To the Editor
Asthma in the elderly has been inadequately studied. The age demographic >65 years old is the fastest growing in the United States, and asthma in the elderly population is frequently underdiagnosed and undertreated(1). Furthermore, elderly asthmatics also have a higher rate of severe exacerbations, emergency department visits, and hospitalizations than younger asthmatics which represents significant disease impairment and risk(2). The airway inflammation in older asthma subjects differs from younger patients, which suggests that the phenotype of asthma in older asthmatics may be different. An understanding of this phenotype of asthma will be important for the diagnosis of the disease, determining optimal therapies, and improvement in the morbidity and mortality in the growing elderly asthmatic population.
The role of neutrophils in asthma has not been fully defined; however, there are phenotypes of asthma recognized that have a predominance of neutrophilic airway inflammation. Specifically, one of the severe asthma phenotypes is characterized by airway neutrophilia with corresponding increases in neutrophil mediators including matrix metalloproteinase (MMP)-9, neutrophil elastase, and interleukin (IL)-8, which are thought to play a pathogenic role in severity of asthma(3–6). Another characteristic of this phenotype of severe asthma is a lack of responsiveness to corticosteroids.
Since previous studies have suggested that aging can result in increased airway neutrophilia in normal healthy subjects(7), we examined the airway neutrophilia and neutrophil associated mediators (including MMP-9, neutrophil elastase activity, and IL-8) in a pilot study of younger and older asthma subjects. The subjects in this study were recruited in a University of Wisconsin IRB-approved protocol into two age groups: younger (20–40 years old; n=15) and older (55–80 years old; n=14) as previously described(8). Induced sputum samples were obtained from subjects at baseline control of asthma. Hypertonic saline was utilized and processing was performed. Briefly, the sputum was incubated with Sputolysin (Calbiochem; La Jolla, CA) for 20 minutes, centrifuged, supernatant collected and stored at −80°C until analysis. A sensitive two-step sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure IL-8 and MMP-9 in the sputum. Neutrophil elastase activity was measured by release of 7-amino-4-methylcoumarin (AMC) utilizing a fluorometric immunocapture assay kit (Calbiochem). Sputolysin was added to all assay standards to represent the equivalent amount in the diluted sputum samples.
The subject characteristics of this cohort have been previously published(8). There were no significant differences in the total serum IgE, peripheral blood eosinophil count or atopy (defined as at least 1 positive skin prick test) between age groups. Both the absolute FEV1 and FVC were significantly lower in the older subjects compared to the younger subjects, which is consistent with lung volume decreases in the aging population. However, the percent of predicted values, for both FEV1 and FVC were comparable between the younger and older subjects, suggesting similar disease severity in both age groups. A comparison of medication usage in both age groups indicated no significant difference in inhaled corticosteroid use. Other medications, including oral contraceptives and bisphosphonates, were used disproportionately in the younger and older groups; however, subgroup analyses with these medications did not correlate with any clinical or in vitro functional measurements.
