Abstract
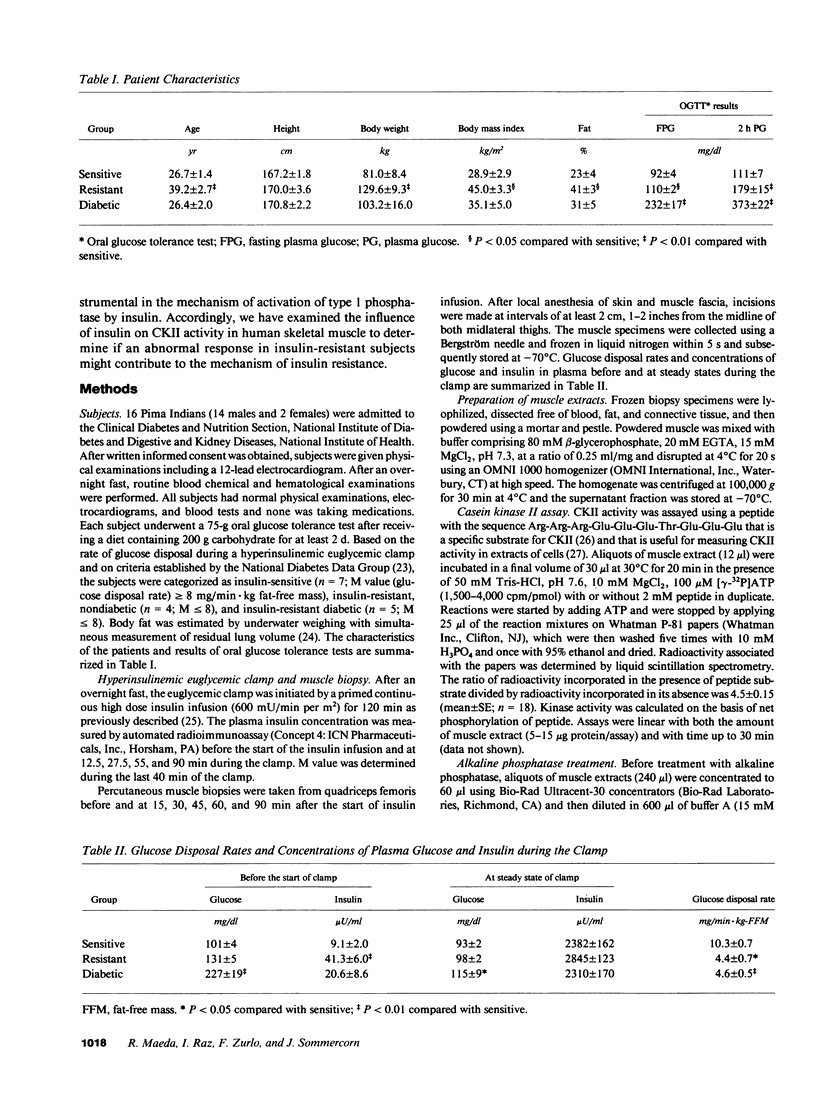
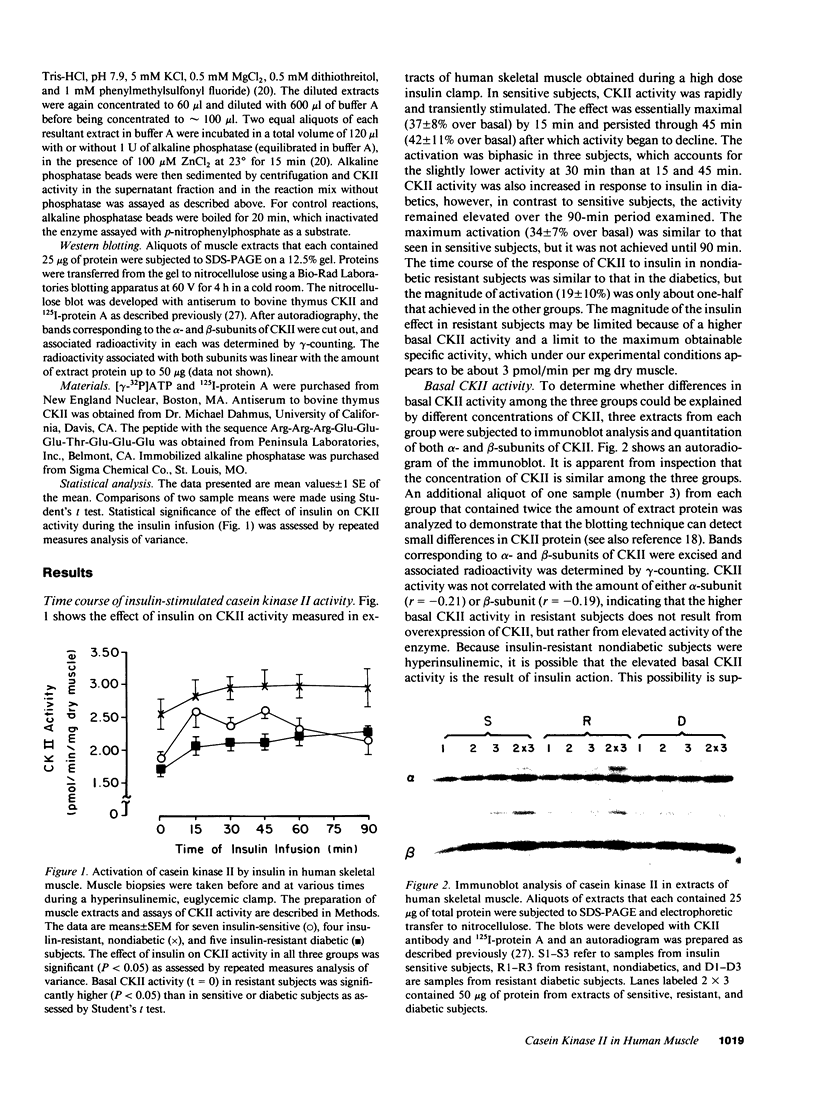
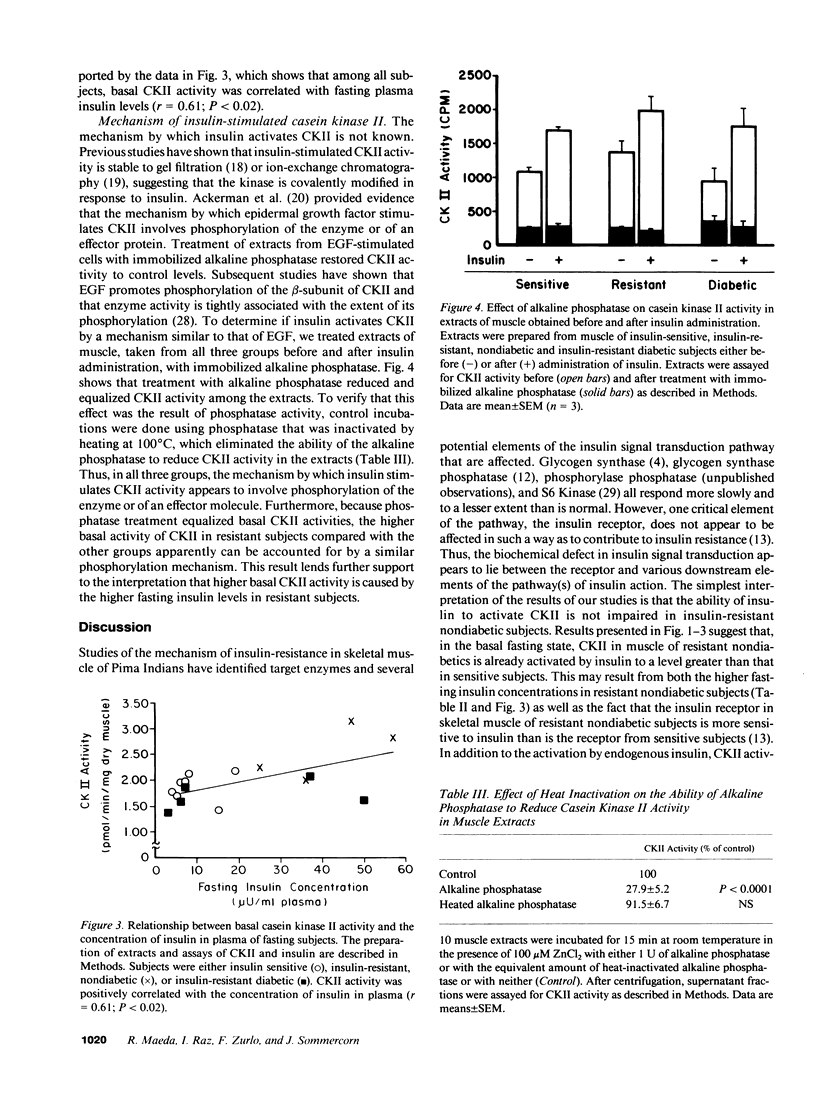
Insulin resistance, which may precede the development of non-insulin-dependent diabetes mellitus in Pima Indians, appears to result from a postreceptor defect in signal transduction in skeletal muscle. To identify the putative postreceptor lesion responsible for insulin resistance in Pima Indians, we investigated the influence of insulin on the activity of casein kinase II (CKII) in skeletal muscle of seven insulin-sensitive, four insulin-resistant, nondiabetic, and five insulin-resistant diabetic Pima Indians during a 2 h hyperinsulinemic, euglycemic clamp. In sensitive subjects, CKII was transiently activated reaching a maximum over basal activity (42%) at 45 min before declining. CKII was also stimulated in resistant (19%) and diabetic (34%) subjects. Basal CKII activity in resistant subjects was 40% higher than in either sensitive or diabetic subjects, although the concentration of CKII protein, as determined by Western blotting, was equal among the three groups. Basal CKII activity was correlated with fasting plasma insulin concentrations, suggesting that the higher activity in resistant subjects resulted from insulin action. Extracts of muscle obtained from all three groups either before or after insulin administration were treated with immobilized alkaline phosphatase, which reduced and equalized CKII activity. These results suggest that insulin stimulates CKII activity in human skeletal muscle by a mechanism involving phosphorylation of either CKII or of an effector molecule, and support the idea that elevated basal activity in resistant subjects results from insulin action. It