIb/IIIa inhibitor-induced thrombocytopenia after initial exposure vary from 0.7% to 2%. After re-exposure, thrombocytopenia event rates have been reported to be as high as 4.6%.11,23,24 Abciximib, unlike eptifibatide and tirofiban, is a large chimeric monoclonal antibody, which directly inhibits GP IIb/IIIa binding to fibrinogen and probably results in the higher rates of thrombocytopenia than do eptifibatide and tirofiban. Because eptifibatide and tirofiban act by binding to recognition sites on the activated GP IIb/IIIa receptor, they are believed to have less propensity for generating an antibody-mediated response.20,25,26
Early clinical trials did not reveal a statistically significant association between eptifibatide and the development of thrombocytopenia.1,2,27 Since the publication of these trials, 13 case reports (encompassing 21 patients) have suggested that the administration of eptifibatide is associated with the development of thrombocytopenia.8,9,28–38 Previous eptifibatide exposure was noted in 7 of the 13 case reports. Three cases involved acute severe thrombocytopenia (platelet count, <50 × 103/mm3 in 24 hr). In correspondence with our patient's presentation, the average platelet nadir among the 21 patients was 13.8 × 103/mm3, and the average time to platelet nadir was 8.2 hours. One case report35 implicated the association of thrombocytopenia with thrombosis and the administration of eptifibatide.
The pathophysiology of eptifibatide-induced thrombocytopenia has not been fully explained. Although the rapid onset suggests a non-immune-related reaction, recent research supports an immune-mediated reaction through the presence of neoantigen-induced drug-dependent antibodies specific for eptifibatide-occupied GP IIb/IIIa receptor sites.39 It is of interest that these antibodies have been observed in the serum of patients not previously exposed to eptifibatide. Typically, eptifibatide antibodies bind to the platelet surface without inducing platelet activation. However, through serum analysis (as described in the only other documented case report of eptifibatide-induced thrombocytopenia and thrombosis), Gao and colleagues40 were able to identify a subset of eptifibatide antibodies that bind to antigens with a topographic orientation that elicits platelet activation and subsequent thrombosis. To date, the occurrence of eptifibatide-induced thrombocytopenia with thrombosis has never been reported in any major clinical trial. The existence of only 1 similar published report (that of Gao) suggests that our patient's clinical presentation is exceedingly rare.
The presence of DIC in this case is notable, because eptifibatide-induced thrombocytopenia with thrombosis and DIC has never before (to our knowledge) been reported. Disseminated intravascular coagulation is a systemic process caused by the pathologic generation of thrombin in combination with suppression of the fibrinolytic system. The widespread deposition of fibrin often compromises blood supply to various organs and thus contributes to multiple-organ failure.41 Concurrent consumption of coagulation proteins and platelets can induce severe bleeding. Typical precipitants of DIC include sepsis, burns, amniotic fluid embolism, abruptio placentae, trauma, reactions to toxins (snake venom, amphetamines), acute leukemia, and adenocarcinoma.42 These conditions share the ability to induce systemic activation of coagulation either by activating cytokines as part of a systemic inflammatory response or by causing the release of, or exposure to, procoagulant substances. Although this recurrence has never been clinically described, GP IIb/IIIa inhibitors have been shown in the laboratory to activate similar coagulation cytokines, and they could, in theory, result in the induction of DIC.43,44 In addition, GP IIb/IIIa antagonist antibodies have been shown