Abstract
We sought to determine whether patients with precapillary pulmonary hypertension show elevated serum levels of prolactin (PRL) and its 16-kDa N-terminal fragment (16-kDa PRL) and whether there is any correlation to measures of prognosis.
Twenty-eight patients with idiopathic pulmonary artery hypertension, 15 with peripheral chronic thromboembolic pulmonary hypertension, and 4 with portopulmonary hypertension Child-Pugh class A were included. Our control subjects were 56 blood donors. Total prolactin was measured with an immunoluminometric assay. Antibodies against epitope C detected only the intact prolactin before it was split. The 16-kDa PRL was calculated from the difference between total and intact prolactin.
Prolactin was significantly (P=0.009) higher in the study group (median, 190 mU/L; interquartile range, 162 mU/L) than in the control group (median, 140 mU/L; interquartile range, 91 mU/L). The 16-kDa PRL was significantly elevated in the study group (P=0.046). Prolactin and 16-kDa PRL correlated inversely with the 6-minute-walk distance (P <0.01) and with peak oxygen uptake during exercise (P <0.005).
Serum levels of prolactin and 16-kDa PRL were significantly higher in patients with precapillary pulmonary hypertension and were inversely correlated with 6-minute-walk distance and peak oxygen uptake.
These results indicate that prolactin and 16-kDa PRL might play a role in the pathophysiology of precapillary pulmonary hypertension.
Key words: Chronic thromboembolic pulmonary hypertension; hyperprolactinemia/blood; hypertension, portal; hypertension, pulmonary; idiopathic pulmonary arterial hypertension; prolactin/blood/chemistry; prolactin 16-kDa fragment, human
Pulmonary artery hypertension (PAH) is a progressive disease with a high morbidity and mortality rate.1 The chief mechanism involved in the pathogenesis of PAH seems to be a dysfunction of the endothelial cells in the form of reduced vasodilatory and enhanced vasoconstrictive mediators. Robust evidence indicates that the 16-kilodalton N-terminal of prolactin (16-kDa PRL) and prolactin (PRL) are of importance in the pathophysiology of endothelial dysfunction,2,3 by different pathways. Previous studies in premenopausal hyperprolactinemic women suggested an association between hyperprolactinemia and endothelial dysfunction.4 An elevated proportion of 16-kDa PRL in serum during the first 10 weeks of pregnancy might play a role in the pathogenesis of preeclampsia.5 This fragment is split from the native PRL (which includes macroprolactin 150–170 kDa PRL, 23-kDa PRL, and 16-kDa PRL) by a cathepsin-like protease and reduction of the disulfide bond.6 Its capacity to bind to the PRL receptor is considerably reduced. Although 16-kDa PRL has significant bioactivity by itself, it contains only 6 of the 14 residues that are thought to participate in the coupling of the intact hormone to its receptor. The 16-kDa PRL downregulates inducible nitric oxide (NO)-synthase expression in endothelial cells, inhibits endothelial urokinase activity, and activates plasminogen activator inhibitor type I (PAI-1) expression.3 This could be relevant for patients with chronic thromboembolic pulmonary hypertension (CTEPH) because differences in the expression of PAI-1 are seen in thrombi of patients with CTEPH when comparison is made with thrombi of patients who have acute pulmonary embolism.7
There are hints of inflammatory and vasoproliferative processes in PAH and CTEPH. Intact human PRL has angiogenic activities8 by stimulation of expression of vascular endothelial growth factor, whereas the proteolytic 16-kDa fragment of native PRL inhibits the development of capillaries as shown in the corneas of rats.9 Proinflammatory cytokines can induce the expression of PRL receptors in pulmonary fibroblasts, and the stimulation of these receptors by PRL downregulates the NO synthase.10 Therefore, 16-kDa PRL might be involved in several pathogenetic mechanisms that seem to be crucial for the development of pulmonary hypertension.
The aim of this trial was to measure serum levels of PRL and 16-kDa PRL in patients with PAH or CTEPH and to evaluate any correlations to exercise capacity and the serum levels of N-terminal pro-brain natriuretic peptide (NT-proBNP).
