Abstract
Colorectal cancer rarely metastasizes to the heart. In the world medical literature, we identified only 7 cases of well-documented colorectal cancer metastasis to the right atrium. Herein, we describe the case of a 72-year-old man in whom metastatic mucinous adenocarcinoma of the colon involved the right atrium and caused superior vena cava syndrome. To our knowledge, this is the first case report of sudden cardiac death due to embolization of metastatic colon cancer from the right atrium. We also present the first comprehensive case series review of this rare entity.
Given improvements in diagnostic and therapeutic methods that have increased the longevity of many cancer patients, the detection of cardiac metastases is likely to increase in frequency. Accordingly, we recommend that previously asymptomatic cancer patients with a history of colorectal cancer who develop cardiac symptoms undergo prompt investigation for possible cardiac metastasis.
Key words: Adenocarcinoma, mucinous/secondary; colonic neoplasms/pathology; diagnosis, differential; heart atria/pathology; neoplasms/diagnosis/secondary; superior vena cava syndrome/pathology
Tumors involving the heart are more commonly metastatic than primary, and the prognosis for patients with metastatic cardiac tumors is typically poor.1 Although clinical recognition of cardiac metastasis from colorectal cancer is rare and continues to present a diagnostic and therapeutic challenge, this neoplasm should be considered among the broad differential of cardiac intracavitary masses. Herein, we report a rare case of mucinous adenocarcinoma of the colon that metastasized to the right atrium (RA) and resulted in superior vena cava syndrome. We also present a comprehensive review of this rare entity, including pertinent diagnostic methods.
Case Report
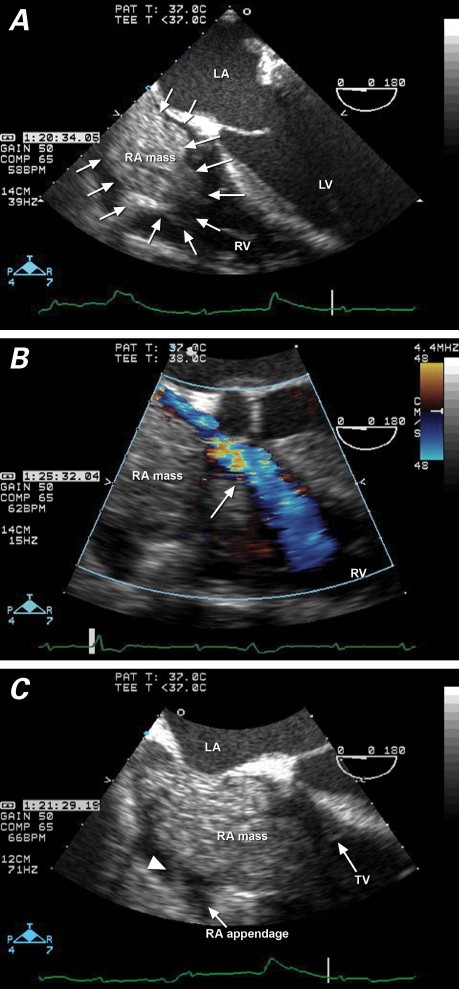
In 2007, a 72-year-old man was transferred to our hospital for surgical treatment of a newly identified RA mass. In 2004, he had undergone hemicolectomy of a T4b N2b M0 mucinous adenocarcinoma of the colon. His medical history included myocardial infarctions, treated with coronary artery bypass grafting; ischemic cardiomyopathy, treated with a prophylactic implantable cardioverter-defibrillator (ICD); lower-extremity peripheral arterial disease, treated with bypass surgery; and type 2 diabetes mellitus, hypertension, hyperlipidemia, and chronic anemia. The patient was admitted to another hospital for evaluation and treatment of a nonhealing right-foot ulcer. During hospitalization, he had mild shortness of breath and facial swelling; however, he had no fever, chest pain, or rash. Transthoracic echocardiography (TTE) revealed an RA mass that prolapsed through the tricuspid valve into the right ventricle. Transesophageal echocardiography (TEE) confirmed the presence of a heterogeneous, lobulated mass attached to the RA by a pedicle (Fig. 1). The mass bore little resemblance to a thrombus, and no other mass, vegetation, or cardiac shunt was detected. Upon transfer to our hospital, the patient remained dyspneic. Infectious process was excluded with serial negative blood, urine, and wound cultures. Duplex Doppler ultrasonography of the lower extremities revealed no deep vein thrombosis. Chest computed tomography (CT) showed multiple bilateral pulmonary nodules of up to 1 cm in size, raising the suspicion of metastasis. Surgical resection of the RA mass was scheduled.