appears that the ability of insulin to activate CKII in skeletal muscle is not impaired in insulin-resistant Pima Indians, and that the biochemical lesion responsible for insulin resistance occurs either downstream from CKII or in a different pathway of insulin action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc Natl Acad Sci U S A. 1990 Jan;87(2):821–825. doi: 10.1073/pnas.87.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman P., Osheroff N. Regulation of casein kinase II activity by epidermal growth factor in human A-431 carcinoma cells. J Biol Chem. 1989 Jul 15;264(20):11958–11965. [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama A., Patterson A. P., Kadowaki T., Kadowaki H., Siegel G., D'Ambrosio D., Lillioja S., Roth J., Taylor S. I. The amino acid sequence of the insulin receptor is normal in an insulin-resistant Pima Indian. J Clin Endocrinol Metab. 1990 Apr;70(4):1155–1161. doi: 10.1210/jcem-70-4-1155. [DOI] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Chan C. P., McNall S. J., Krebs E. G., Fischer E. H. Stimulation of protein phosphatase activity by insulin and growth factors in 3T3 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6257–6261. doi: 10.1073/pnas.85.17.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Klarlund J. K., Yagaloff K. A., Bradford A. P., Lewis R. E. Insulin receptor signaling. Activation of multiple serine kinases. J Biol Chem. 1988 Aug 15;263(23):11017–11020. [PubMed] [Google Scholar]
- DePaoli-Roach A. A. Synergistic phosphorylation and activation of ATP-Mg-dependent phosphoprotein phosphatase by F A/GSK-3 and casein kinase II (PC0.7). J Biol Chem. 1984 Oct 10;259(19):12144–12152. [PubMed] [Google Scholar]