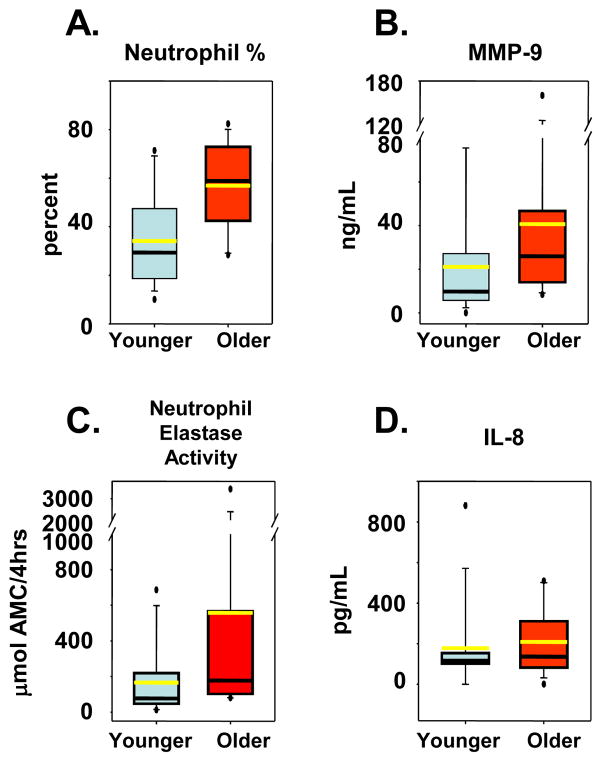
As previously shown, sputum cell differentials revealed a significant increase in the percentage of neutrophils in older asthma subjects compared to the younger asthma subjects (57% ± 16% vs. 37% ± 21%; p=0.008; Figure 1A)(8). However, there was no difference in percentage or total number of eosinophils between each group. There were significant increases in MMP-9 levels (406 ± 109 ng/mL vs. 211 ± 70 ng/mL; p=0.02; Figure 1B) and neutrophil elastase activity in the older asthma subjects (557 ± 243 μmol AMC/4hrs vs. 166 ± 52μmol AMC/4hrs; p=0.02; Figure 1C). Mean sputum IL-8 levels appeared higher in older subjects than younger subjects, but this did not reach statistical significance (209 ± 44 pg/mL vs. 128 ± 24 pg/mL; p=0.3; Figure 1D) likely due to an outlier in the younger group and inadequate sample size.
Figure 1.
Sputum Neutrophil and Neutrophil Mediator Levels. Young asthma subjects (blue), Older asthma subjects (red). A, Box plot of sputum neutrophil percentage. B, Sputum MMP-9 measured by ELISA. C, Sputum neutrophil elastase activity measured by immunocapture assay kit. D, Sputum IL-8 measured by ELISA. Black line represents median and the yellow represents the mean.
Our comparisons of neutrophil numbers and their mediators (MMP-9, neutrophil elastase, and IL-8) in the airways of older versus younger asthma subjects exhibit parallels to previous comparisons of neutrophil-predominant severe asthma and non-severe asthma(6). Although previous studies in healthy older adults have also shown an increase in airway neutrophils and its mediators(7), the impact of these age-related changes in airway inflammation on exacerbations of asthma is not known. Furthermore, it is not known whether this difference in older asthmatics has implications for treatment, particularly with respect to a decreased responsiveness to glucocorticoids as is seen in neutrophil-predominant asthma. Since a majority of clinical studies on the efficacy of asthma therapies often exclude elderly asthmatics, we propose that studies focusing on this population are required to provide truly evidence-based recommendations.
One limitation of this study is that we did not include healthy controls, so we cannot assess whether the magnitude of these findings are different in asthma versus healthy normal aging. Also, whether these changes are responsible for the apparent increased severity of asthma exacerbations in the elderly with increased frequency of hospital visits and increased rates of hospitalization should be addressed(2;9). In summary, further study of asthma in the elderly with respect to both clinical features and mechanisms of inflammation are necessary to provide an evidence-based rationale for the effective management of this growing population.
References
- 1.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Underdiagnosis and Undertreatment of Asthma in the Elderly. Chest. 1999;116(3):603–13. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National Surveillance for Asthma- United States, 1980–2004. MMWR Surveillance Summaries. 2007;56(SS08):1–54. [PubMed] [Google Scholar]
- 3.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160(5):1532–9. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 4.Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172(5):559–65. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 5.Cundall M, Sun YC, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucortcoids. Journal of Allergy and Clinical Immunology. 2003;112(6):1064–71. doi: 10.1016/j.jaci.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368(9537):804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 7.Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mechanisms of Ageing and Development. 1998;104(2):169–81. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 8.Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008;133(2):412–9. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerji A, Clark S, Afilalo M, Blanda MP, Cydulka RK, Camargo CA. Prospective multicenter study of acute asthma in younger versus older adults presenting to the emergency department. Journal of the American Geriatrics Society. 2006;54(1):48–55. doi: 10.1111/j.1532-5415.2005.00563.x. [DOI] [PubMed] [Google Scholar]