to suppress the fibrinolytic system through the binding and cross-bridging of fibrinogren and to increase the proinflammatory generation of thrombin. This results in a paradoxical bleeding-prothrombotic state,45 clinically recognized as the constellation of thrombocytopenia, thrombosis, and bleeding. No single laboratory test can establish the diagnosis of DIC. The diagnosis takes into account the clinical presentation and the laboratory data of low platelet counts, poor platelet survival after platelet transfusion, elevated levels of prothrombin time, activated partial thromboplastin time, and D-dimer, and/or low levels of fibrinogen. The diagnosis of DIC should encompass both clinical and laboratory information and is often difficult. The International Society on Thrombosis and Haemostasis (ISTH) DIC scoring system was therefore constructed to provide objective measurement of DIC. The scoring system correlates with key clinical observations and outcomes and is the primary means for the definitive diagnosis of DIC.46 Our patient met the ISTH diagnostic criteria for DIC with noted elevations in prothrombin time, activated partial thromboplastin time, and D-dimer, and with low fibrinogen levels (Fig. 2).46 Given the temporal relationship between the onset of thrombocytopenia and DIC in the absence of other identified precipitants of DIC, it is plausible that the 2 events were related and were associated with the administration of eptifibatide. The cornerstone of the successful treatment of DIC is treatment of the underlying disorder. In many cases (as this presentation shows), DIC will resolve spontaneously when the underlying disorder is properly managed. Guidelines suggest that additional supportive treatment (directed at the specific coagulation abnormalities) might be needed and that this should be evaluated on the basis of the individual case.46,47
Conclusion
This case is unique in that (to the best of our knowledge) no case of eptifibatide-induced thrombocytopenia with the constellation of thrombosis and DIC has been previously reported. It highlights the importance of routinely obtaining platelet counts at baseline and within 2 to 6 hours after the administration of eptifibatide. Further investigation to determine the incidence and pathophysiology of eptifibatide-induced thrombocytopenia with thrombosis and DIC is warranted.
Footnotes
Address for reprints: Michael W. Tempelhof, MD, 676 N. Saint Clair St., Ste. 600, Chicago, IL 60611-2981
E-mail: m-tempelhof@md.northwestern.edu
References
- 1.Tcheng JE. Impact of eptifibatide on early ischemic events in acute ischemic coronary syndromes: a review of the IMPACT II trial. Integrilin to Minimize Platelet Aggregation and Coronary Thrombosis. Am J Cardiol 1997;80(4A):21B-28B. [DOI] [PubMed]
- 2.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med 1998;339(7):436–43. [DOI] [PubMed]
- 3.ESPRIT Investigators. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): a randomised, placebo-controlled trial [published erratum appears in Lancet 2001;357(9265):1370]. Lancet 2000;356(9247): 2037–44. [DOI] [PubMed]
- 4.ADVANCE MI Investigators. Facilitated percutaneous coronary intervention for acute ST-segment elevation myocardial infarction: results from the prematurely terminated ADdressing the Value of facilitated ANgioplasty after Combination therapy or Eptifibatide monotherapy in acute Myocardial Infarction (ADVANCE MI) trial [published erratum appears in Am Heart J 2005;150(3):391]. Am Heart J 2005;150(1):116–22. [DOI] [PubMed]
- 5.Alexander KP, Chen AY, Roe MT, Newby LK, Gibson CM, Allen-LaPointe NM, et al. Excess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromes [published erratum appears in JAMA 2006;295(6):628]. JAMA 2005;294(24):3108–16. [DOI] [PubMed]
- 6.Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 2009;360(21):2176–90. [DOI] [PubMed]
