Abstract
Citalopram is a selective serotonin reuptake inhibitor with a favorable cardiac-safety profile. Corrected QT interval (QTc) prolongation and cardiac arrhythmias have not been previously reported in association with citalopram use except in the presence of overdose, abnormal electrolyte values, or renal or liver failure. Herein, we report the case of a 40-year-old woman with mental depression who presented with a prolonged QTc interval and torsades de pointes after the initiation of citalopram at therapeutic doses. The QTc interval improved when citalopram therapy was discontinued. We recommend that clinicians investigate the family history for sudden deaths and perform baseline electrocardiography before prescribing citalopram. We also recommend routine electrocardiographic testing during citalopram therapy, and that patients with long QT syndrome avoid taking citalopram.
Key words: Citalopram/adverse effects/therapeutic use, depression/drug therapy, long QT syndrome/chemically induced/diagnosis/physiopathology, serotonin uptake inhibitors/adverse effects/therapeutic use, torsades de pointes/chemically induced/diagnosis/physiopathology
Citalopram is a selective serotonin reuptake inhibitor that has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of mental depression. Due to its lack of QT-interval–prolonging effects, citalopram has a better cardiac-safety profile than do tricyclic antidepressants (TCAs). Herein, we describe the case of a patient with a history of depression who presented with a prolonged QT interval and episodes of torsades de pointes while taking therapeutic doses of citalopram.
Case Report
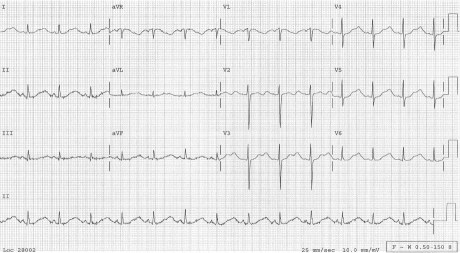
In March 2010, a 40-year-old white woman presented at the emergency department (ED) after 2 syncopal episodes. The first episode had occurred at 2 AM. It was preceded by 3 days of lightheadedness and palpitations. During this episode, she lost consciousness, fell down some stairs, and awakened later with a scalp laceration. She continued to have spells of lightheadedness and drove to her mother's house, where she had another syncopal episode. She was then taken to the ED. On examination, her temperature was 99°F; heart rate, 90 beats/min; blood pressure, 130/90 mmHg; and oxygen saturation, 96% on room air. Physical examination revealed nothing unusual except for a minor scalp laceration. A 12-lead electrocardiogram (ECG) showed a prolonged corrected QT interval (QTc) of 535 ms, with notched T waves in leads V2 and V3 (Fig. 1).
Fig. 1 Electrocardiogram upon the patient's admission shows a prolonged corrected QT interval of 535 ms.
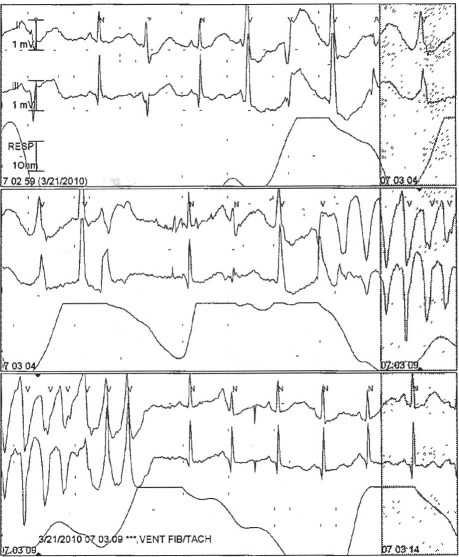
The patient had multiple episodes of torsades de pointes during her hospitalization. Although most of these were asymptomatic, she had a recurrence of syncope in the ED during one such episode (Fig. 2).
Fig. 2 Telemetry records in the emergency department show an episode of torsades de pointes.