Patients and Methods
Study Subjects and Study Design
From January 2007 through April 2008, serum samples were collected prospectively and consecutively from 47 patients with known PAH, including 32 with portopulmonary hypertension (PoPH) or idiopathic PAH, and 15 with CTEPH. The eligibility criteria were a proven diagnosis of idiopathic PAH, PoPH, or inoperable peripheral CTEPH in patients between the ages of 18 and 80 years. In the CTEPH patients, we included only those with peripheral CTEPH, in order to limit the study to patients with precapillary pulmonary hypertension due to distal small-vessel vasculopathy. Diagnosis was performed in accordance with the diagnostic criteria defined by the Fourth World Symposium on Pulmonary Hypertension 2008.11,12 Other forms of pulmonary hypertension were excluded by echocardiography and right-sided heart catheterization (for left-sided heart disease and congenital systemic-to-pulmonary shunts), computed tomography of the lung and pulmonary function studies (for lung disease), and serologic testing (for connective-tissue disease and human immunodeficiency virus infection), according to the European guidelines for the diagnosis and treatment of pulmonary hypertension.13 Lung scintigraphy with a ventilation/perfusion scan was performed in each patient to distinguish PAH from CTEPH.
In patients, we performed right-sided heart catheterization independently of the study within 11 ± 7 months (range, 0–33 mo) of blood-sample collection. Our control group of 56 healthy blood donors had no known cardiovascular diseases and was taking no medication.
Predetermined exclusion criteria for the patients were the following: pregnancy; lactation; macro- or microprolactinoma; diseases of the pituitary gland; and medication with neuroleptic agents, antidepressants, or substances with dopaminergic or antidopaminergic effects (for example, lisuride, methysergide, and ergotamine). Patients with known risk factors for hyperprolactinemia, such as hypothyroidism and moderate-to-severe hepatic cirrhosis (Child-Pugh classes B and C), or who regularly consumed alcohol or nicotine, were also excluded from the study.
The study protocol was approved by the local ethics committee, and written informed consent was obtained from the participants.
In the case group, the following data were collected on the day that blood was drawn: serum levels of PRL and 16-kDa PRL, serum levels of NT-proBNP, 6-minute walking distance (6-MWT), ergospirometric values like peak oxygen uptake (VO2max), and those obtained by transthoracic echocardiography. The 6-MWT was performed (to evaluate submaximal levels of functional capacity) according to American Thoracic Society recommendations, and cardiopulmonary exercise testing was performed (symptom-limited) on a cycle ergometer, following a standardized protocol.1,14 In regard to echocardiographic measurements, we recorded right atrial diameter, right ventricular Tei (myocardial performance index), and right ventricular end-systolic pressure.
Methods
Immediately after the sample collection, measurement of PRL and 16-kDa PRL was performed by the LIAISON® automated analyzer (DiaSorin Deutschland GmbH; Dietzenbach, Germany).15
Total PRL (including macroprolactin 150–170 kDa, 23-kDa-PRL, and the 16-kDa PRL) was measured with a Conformité Européenne-certified kit (DiaSorin). This is a double-sided immunoluminometric assay kit (LIAISON-Prolactin®), with a measurement range from 10 to 8,000 mIU/L. The intra-assay variance was 2.6% ± 0.3%, and the inter-assay variance was 4.6% ± 1.2%. The lowest detection limit was less than 10 mIU/L (mean value + 2 standard deviations).15 Cross-reactivity with luteinizing hormone, follicle-stimulating hormone, thyroid-stimulating hormone, human chorionic gonadotropin, human growth hormone, and human placental lactogen was not found within a physiologic range of concentration. The recovery during admixture experiments was between 97% and 100%. Isoluminol was used as the reagent.
To detect only the intact molecule of PRL, mouse antibodies against epitope C of the human PRL (US Biological; Swampscott, Mass) were bound to magnetic particles coated with steptavidin by means of biotinylation. The final concentration was 80 µg of anti-PRL-epitope C-antibodies to 1.83 mg of magnetic particles in 1 mL of suspension.