- 7.McClure MW, Berkowitz SD, Sparapani R, Tuttle R, Kleiman NS, Berdan LG, et al. Clinical significance of thrombocytopenia during a non-ST-elevation acute coronary syndrome. The platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial experience. Circulation 1999;99(22):2892–900. [DOI] [PubMed]
- 8.Hongo RH, Brent BN. Association of eptifibatide and acute profound thrombocytopenia. Am J Cardiol 2001;88(4):428–31. [DOI] [PubMed]
- 9.Tanaka KA, Vega JD, Kelly AB, Hanson SR, Levy JH. Eptifibatide-induced thrombocytopenia and coronary bypass operation. J Thromb Haemost 2003;1(2):392–4. [DOI] [PubMed]
- 10.Suleiman M, Gruberg L, Hammerman H, Aronson D, Halabi M, Goldberg A, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, eptifibatide and abciximab: outcomes, complications and thrombocytopenia during percutaneous coronary intervention. J Invasive Cardiol 2003;15(6):319–23. [PubMed]
- 11.Fahdi IE, Saucedo JF, Hennebry T, Ghani M, Sadanandan S, Garza-Arreola L. Incidence and time course of thrombocytopenia with abciximab and eptifibatide in patients undergoing percutaneous coronary intervention. Am J Cardiol 2004;93 (4):453–5. [DOI] [PubMed]
- 12.Berkowitz SD, Harrington RA, Rund MM, Tcheng JE. Acute profound thrombocytopenia after C7E3 Fab (abciximab) therapy. Circulation 1997;95(4):809–13. [DOI] [PubMed]
- 13.Sane DC, Damaraju LV, Topol EJ, Cabot CF, Mascelli MA, Harrington RA, et al. Occurrence and clinical significance of pseudothrombocytopenia during abciximab therapy. J Am Coll Cardiol 2000;36(1):75–83. [DOI] [PubMed]
- 14.Warkentin TE, Greinacher A, Koster A, Lincoff AM; American College of Chest Physicians. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008;133(6 Suppl):340S-380S. [DOI] [PubMed]
- 15.Dager WE, Dougherty JA, Nguyen PH, Militello MA, Smythe MA. Heparin-induced thrombocytopenia: treatment options and special considerations. Pharmacotherapy 2007;27 (4):564–87. [DOI] [PubMed]
- 16.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med 2001; 135(7):502–6. [DOI] [PubMed]
- 17.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy [published erratum appears in Chest 2005;127(1): 416]. Chest 2004;126(3 Suppl):311S-337S. [DOI] [PubMed]
- 18.Kilickiran Avci B, Oto A, Ozcebe O. Thrombocytopenia associated with antithrombotic therapy in patients with cardiovascular diseases: diagnosis and treatment. Am J Cardiovasc Drugs 2008;8(5):327–39. [DOI] [PubMed]
- 19.Bennett CL, Kim B, Zakarija A, Bandarenko N, Pandey DK, Buffie CG, et al. Two mechanistic pathways for thienopyridine-associated thrombotic thrombocytopenic purpura: a report from the SERF-TTP Research Group and the RADAR Project. J Am Coll Cardiol 2007;50(12):1138–43. [DOI] [PMC free article] [PubMed]
- 20.Pytela R, Pierschbacher MD, Ginsberg MH, Plow EF, Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp–specific adhesion receptors. Science 1986;231(4745):1559–62. [DOI] [PubMed]
- 21.Topol EJ, Ferguson JJ, Weisman HF, Tcheng JE, Ellis SG, Kleiman NS, et al. Long-term protection from myocardial ischemic events in a randomized trial of brief integrin beta3 blockade with percutaneous coronary intervention. EPIC Investigator Group. Evaluation of Platelet IIb/IIIa Inhibition for Prevention of Ischemic Complication. JAMA 1997;278(6): 479–84. [DOI] [PubMed]
- 22.Aster RH. Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest 2005;127(2 Suppl):53S-59S. [DOI] [PubMed]
- 23.Tcheng JE, Kereiakes DJ, Lincoff AM, George BS, Kleiman NS, Sane DC, et al. Abciximab readministration: results of the ReoPro Readministration Registry. Circulation 2001;104 (8):870–5. [DOI] [PubMed]
- 24.Jubelirer SJ, Koenig BA, Bates MC. Acute profound thrombocytopenia following C7E3 Fab (abciximab) therapy: case reports, review of the literature and implications for therapy. Am J Hematol 1999;61(3):205–8. [DOI] [PubMed]