Fig. 1 Transesophageal echocardiography in a patient with superior vena cava syndrome shows A) a mass filling the entire right atrium (RA) and protruding through the tricuspid valve (4-chamber view); B) restricted venous return (arrow) around the RA mass into the right ventricle (RV) (color-flow Doppler), and C) attachment by pedicle (arrowhead) of the RA mass to the RA free wall. LA = left atrium; LV = left ventricle; TV = tricuspid valve
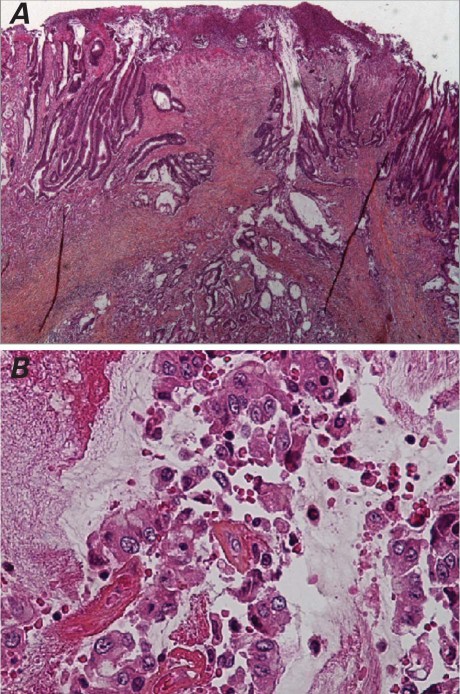
On the evening before the planned excision of the mass, the patient was found on the bathroom floor, minimally responsive and pulseless, with ventricular tachycardia on telemetry. Advanced cardiac life support was initiated. His ICD fired 3 times. Subsequently, he developed electromechanical dissociation and was pronounced dead. Gross autopsy findings included a friable mass, composed of tan mucinous tissue admixed with blood; the mass was attached to the endocardial surface of the RA free wall by a fine stalk and extended into the superior vena cava. Microscopic pathologic examination of this mass confirmed metastatic mucinous adenocarcinoma that resembled the histologic appearance of his primary adenocarcinoma of the colon (Fig. 2). Multiple tumor emboli recovered from the bilateral pulmonary arteries were the proximate cause of the patient's sudden cardiac death.
Fig. 2 Photomicrographs (H & E) show A) an infiltrating, moderately to poorly differentiated primary colonic adenocarcinoma, with desmoplastic response in the bowel wall (orig. ×2); and B) a metastatic tumor thrombus in the right atrium, with scattered, preserved tumor cells floating in mucin and fibrin (orig. ×20).