The potential of macroprolactin inclusion was eliminated by polyethylene glycol precipitation. The proportion of 16-kDa PRL was calculated from the difference between the concentration of total PRL and the concentration of intact PRL, because possible contributions of other PRL metabolites are extremely small and would not influence the assay.15
For measurement of NT-proBNP, we used an electrochemoluminescence assay (Roche Diagnostics GmbH; Mannheim, Germany) with the biochemistry analyzer Modular PPE (Roche). The NT-proBNP was measured simultaneously with PRL and 16-kDa PRL.
Statistical Analysis
Analyses were performed with SPSS software, version 16.0 (IBM Corporation; Somers, NY). P values of less than 0.05 2-sided were considered statistically significant. The Student t test was used to determine differences in mean age and the Fisher exact test to determine sex differences. Median differences in the PRL and the 16-kDa PRL fragment between the study groups were analyzed by analysis of covariance, with age and sex as the covariates. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to check whether data had a normal or a skewed distribution; a skewed distribution was shown for NT-proBNP, PRL, and 16-kDa PRL. The associations between PRL and 16-kD PRL and measurements of disease severity were described by the Spearman rank correlation coefficient. Diagnostic value for PRL and 16-kDa PRL concerning the prediction of PAH was described by receiver operating characteristic (ROC) curve analysis after adjusting for sex and age.
Results
Of 47 patients with precapillary pulmonary hypertension who were recruited, 28 had idiopathic PAH, 15 CTEPH, and 4 PoPH (Table I). Most patients (90%) were in World Health Organization functional class III. Thirty of 32 patients with PAH and 5 of 10 patients with CTEPH were receiving PAH-specific therapy. Twenty-seven patients were on monotherapy with a phosphodiesterase V inhibitor (PDE-V-I: sildenafil, n=10), an endothelin receptor antagonist (ERA: bosentan, n=13 or sitaxentan, n=3) or a stimulator of cyclic guanylatecylase (riociguat, n=3). Six patients were on double combination therapy (3 patients: ERA plus PDE-V-I, 3 patients: ERA plus inhaled iloprost) and 5 patients were on triple therapy (ERA plus PDE-V-I plus either inhaled iloprost or subcutaneous treprostinil). Most patients had normal renal function; 1 CTEPH patient had an advanced state of chronic renal insufficiency (creatinine, 430 µmol/L), and 4 patients had elevated creatinine values (range, 120–180 µmol/L). Patients and control participants differed in mean age (P <0.0005) and in the proportion of males to females (P=0.003).
TABLE I. Data from the Study and Control Groups
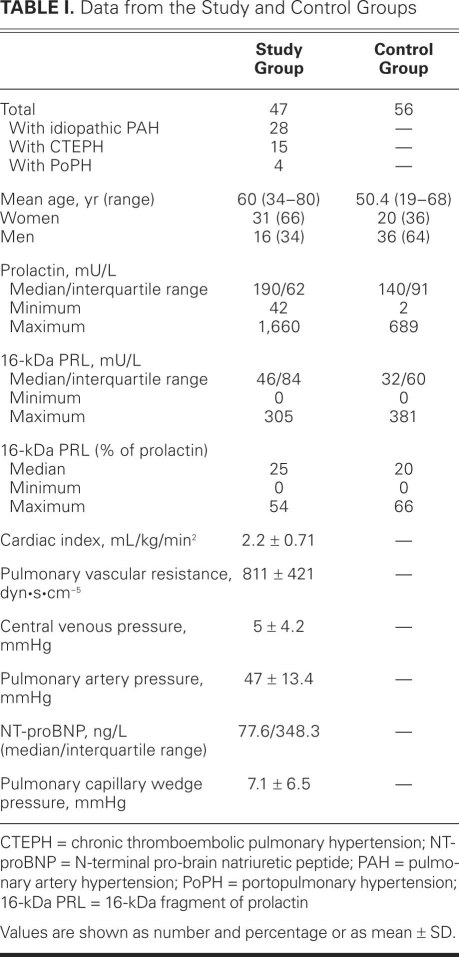
There was a significant correlation between the serum level of PRL and the level of 16-kDa PRL (Spearman rho correlation coefficient 0.789 in control participants and 0.841 in patients; P <0.01), both in the study and control groups. Increasing age was significantly correlated with lower levels of 16-kDa PRL (Spearman coefficient of correlation, −0.226; P=0.002). Age- and sex-adjusted values of native PRL among patients (median, 190 mU/L; interquartile range [IQR], 162 mU/L) were significantly higher (P=0.009) than among control participants (140 mU/L; IQR, 91 mU/L) (Fig. 1).