- 25.Scarborough RM, Rose JW, Hsu MA, Phillips DR, Fried VA, Campbell AM, et al. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem 1991;266(15):9359–62. [PubMed]
- 26.Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med 2001;344(25):1888–94. [DOI] [PubMed]
- 27.O'Shea JC, Hafley GE, Greenberg S, Hasselblad V, Lorenz TJ, Kitt MM, et al. Platelet glycoprotein IIb/IIIa integrin blockade with eptifibatide in coronary stent intervention: the ESPRIT trial: a randomized controlled trial. JAMA 2001;285 (19):2468–73. [DOI] [PubMed]
- 28.Yoder M, Edwards RF. Reversible thrombocytopenia associated with eptifibatide. Ann Pharmacother 2002;36(4):628–30. [DOI] [PubMed]
- 29.Nagge J, Jackevicius C, Dzavik V, Ross JR, Seidelin P. Acute profound thrombocytopenia associated with eptifibatide therapy. Pharmacotherapy 2003;23(3):374–9. [DOI] [PubMed]
- 30.Morel O, Jesel L, Chauvin M, Freyssinet JM, Toti F. Eptifibatide-induced thrombocytopenia and circulating procoagulant platelet-derived microparticles in a patient with acute coronary syndrome. J Thromb Haemost 2003;1(12):2685–7. [DOI] [PubMed]
- 31.Rezkalla SH, Hayes JJ, Curtis BR, Aster RH. Eptifibatide-induced acute profound thrombocytopenia presenting as refractory hypotension. Catheter Cardiovasc Interv 2003;58(1): 76–9. [DOI] [PubMed]
- 32.Salengro E, Mulvihill NT, Farah B. Acute profound thrombocytopenia after use of eptifibatide for coronary stenting. Catheter Cardiovasc Interv 2003;58(1):73–5. [DOI] [PubMed]
- 33.Coons JC, Barcelona RA, Freedy T, Hagerty MF. Eptifibatide-associated acute, profound thrombocytopenia. Ann Pharmacother 2005;39(2):368–72. [DOI] [PubMed]
- 34.Cheema AA, Teklinski AH, Maria V, Chilukuri K, Frank JJ, Gosselin MO. Recurrent acute profound thrombocytopenia related to readministration of eptifibatide. J Interv Cardiol 2006;19(1):99–103. [DOI] [PubMed]
- 35.Epelman S, Nair D, Downey R, Militello M, Askari AT. Eptifibatide-induced thrombocytopenia and thrombosis. J Thromb Thrombolysis 2006;22(2):151–4. [DOI] [PubMed]
- 36.Paradiso-Hardy FL, Madan M, Radhakrishnan S, Hurden S, Cohen EA. Severe thrombocytopenia possibly related to readministration of eptifibatide. Catheter Cardiovasc Interv 2001;54(1):63–7. [DOI] [PubMed]
- 37.Khaykin Y, Paradiso-Hardy FL, Madan M. Acute thrombocytopenia associated with eptifibatide therapy. Can J Cardiol 2003;19(7):797–801. [PubMed]
- 38.Onitilo AA. Delayed profound thrombocytopenia associated with eptifibatide. Am J Hematol 2006;81(12):984. [DOI] [PubMed]
- 39.Bougie DW, Wilker PR, Wuitschick ED, Curtis BR, Malik M, Levine S, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood 2002;100 (6):2071–6. [PubMed]
- 40.Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, et al. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcgammaRIIa and the integrin beta3 cytoplasmic domain. J Clin Invest 2009;119(3):504–11. [DOI] [PMC free article] [PubMed]
- 41.Suffredini AF, Harpel PC, Parrillo JE. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med 1989;320(18):1165–72. [DOI] [PubMed]
- 42.Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med 1999;341(8):586–92. [DOI] [PubMed]
- 43.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol 2009;85(2):195–204. [DOI] [PubMed]
- 44.Neumann FJ, Zohlnhofer D, Fakhoury L, Ott I, Gawaz M, Schomig A. Effect of glycoprotein IIb/IIIa receptor blockade on platelet-leukocyte interaction and surface expression of the leukocyte integrin Mac-1 in acute myocardial infarction. J Am Coll Cardiol 1999;34(5):1420–6. [DOI] [PubMed]
- 45.Kereiakes DJ. Effects of GP IIb/IIIa inhibitors on vascular inflammation, coronary microcirculation, and platelet function. Rev Cardiovasc Med 2006;7 Suppl 4:S3–11. [PubMed]
- 46.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 2009;145(1):24–33. [DOI] [PubMed]
- 47.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care 2006;10(4):222. [DOI] [PMC free article] [PubMed]