Discussion
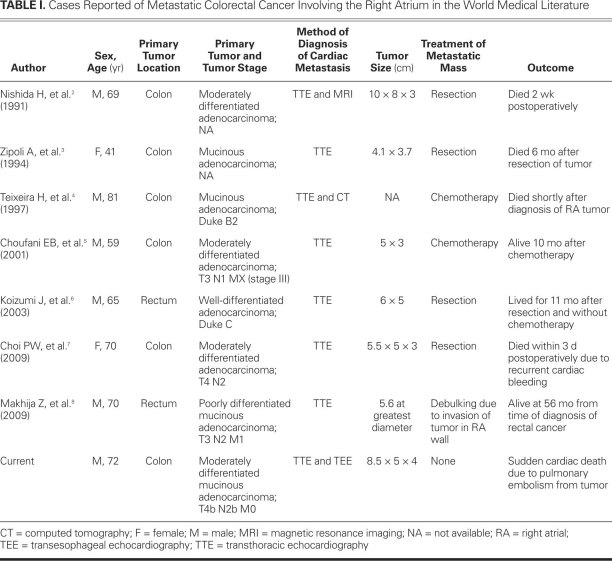
We found only 7 cases of well-documented metastatic colorectal cancer that had involved the RA (Table I).2–8 To our knowledge, ours is the first reported case of sudden cardiac death due to embolization of metastatic colon cancer from the RA.
TABLE I. Cases Reported of Metastatic Colorectal Cancer Involving the Right Atrium in the World Medical Literature
Metastatic Cardiac Tumors
In autopsy series of cancer patients, metastatic cardiac tumors have a prevalence of 1.5% to 20%.1 The most common cancers with cardiac metastatic potential are melanoma, lymphoma, carcinomas of the lung, breast, and esophagus, and leukemia.1,9 The metastatic pathway of the primary tumor can be by the lymphatic system,10,11 bloodstream, transvenous extension, or direct contiguous extension.11 In decreasing order of frequency, metastatic cardiac tumors involve the pericardium, myocardium, epicardium, endocardium, and intracavitary regions.11 Right-sided cardiac involvement, mainly ventricular, is more common than left-sided involvement.10,12 Metastases to the valvular endocardium, known as marantic endocarditis, are rare and are associated with adenocarcinomas and hypercoagulable states.13,14 The clinical manifestations of cardiac metastases are protean and depend on the location and size of the metastatic tumor; however, extensive involvement may occur with few or no symptoms11—and more than 90% of metastatic cardiac tumors are clinically silent.1
The lymph nodes, liver, and lungs are the most common sites of metastases from colorectal cancer. Colorectal cancer rarely metastasizes to the heart (in autopsy series, the prevalence is 1.4%–2% vs 23%–31% for bronchogenic carcinomas) and most commonly involves the pericardium.5
Review of Reported Cases
From the world medical literature, we identified only 7 cases of well-documented metastatic colorectal cancer that involved the RA.2–8 Similar to our case, metastatic involvement of the RA from colorectal cancer was found predominantly in men aged 65 to 72 years. The mass in the RA was diagnosed as early as 8 months2 and up to 5 years4 after treatment of the primary tumor. Choi and colleagues7 reported a case in which primary colon cancer and RA involvement were diagnosed concurrently. In most of these reported cases, the metastatic adenocarcinomas were of moderately differentiated histology. The primary tumor in 3 cases,3,4,8 as in ours, was mucinous adenocarcinoma; characterization was not specified in the other reports. Of note, in about half of the cases, isolated RA involvement was the only clinical evidence of metastasis.2,3,6,7
The clinical manifestations of RA involvement from metastatic colorectal cancer are nonspecific and include symptoms of right-sided heart failure,4 shortness of breath, arrhythmias, pulmonary thromboembolism, and—as in our patient—superior vena cava syndrome.2 Differential diagnosis is broad and includes thrombus, vegetation, or primary tumors such as myxoma. As a tumor marker, the carcinoembryonic antigen assay (CEA) can be helpful in detecting recurrence of primary or metastatic disease; however, this test is nonspecific for the diagnosis of cardiac metastatic disease.5 In our patient, the CEA values were within normal range during serial follow-up after hemicolectomy, including the time of diagnosis of the RA mass. This can be explained by the mucinous nature of adenocarcinoma, which yields relatively low amounts of the tumor marker. The CEA levels were not specified in the other reports of mucinous adenocarcinoma.3,4,8
In all of the reported cases,2–8 the primary tumor was surgically treated at the time of diagnosis; however, only 3 patients underwent postoperative adjuvant chemotherapy.5,6,8 In 2 of those patients, the RA mass was found at 6 months6 and 16 months5 after the discontinuation of chemotherapy; in the 3rd instance,8 the RA mass was detected while the patient was undergoing chemotherapy. Our patient had refused further chemotherapy after 3 cycles due to intolerable side effects, and the mass was discovered within 3 years after his hemicolectomy.