She was given a 150-mg bolus of amiodarone in the ED. Results of laboratory evaluations, including a complete metabolic panel, serum magnesium test, complete blood count, and thyroid-stimulating hormone test, were normal. The serum potassium level was 3.8 mg/dL, creatinine was 0.7 mg/dL, and magnesium was 1.6 mg/dL. The creatinine clearance was >60 mL/min. Results of chest radiography, computed tomography (CT) of the head, and urine drug-screening were not unusual. A 2-g bolus of magnesium sulfate followed by a 3 mg/min infusion resulted in a substantial decrease in the dysrhythmia.
The patient had a long-standing history of depression, which had been treated with fluoxetine. Four weeks before the current presentation, her therapy had been switched to citalopram 40 mg twice daily, due to worsening symptoms of depression. She did not report having ingested extra doses of citalopram or having used illicit drugs. She was not taking any other prescribed medications. Her over-the-counter herbal medications had no QT-interval–prolonging effects. She had no known childhood health problems and reported no previous syncope, palpitations, dyspnea, or seizures. She could not recall having undergone ECG in the past. There was no family history of sudden cardiac or unexplained deaths.
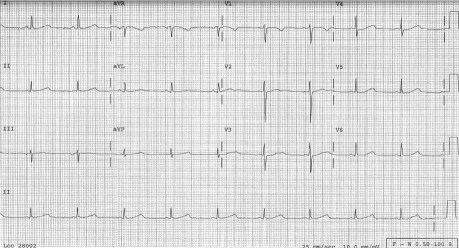
A transthoracic echocardiogram showed normal left ventricular thickness and function, with no obvious structural heart disease. The citalopram therapy was discontinued, and a 12-lead ECG was performed daily to evaluate the QTc interval. The QTc interval improved appropriately upon the discontinuation of citalopram, eliminating the possible need for an implantable cardioverter-defibrillator. When the patient was discharged from the hospital 3 days later, the QTc interval was 469 ms (Fig. 3). Because concealed long QT syndrome (LQTS) could not be ruled out, she was started on 40 mg/d of nadolol. Genetic screening was offered to the patient, but she decided against it. Psychiatric consultation was obtained for the patient. She was started on 15 mg/d of mirtazapine for depression, and she tolerated this therapy very well.
Fig. 3 Electrocardiogram upon the patient's discharge from the hospital shows a corrected QT interval of 469 ms.
In the absence of citalopram overdose or liver or renal dysfunction, our presumptive diagnosis was concealed congenital LQTS that was unmasked by citalopram. Upon follow-up 6 months later, the patient's QTc interval remained stable and she had experienced no further syncopal episodes. The dose of nadolol was increased to 80 mg/d. It was recommended that the patient start taking fish oil supplements and begin a high-potassium diet. At this examination, she was again offered genetic screening for congenital LQTS, but she declined.
Discussion
Citalopram is one of the newer and more selective serotonin reuptake inhibitors that the FDA has approved as therapy for major depression.1 The drug's half-life is 36 hours, and it attains maximum levels 2 hours after administration. It is metabolized by the liver, and it has a systemic clearance rate of 330 mL/min with 20% clearance by the kidneys. Citalopram has a better cardiac-safety profile in regard to QT-interval prolongation and cardiac arrhythmias than do TCAs at concentrations in the clinical range. In animal studies, citalopram has been associated with the inhibition of delayed rectifier potassium current. However, this effect is counteracted by its inhibition of depolarizing current mediated by L-type calcium channels. Accordingly, citalopram prevents QT-interval prolongation and arrhythmias that would occur as a result of the selective blockade of delayed rectifier potassium current that often occurs with TCAs.2
There have been several studies and case reports of cardiac arrhythmias and deaths related to citalopram. However, these studies were reported in patients with citalopram overdose3–5 or in combination with other antidepressant medicines and antiarrhythmic agents.6,7 Citalopram-induced QT-interval prolongation and fatal arrhythmias are presumed to result from one of its metabolites, didemethylcitalopram.8 However, the dose of citalopram required to produce fatal arrhythmias in human beings is approximately 240 mg, which is 4 times the maximum recommended dose. The Swedish Poisons Information Centre analyzed 108 cases of acute citalopram overdose and concluded that most had an uneventful cardiac course.9 Cases have been reported of citalopram-induced torsades de pointes in patients with end-stage renal disease or abnormal electrolyte levels.10,11 However, the torsades de pointes in our patient occurred in the absence of overdose, electrolyte imbalance, and renal or liver failure. We suspect that she had concealed LQTS, which was unmasked by citalopram.