Fig. 1 Box plots show serum concentrations (median and inter-quartile range) of prolactin (mU/L) in the control and study groups. Four extremes for prolactin are not shown in the figure, due to the scaling.
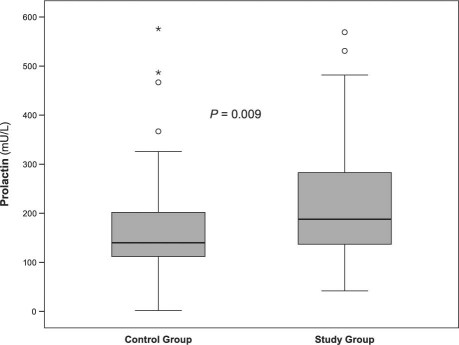
In the control group, the age- and sex-adjusted values of 16-kDa PRL (median, 32 mU/L; IQR, 60 mU/L) were significantly lower than in the patient group (median, 46 mU/L; IQR, 84 mU/L; P=0.046) (Fig. 2). The percentage of 16-kDa PRL in relation to total PRL in both groups did not show any significant difference.
Fig. 2 Box plots show serum concentrations (median and interquartile range) of 16-kDa prolactin (mU/L) in the control and study groups. Six extremes for 16-kDa prolactin are not shown in the figure due to the scaling.° = outliers; PRL = prolactin
In our study group, prolactin and 16-kDa PRL showed no significant correlation with NT-proBNP. However, prolactin as well as 16-kDa PRL correlated inversely to the 6-MWT distance (Spearman rho correlation coefficient, −0.418, P=0.005; and −0.339, P=0.026, respectively). Peak oxygen uptake (VO2 peak) during spiroergometry showed a negative correlation to PRL and 16-kDa PRL (Spearman rho coefficient of correlation, −0.491, P=0.001; and −0.413, P=0.005, respectively). We did not find any significant correlation between PRL or 16-kDa PRL and the hemodynamic values obtained by right-sided heart catheterization.
The normal range for PRL was defined as 86–324 mU/L for men and 102–496 mU/L for nonpregnant women. We found hyperprolactinemia in 4 (7.3%, 3 men and 1 woman) among the control participants and in 6 (13.3%, 5 women and 1 man, 3 with idiopathic PAH, 2 with CTEPH, 1 with PoPH) among the patients, but this was still without a significant difference (Fisher exact test).
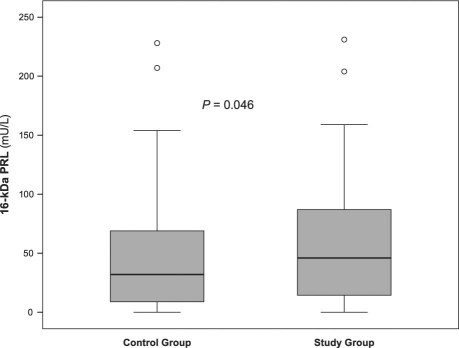
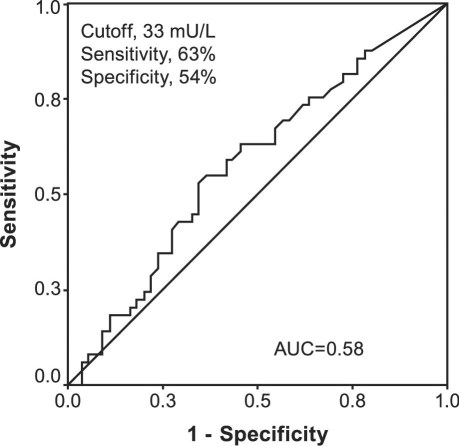
Measurement of PRL and 16-kDa PRL in serum displayed little diagnostic relevance for the prediction of pulmonary hypertension as shown by ROC curve analysis (areas under the curve, 0.66 for PRL and 0.58 for 16-kDa PRL) (Figs. 3 and 4). As determined by ROC curve analysis, a PRL cutoff value of 150 mU/L had a sensitivity of 69% and a specificity of 57%. For 16-kDa PRL, a cutoff value of 33 mU/L showed the best sensitivity (63%) and specificity (54%).