Chemotherapy treatment of the RA mass was attempted in 2 patients4,5; in the younger patient, a favorable response was evidenced by the resolution of the mass.5 In the other instance, chemotherapy was discontinued due to disease progression, and the patient died shortly thereafter.4 Surgical resection was attempted in 4 cases of isolated solitary RA mass, and debulking was successful in a circumstance of RA wall invasion by the tumor.8 The tumor was successfully excised in 2 patients.3,6 A 3rd patient could not be weaned from mechanical ventilation and died postoperatively as a result of presumed pulmonary embolization of the tumor,2 and a 4th patient died due to postoperative recurrent cardiac bleeding.7 Of note, we learned that the patient who underwent debulking8 was still alive with the aid of chemotherapy 56 months after the diagnosis of the RA mass. We had advised our patient to undergo surgical removal of the large RA mass, to alleviate superior vena cava syndrome and prevent impending massive pulmonary embolism.
Diagnostic Imaging Methods
On TTE, the characteristics of RA masses are variable and may be homogeneous2,4 or heterogeneous5,7 with variable mobility and echogenicity. Contrast-enhanced echocardiography with microbubble agents has been found to be useful in differentiating malignant tumor from benign tumor or avascular thrombus.15 Typically, the increased vascularity of malignant tumor enhances preferentially more than does the surrounding myocardium. More recently, 3-dimensional TTE has become the diagnostic method of choice for the characterization of cardiac metastases.16 Of note, the use of TEE was not described in any of the other reports.
Transesophageal echocardiography can distinguish among solid, liquid, and hemorrhagic lesions, and can be useful in confirming metastatic disease. In our patient, TEE revealed a lobulated, multicystic mass with a clearly delineated pedicle that was attached to the RA free wall (Fig. 1C). In light of the negative serial blood cultures and chest CT description of bilateral pulmonary nodular masses, the TEE findings suggested metastatic disease. Compared with echocardiography, contrast-enhanced CT or cardiovascular magnetic resonance (CMR) will provide a broader field of view for the evaluation of cardiothoracic disease, and the contrast resolution of CMR enables the clear distinction of a tumor from myocardium.16 Both are useful to diagnose a mass noninvasively on the basis of enhancement and tissue density.2,4,16 Enhancement of the RA mass after the injection of intravenous contrast agents distinguishes a tumor from a thrombus: nonnecrotic tumors typically enhance, whereas thrombus does not.
Conclusion
Metastatic involvement of the RA from colorectal cancer is rarely reported. Because improved diagnostic and therapeutic methods have extended the lives of many cancer patients, the detection of cardiac metastases is likely to increase in the future. Any patient with a history of colorectal cancer who presents with cardiopulmonary symptoms should undergo prompt evaluation for possible cardiac metastasis. A multidisciplinary approach involving cardiologists, oncologists, and cardiothoracic surgeons is crucial for deciding the best therapeutic regimen for such patients. We believe that an isolated RA mass in the absence of other metastases has important therapeutic implications for adjunctive surgical treatment. On the basis of the current incidence and prevalence of colorectal cancer, we postulate that a multicenter registry would provide more conclusive recommendations for the best diagnostic and treatment approaches in this unusual subgroup of patients.
Acknowledgments
We thank Dr. Nino Marino for his translations of Italian medical literature into English, and Dr. Antonio Mendoza-Ladd for his translations of Portuguese medical literature into English.
