Abstract
Purpose
To compare the effectiveness of intravitreal injection of bevacizumab and ranibizumab in patients with treatment-naïve polypoidal choroidal vasculopathy (PCV).
Methods
A total of 66 and 60 eyes of 121 consecutive patients who received intravitreal bevacizumab (1.25 mg) or ranibizumab (0.5 mg) injection for treatment of PCV were retrospectively reviewed. After initial three loading injections by month, injection was performed as needed. Main outcome measures included best corrected visual acuity (BCVA), foveal center thickness (FCT) as assessed by spectral domain optical coherence tomography (SD-OCT), and change in polypoidal lesion on indocyanine green angiography (ICGA).
Results
At 12 months, average number of injections was 4.72±1.84 in the bevacizumab group and 5.52±1.54 in the ranibizumab group. Mean logarithm of the minimum angle of resolution of BCVA from baseline at 12 months after injection improved by 0.11 in the bevacizumab group (P=0.02) and by 0.14 in the ranibizumab group (P=0.01). Average FCT decreased from 368±62.48 to 298±40.77 μm in the bevacizumab group (P=0.01) and from 371±50.79 to 286±36.93 μm in the ranibizumab group (P=0.01). Polyp regression rate was 24.2% (16 eyes out of 66 eyes) in the bevacizumab group and 23.3% (14 eyes out of 60 eyes) in the ranibizumab group. There was no statistically significant difference in BCVA improvement achieved, FCT improvement achieved, and polyp regression rate between groups.
Conclusion
Intravitreal injections of bevacizumab and ranibizumab have similar effects in stabilization of visual acuity, macular edema, and regression of polypoidal complex with PCV eyes.
Keywords: bevacizumab, polypoidal choroidal vasculopathy, ranibizumab
Introduction
Polypoidal choroidal vasculopathy (PCV) is characterized by a branching vascular network with polypoidal-shaped choroidal vascular lesions that result in subretinal leakage, subretinal hemorrhage, and pigment epithelial detachment.1, 2, 3, 4 When pathological changes associated with PCV are extended to the subfoveal area, visual prognosis may be poor. Sho et al3 reported that severe vision loss, caused by persistent serous detachment, atrophy of retinal pigment epithelium (RPE), and submacular hemorrhage was noted in 34.5% (38 of 110 eyes) of the PCV patients.
Currently, there are several effective methods of treatment for PCV. Photodynamic therapy (PDT) with verteporfin or intravitreal injection of anti-vascular endothelial growth factor (VEGF) has recently been administered for treatment of PCV eyes, and encouraging results have been reported.5, 6, 7, 8, 9, 10
Pathogenesis of PCV is not fully understood. However, VEGF may have a role in pathogenesis. Compared with normal controls, VEGF concentrations in the aqueous were found to be markedly increased in PCV eyes11 and strong expression of VEGF was observed in RPE cells of PCV specimens.12 These reports support the use of anti-VEGF treatment of PCV eyes.
Ranibizumab is a humanized anti-VEGF antibody that inhibits all forms of biologically active VEGF-A; treatment with ranibizumab appears to significantly decrease bleeding and exudation in PCV.10 Bevacizumab (a humanized full-length anti-VEGF antibody) also appears to have a treatment effect in PCV eyes.8, 9 Kokame et al13 reported that continuous monthly intravitreal ranibizumab injection was well tolerated in PCV patients; in addition, patients showed stabilized vision and decreased macular edema.
Recently, equivalence in the therapeutic effect between bevacizumab and ranibizumab in neovascular age-related macular degeneration (AMD) was reported.14 However, to the best of our knowledge, there have been no reports on the differences between bevacizumab and ranibizumab for treatment of PCV. The purpose of this study is to determine whether or not there are differences between bevacizumab and ranibizumab for treatment of PCV.
Materials and methods
After obtaining approval by the Institutional Review Board, a retrospective medical record review was performed on 121 consecutive patients (126 eyes) who were treated with intravitreal anti-VEGF agents for PCV at the Kim's Eye Hospital, Konyang University College of Medicine from October 2008 to October 2010.
Inclusion and exclusion criteria
Patients were included if they met all of the following criteria: (1) confirmation of PCV with fluorescein angiography (FA) and indocyanine green angiography (ICGA), performed using a confocal laser scanning system (HRA-2, Heidelberg Engineering, Dossenheim, Germany) at the first visit. We only included patients whose ICGA showed a branching vascular network with polypoidal-shaped choroidal vascular lesions; (2) patients who were treatment naïve; (3) patients who were treated with only one type of anti-VEGF agent (either bevacizumab or ranibizumab); (4) a minimum follow-up period of 12 months; (5) availability of result from an optical coherence tomography (OCT) examination at baseline, 1, 3, 6, 9, and 12 months.