Fig. 3 Receiver operating characteristic curve predicts pulmonary hypertension by calculated serum prolactin level (>150 mU/L). AUC = area under the curve
Fig. 4 Receiver operating characteristic curve predicts pulmonary hypertension by calculated serum 16-kDa prolactin level (>33 mU/L). AUC = area under the curve
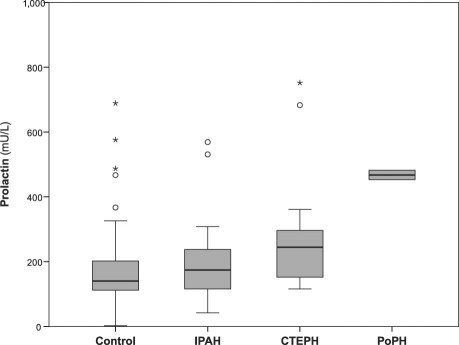
Subgroup Analysis. Subgroup analysis revealed a significant difference in PRL serum concentrations: elevated levels appeared in patients with CTEPH (P=0.005 in the Mann-Whitney U test) and with PoPH (P=0.024) (Fig. 5), in comparison with control participants. There was no significant difference in 16-kDa PRL concentrations when the control group was compared with any subgroup. We found no significant differences in PRL and 16-kDa PRL serum levels between the 3 subgroups of patients with idiopathic PAH, CTEPH, and PoPH. In patients with idiopathic PAH, we found a clear correlation between PRL and the right ventricular systolic pressure (Spearman rho coefficient of correlation, 0.389; P=0.04) and a robust inverse correlation with VO2 peak (Spearman rho, −0.569; P=0.002). In the CTEPH group, a robust inverse correlation with VO2 peak and 6-MWT could be detected (VO2 peak: Spearman rho = −0.779; P=0.002 and 6-MWT: Spearman rho = −0.7; P=0.008, respectively).
Fig. 5 Box plot shows median and interquartile range of serum levels of prolactin in pulmonary hypertension subgroups: control, idiopathic pulmonary artery hypertension (IPAH), chronic thromboembolic pulmonary hypertension (CTEPH), and portopulmonary hypertension (PoPH). Four extremes for prolactin are not shown in the figure due to the scaling.° = outliers; * = extremes
Discussion
We present the first data to show that serum levels of PRL are higher (but often within the normal range) in patients with precapillary pulmonary hypertension when compared with healthy control participants and that these levels correlate with such indicators of severity and prognosis of pulmonary hypertension as VO2 peak and 6-MWT.1 Serum levels of 16-kDa PRL were also significantly elevated in patients with precapillary PAH in comparison with our control group, but 16-kDa PRL normal serum values are not yet established. Only a small number of our patients showed pathologic hyperprolactinemia, and the difference from the control group was not significant. The serum levels of PRL were significantly elevated, especially in patients with CTEPH and PoPH; patients with idiopathic PAH tended to have elevated levels of PRL, but without significant difference from those of the control group.
To date, the pathophysiologic impact of PRL in patients with pulmonary hypertension is unknown. Endothelial dysfunction with an imbalance of vasoconstrictive and vasodilatory mediators plays an important role in developing pulmonary hypertension.
Robust evidence exists that 16-kDa PRL and PRL are important in the pathophysiology of endothelial dysfunction2,3 by different mechanisms; 16-kDa PRL downregulates inducible NO synthase expression in endothelial cells, inhibits endothelial urokinase activity, and activates PAI-1 expression.16 In vitro, infusion of PRL can cause regional vasoconstriction by the inhibition of a vasodilatory β2-adrenergic receptor-mediated effect.17 Impaired endothelial function measured by flow-mediated dilation on a brachial artery with high-resolution ultrasonography could be seen in women with hyperprolactinemia.4
There are hints of inflammatory processes in PAH and CTEPH. Proinflammatory cytokines can induce the expression of PRL receptors in pulmonary fibroblasts, and the stimulation of these receptors by PRL inhibits cytokine-induced NO production. Prolactin stimulates the synthesis of immunoglobulins and the production of anti-dsDNA antibodies and immunoglobulin M rheumatoid factors.18 This effect is more distinctive in physiologic than in supraphysiologic concentrations and could be in line with the observed normal range of PRL, especially in patients with idiopathic PAH. Proinflammatory cytokines can induce the expression of PRL receptors in pulmonary fibroblasts, thereby enabling PRL to inhibit cytokine-induced NO production and the expression of the inducible NO synthase (iNOS).10 In this manner, PRL and PRL receptors may play a modulating role during immune-inflammatory processes.