- Debant A., Clauser E., Ponzio G., Filloux C., Auzan C., Contreres J. O., Rossi B. Replacement of insulin receptor tyrosine residues 1162 and 1163 does not alter the mitogenic effect of the hormone. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8032–8036. doi: 10.1073/pnas.85.21.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M. Early events in insulin actions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:293–341. [PubMed] [Google Scholar]
- Freymond D., Bogardus C., Okubo M., Stone K., Mott D. Impaired insulin-stimulated muscle glycogen synthase activation in vivo in man is related to low fasting glycogen synthase phosphatase activity. J Clin Invest. 1988 Nov;82(5):1503–1509. doi: 10.1172/JCI113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y., Esposito-Del Puente A., Bogardus C., Mott D. M. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest. 1990 Feb;85(2):476–481. doi: 10.1172/JCI114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K., Czech M. P. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988 Nov 5;263(31):15872–15875. [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Hiken J., Burnette B., DePaoli-Roach A. A. Phosphorylation of phosphoprotein phosphatase inhibitor-2 (I-2) in rat fat cells. Biochem Biophys Res Commun. 1988 Jan 15;150(1):197–203. doi: 10.1016/0006-291x(88)90505-0. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- López-Alarcón L., Mojena M., Monge L., Felíu J. E. Stimulation of pyruvate kinase phosphatase activity by insulin in isolated rat hepatocytes. Biochem Biophys Res Commun. 1986 Jan 14;134(1):292–298. doi: 10.1016/0006-291x(86)90561-9. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Yokota A., Flier J. S. Normal insulin-receptor cDNA sequence in Pima Indians with NIDDM. Diabetes. 1989 Nov;38(11):1496–1500. doi: 10.2337/diab.38.11.1496. [DOI] [PubMed] [Google Scholar]