We recommend that all occurrences of long QT interval and torsades de pointes associated with citalopram be reported, and that citalopram be changed from a conditional to possible risk of torsades de pointes on the Arizona Center for Education & Research on Therapeutics' list of drugs (www.azcert.org) that are known or suspected to cause long QT interval and torsades de pointes.12 We recommend that clinicians thoroughly investigate family history for sudden deaths and perform a baseline ECG before prescribing citalopram. We also recommend routine ECG testing during citalopram therapy, as well as the avoidance of citalopram by patients with the diagnosis of LQTS.
Footnotes
Address for reprints: Anand Deshmukh, MD, The Cardiac Center of Creighton University, 3006 Webster St., Omaha, NE 68131
E-mail: drananddeshmukh@yahoo.com
References
- 1.Tan JY, Levin GM. Citalopram in the treatment of depression and other potential uses in psychiatry. Pharmacotherapy 1999;19(6): 675–89. [DOI] [PubMed]
- 2.Witchel HJ, Pabbathi VK, Hofmann G, Paul AA, Hancox JC. Inhibitory actions of the selective serotonin re-uptake inhibitor citalopram on HERG and ventricular L-type calcium currents. FEBS Lett 2002;512((1–3):59–66. [DOI] [PubMed]
- 3.Catalano G, Catalano MC, Epstein MA, Tsambiras PE. QTc interval prolongation associated with citalopram overdose: a case report and literature review. Clin Neuropharmacol 2001; 24(3):158–62. [DOI] [PubMed]
- 4.Ostrom M, Eriksson A, Thorson J, Spigset O. Fatal overdose with citalopram. Lancet 1996;348(9023):339–40. [DOI] [PubMed]
- 5.Grundemar L, Wohlfart B, Lagerstedt C, Bengtsson F, Eklundh G. Symptoms and signs of severe citalopram overdose. Lancet 1997;349(9065):1602. [DOI] [PubMed]
- 6.Musshoff F, Schmidt P, Madea B. Fatality caused by a combined trimipramine-citalopram intoxication. Forensic Sci Int 1999;106(2):125–31. [DOI] [PubMed]
- 7.Fayssoil A, Issi J, Guerbaa M, Raynaud JC, Heroguelle V. Torsade de pointes induced by citalopram and amiodarone. Ann Cardiol Angeiol (Paris) 2011;60(3):165–8. [DOI] [PubMed]
- 8.Kelsey JE, Nemeroff CB. Selective serotonin reuptake inhibitors: citalopram. In: Sadock BJ, Sadock VA, editors. Kaplan and Sadock's comprehensive textbook of psychiatry. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 2435–8.
- 9.Personne M, Persson H, Sjoberg G. Citalopram toxicity. Lancet 1997;350(9076):518–9. [DOI] [PubMed]
- 10.Kanjanauthai S, Kanluen T, Chareonthaitawee P. Citalopram induced torsade de pointes, a rare life threatening side effect. Int J Cardiol 2008;131(1):e33–4. [DOI] [PubMed]
- 11.de Gregorio C, Morabito G, Cerrito M, Dattilo G, Oreto G. Citalopram-induced long QT syndrome and torsade de pointes: role for concomitant therapy and disease. Int J Cardiol 2011;148(2):226–8. [DOI] [PubMed]
- 12.Resources for professionals: QT drug lists by risk group [Internet]. Tucson (AZ): Arizona Center for Education & Research on Therapeutics; 2011. Available from: http://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm [updated 2011 Dec 21; cited 2012 Jan 13.].