Intact human PRL has angiogenic activities, whereas 16-kDa PRL inhibits the development of capillaries, as shown in rat corneas.9 Whether there are similar effects on pulmonary capillaries is unknown to date, but this could be of interest in regard to a conceptual shift: understanding the pathogenesis of PAH as vasoproliferative remodeling, rather than a predominantly vasoconstrictive process.
Differences in the expression of PAI-1 in CTEPH thrombi (compared with thrombi seen in acute pulmonary embolism) have been shown in patients with CTEPH.7 Moreover, 16-kDa PRL can activate PAI-1.16
The intracellular transduction after activation of the PRL receptors is performed by a tyrosine kinase.19 In line with this, substances like imatinib, which have blocking effects on the activity of tyrosine kinase, are examined for the treatment of PAH.20
Underlining the possible relevance of prolactin in the pathophysiology of PAH, terguride, approved for ovulation disorders due to hyperprolactinemia,21,22 ameliorates monocrotaline-induced pulmonary hypertension in rats, as previously published.23 The animal monocrotaline precapillary pulmonary hypertension model is typically used to examine the pathophysiology of human PAH. Consequently, it might be speculated that prolactin is a factor involved in the pathogenesis of PAH.
Limitations. Our study was limited by the relatively small number of patients. The cohort of patients under study was quite inhomogeneous in regard to the underlying type of pulmonary hypertension. Patients with comorbid conditions and medication known to affect PRL levels were excluded from the study. However, because our patients received different PAH medications and co-medications (like diuretics), unknown interaction of medications and comorbidities with PRL levels cannot be ruled out.
In addition, the hemodynamic values obtained during right-sided heart catheterization were not measured at the time of drawing the blood samples. Furthermore, follow-up measurements of PRL in each individual patient were not performed but might be of interest to gain insights into the natural course of PRL concentration in patients with emerging pulmonary hypertension.
The impact of PRL in the pathogenesis of pulmonary hypertension is unknown, and our data describe only the association. In summary, PRL and 16-kDa PRL might be involved in several pathophysiologic processes that play a role in developing pulmonary hypertension and offer starting points for new treatments. Due to the fact that terguride,23 an approved drug for treatment of disorders due to hyperprolactinemia,21,22 ameliorates experimental precapillary pulmonary hypertension, one might speculate that PRL is a factor involved in the pathogenesis of PAH. Further studies are necessary to clarify the role of PRL.