- Nyomba B. L., Ossowski V. M., Bogardus C., Mott D. M. Insulin-sensitive tyrosine kinase: relationship with in vivo insulin action in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E964–E974. doi: 10.1152/ajpendo.1990.258.6.E964. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L., Herrera R., Rosen O. M. Insulin receptor is an insulin-dependent tyrosine protein kinase: copurification of insulin-binding activity and protein kinase activity to homogeneity from human placenta. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3327–3331. doi: 10.1073/pnas.81.11.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio G., Contreres J. O., Debant A., Baron V., Gautier N., Dolais-Kitabgi J., Rossi B. Use of an anti-insulin receptor antibody to discriminate between metabolic and mitogenic effects of insulin: correlation with receptor autophosphorylation. EMBO J. 1988 Dec 20;7(13):4111–4117. doi: 10.1002/j.1460-2075.1988.tb03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommercorn J., Krebs E. G. Induction of casein kinase II during differentiation of 3T3-L1 cells. J Biol Chem. 1987 Mar 15;262(8):3839–3843. [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies R. S., Ullrich A., McClain D. A. Augmented mitogenesis and impaired metabolic signaling mediated by a truncated insulin receptor. J Biol Chem. 1989 Aug 5;264(22):12820–12825. [PubMed] [Google Scholar]
- Zick Y., Whittaker J., Roth J. Insulin stimulated phosphorylation of its own receptor. Activation of a tyrosine-specific protein kinase that is tightly associated with the receptor. J Biol Chem. 1983 Mar 25;258(6):3431–3434. [PubMed] [Google Scholar]