Footnotes
Address for reprints: Marc A. Nolan, MD, FACC, 90 East End Ave., New York, NY 10028
E-mail: mnolan.pma@gmail.com
Dr. Patel is now with the Division of Cardiology, Department of Internal Medicine, Advocate Lutheran General Hospital, Park Ridge, Illinois. Dr. Nolan is now with the Division of Cardiology, Department of Medicine, New York University, New York, New York.
References
- 1.Al-Mamgani A, Baartman L, Baaijens M, de Pree I, Incrocci L, Levendag PC. Cardiac metastases. Int J Clin Oncol 2008; 13(4):369–72. [DOI] [PubMed]
- 2.Nishida H, Grooters RK, Coster D, Soltanzadeh H, Thieman KC. Metastatic right atrial tumor in colon cancer with superior vena cava syndrome and tricuspid obstruction. Heart Vessels 1991;6(2):125–7. [DOI] [PubMed]
- 3.Zipoli A, Bartoli P, Fradella G, Sansoni M, Brandinelli A, Mazza F, Ieri A. Right atrial metastasis as an initial clinical manifestation of adenocarcinoma of the colon [in Italian]. Ann Ital Med Int 1994;9(3):150–2. [PubMed]
- 4.Teixeira H, Timoteo T, Marcao I. Cardiac metastases from a colonic tumor [in Portuguese]. Acta Med Port 1997;10(4): 331–4. [PubMed]
- 5.Choufani EB, Lazar HL, Hartshorn KL. Two unusual sites of colon cancer metastases and a rare thyroid lymphoma. Case 2. Chemotherapy-responsive right atrial metastasis from colon carcinoma. J Clin Oncol 2001;19(15):3574–5. [DOI] [PubMed]
- 6.Koizumi J, Agematsu K, Ohkado A, Shiikawa A, Uchida T. Solitary cardiac metastasis of rectal adenocarcinoma. Jpn J Thorac Cardiovasc Surg 2003;51(7):330–2. [DOI] [PubMed]
- 7.Choi PW, Kim CN, Chang SH, Chang WI, Kim CY, Choi HM. Cardiac metastasis from colorectal cancer: a case report. World J Gastroenterol 2009;15(21):2675–8. [DOI] [PMC free article] [PubMed]
- 8.Makhija Z, Deshpande R, Desai J. Unusual tumours of the heart: diagnostic and prognostic implications. J Cardiothorac Surg 2009;4:4. [DOI] [PMC free article] [PubMed]
- 9.Burke A, Virmani R. Tumors of the heart and great vessels. Fascicle 16, 3rd Series. In: Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1996.
- 10.Lymburner RM. Tumours of the heart: histopathological and clinical study. Can Med Assoc J 1934;39(4):368–73. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC403303/pdf/canmedaj00142-0016.pdf [cited 2012 Jan 17]. [PMC free article] [PubMed]
- 11.Hanfling SM. Metastatic cancer to the heart. Review of the literature and report of 127 cases. Circulation 1960;22(3):474–83. [DOI] [PubMed]
- 12.Prichard RW. Tumors of the heart; review of the subject and report of 150 cases. AMA Arch Pathol 1951;51(1):98–128. [PubMed]
- 13.Blanchard DG, Ross RS, Dittrich HC. Nonbacterial thrombotic endocarditis. Assessment by transesophageal echocardiography. Chest 1992;102(3):954–6. [DOI] [PubMed]
- 14.Lopez JA, Fishbein MC, Siegel RJ. Echocardiographic features of nonbacterial thrombotic endocarditis. Am J Cardiol 1987;59(5):478–80. [DOI] [PubMed]
- 15.Yelamanchili P, Wanat FE, Knezevic D, Nanda NC, Patel V. Two-dimensional transthoracic contrast echocardiographic assessment of metastatic left ventricular tumors. Echocardiography 2006;23(3):248–50. [DOI] [PubMed]
- 16.Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J 2011;38(3):261–2. [PMC free article] [PubMed]