Footnotes
Address for reprints: Uta Hönicke, Department of Pulmonology, University Hospital Carl Gustav Carus, Fetscherstr. 74, 01307 Dresden, Germany
E-mail: Uta.Hoenicke@uniklinikum-dresden.de
References
- 1.Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, et al. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation 2002;106(3):319–24. [DOI] [PubMed]
- 2.Gonzalez C, Corbacho AM, Eiserich JP, Garcia C, Lopez-Barrera F, Morales-Tlalpan V, et al. 16K-prolactin inhibits activation of endothelial nitric oxide synthase, intracellular calcium mobilization, and endothelium-dependent vasorelaxation. Endocrinology 2004;145(12):5714–22. [DOI] [PubMed]
- 3.Lee SH, Nishino M, Mazumdar T, Garcia GE, Galfione M, Lee FL, et al. 16-kDa prolactin down-regulates inducible nitric oxide synthase expression through inhibition of the signal transducer and activator of transcription 1/IFN regulatory factor-1 pathway. Cancer Res 2005;65(17):7984–92. [DOI] [PubMed]
- 4.Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gozu H, Sezgin O, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol 2003;149(3):187–93. [DOI] [PubMed]
- 5.Parra A, Ramirez-Peredo J. The possible role of prolactin in preeclampsia: 2001, a hypothesis revisited a quarter of century later. Med Hypotheses 2002;59(4):378–84. [DOI] [PubMed]
- 6.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80(4):1523–631. [DOI] [PubMed]
- 7.Lang I, Kerr K. Risk factors for chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc 2006;3(7):568–70. [DOI] [PubMed]
- 8.Goldhar AS, Vonderhaar BK, Trott JF, Hovey RC. Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol Cell Endocrinol 2005;232((1–2):9–19. [DOI] [PubMed]
- 9.Duenas Z, Torner L, Corbacho AM, Ochoa A, Gutierrez-Ospina G, Lopez-Barrera F, et al. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest Ophthalmol Vis Sci 1999;40(11): 2498–505. [PubMed]
- 10.Corbacho AM, Macotela Y, Nava G, Eiserich JP, Cross CE, Martinez de la Escalera G, Clapp C. Cytokine induction of prolactin receptors mediates prolactin inhibition of nitric oxide synthesis in pulmonary fibroblasts. FEBS Lett 2003; 544((1–3):171–5. [DOI] [PubMed]
- 11.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54(1 Suppl):S55–66. [DOI] [PubMed]
- 12.McGoon MD, Kane GC. Pulmonary hypertension: diagnosis and management [published erratum appears in Mayo Clin Proc 2009;84(4):386]. Mayo Clin Proc 2009;84(2):191–207. [DOI] [PMC free article] [PubMed]
- 13.Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European Respiratory Society (ERS), International Society of Heart and Lung Transplantation (ISHLT), Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34(6):1219–63. [DOI] [PubMed]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1):111–7. [DOI] [PubMed]
- 15.Kavanagh L, McKenna TJ, Fahie-Wilson MN, Gibney J, Smith TP. Specificity and clinical utility of methods for the detection of macroprolactin. Clin Chem 2006;52(7):1366–72. [DOI] [PubMed]
- 16.Lee H, Struman I, Clapp C, Martial J, Weiner RI. Inhibition of urokinase activity by the antiangiogenic factor 16K prolactin: activation of plasminogen activator inhibitor 1 expression. Endocrinology 1998;139(9):3696–703. [DOI] [PubMed]
- 17.Molinari C, Grossini E, Mary DA, Uberti F, Ghigo E, Ribichini F, et al. Prolactin induces regional vasoconstriction through the beta2-adrenergic and nitric oxide mechanisms. Endocrinology 2007;148(8):4080–90. [DOI] [PubMed]
- 18.Gutierrez MA, Molina JF, Jara LJ, Garcia C, Gutierrez-Urena S, Cuellar ML, et al. Prolactin-induced immunoglobulin and autoantibody production by peripheral blood mononuclear cells from systemic lupus erythematosus and normal individuals. Int Arch Allergy Immunol 1996;109(3):229–35. [DOI] [PubMed]
- 19.Swaminathan G, Varghese B, Thangavel C, Carbone CJ, Plotnikov A, Kumar KG, et al. Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J Endocrinol 2008; 196(2):R1–7. [DOI] [PubMed]
- 20.Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 2010;182(9):1171–7. [DOI] [PMC free article] [PubMed]
- 21.Mizokawa T, Akai T, Nakada Y, Yamaguchi M, Nakagawa H, Hasan S, et al. Terguride as a new anti-hyperprolactinemic agent: characterization in rats and dogs in comparison with bromocriptine. Jpn J Pharmacol 1993;63(3):269–78. [DOI] [PubMed]
- 22.Venturini PL, Horowski R, Valenzano M, Costantini S, Fasce V, Gorlero F, et al. Effect of terguride on prolactin levels in normal, puerperal and hyperprolactinaemic women. Eur J Clin Pharmacol 1986;30(2):195–7. [DOI] [PubMed]
- 23.Dumitrascu R, Kulcke C, Konigshoff M, Kouri F, Yang X, Morrell N, et al. Terguride ameliorates monocrotaline-induced pulmonary hypertension in rats. Eur Respir J 2011;37 (5):1104–18. [DOI] [PubMed]