Exclusion criteria included the following: combination therapy of more than one anti-VEGF agent, prior treatment with PDT, AMD, pathological myopia, idiopathic choroidal neovascularization (CNV), and other secondary CNV, other ocular disease that could affect visual acuity, trauma during the study or in the fellow eye, aphakia, or previous vitreoretinal surgery.
Anti-VEGF treatment
Treatment and re-treatment protocols were the same for both groups of patients. Evidence of PCV with recent visual deterioration was an indication for treatment. We performed three consecutive monthly loading dose injections. After performing three loading injections, retreatment for each patient was performed as a ‘retreat as needed' protocol if any of the following were observed: (1) visual deterioration of more than two lines; (2) evidence of persistent fluid or hemorrhage involving macula on OCT at least 1 month after the previous injection; (3) newly developed macular hemorrhage; and (4) evidence of an active PCV lesion, as found on FA, ICGA, or OCT.
Visual acuity and central foveal center thickness (FCT) measurement
Best corrected visual acuity (BCVA) was measured with a Snellen chart at baseline at 1, 3, 6, 9, and 12 months after initial anti-VEGF treatment. The Snellen BCVA was converted to logarithm of the minimum angle of resolution (logMAR) values. The logMAR value for counting fingers visual acuity was assigned as +2.0 logMAR (0.01) according to the methods published by Holladay.15
FCT was assessed by spectral domain OCT (Spectral OCT/SLO; OTI Ophthalmic Technologies Inc. Miami, FL, USA) using six diagonal fast and slow 6-mm scans. Retinal thickness of the 1-mm central retina was obtained by fast macular scan. Only well-centered scans without overt algorithm failure messages were selected for analysis.
Intravitreal anti-VEGF injection
The off-label nature of the treatment and its potential risks and benefits were discussed in detail. A signed informed consent was obtained from all patients. Patients received 1.25 mg of bevacizumab or 0.5 mg of ranibizumab. Topical anesthesia was applied, and 10% povidone–iodine was used for scrubbing of eyelid and lashes. Following the application of povidone–iodine eyedrops (1.25%), a sterile lid speculum was put into place. Intravitreal injection was performed with a 30-gauge needle at 3.5–4 mm from the inferotemporal limbus. Pressure was applied to the injection site, using a sterile cotton swab, for 1 min. All patients were instructed to apply antibiotic eye drops for a period of 1 week.
Statistical analysis
SAS programming language (SAS Institute, Cary, NC, USA) was used for all analyses. The study was powered to detect a 20% difference. Frequencies were compared between treatment groups using χ2-tests. The changes in BCVA and FCT between baseline and month 6 follow-up were analyzed with a 1-tailed, paired t-test. For continuous variables, medians for baseline, final values, change, and percent change were compared between bevacizumab and ranibizumab treatment groups using t-test or Wilcoxon's rank sum tests. A P-value <0.05 was considered significant.
Results
Patient characteristics and number of injections
Patient demographics and comparisons of data at baseline are summarized in Table 1. Bevacizumab-treated and ranibizumab-treated patients had similar baseline characteristics for age, sex, distribution of baseline BCVA, FCT, mean greatest linear of dimension on ICGA, classification of PCV,16 and location of lesion (Table 1). All patients were Korean and no systemic adverse events were recorded for any of the patients treated with intravitreal injection. No complications, including endophthalmitis, traumatic lens injury, retinal detachment, and so on, were associated with intravitreal injection. There was a difference in the mean number of injections for each group. The average number of injections was 4.72±1.84 in the bevacizumab group and 5.52±1.54 in the ranibizumab group over 12 months (Table 2).
Table 1. Bevacizumab vs ranibizumab for PCV: patient demographics and comparisons at baseline.
| Bevacizumab (66 eyes of 63 patients)-treated group | Ranibizumab (60 eyes of 58 patients)-treated group | P | |
|---|---|---|---|
| Age (years; mean±SD) | 61.62±10.42 | 60.22±11.48 | 0.86a |
| Gender | 0.77b | ||
| Male | 46 (73.0%) | 40 (69.1%) | |
| Female | 17 (27.0%) | 18 (30.9%) | |
| BCVA (logMAR) | 0.91b | ||
| ≤0.3 | 17 (25.8%) | 12 (20.0%) | |
| >0.3∼≤0.7 | 29 (44.0%) | 31 (52.7%) | |
| >0.7 | 20 (30.2%) | 17 (27.3%) | |
| Mean initial GLD on ICGA (mean±SD), μm | 3213.5±1224.6 | 3421.8±1332.4 | 0.56a |
| PCV Classification16 | 0.64b | ||
| Exudative | 8 (12.1%) | 7 (11.7%) | |
| Hemorrhagic | 58 (87.9%) | 53 (88.3%) | |
| Location of lesions | 0.73b | ||
| Macular | 59 (89.4%) | 52 (86.6%) | |
| Peripapillary | 6 (9.1%) | 7 (11.7%) | |
| Peripheral | 1 (1.5%) | 1 (1.7%) |
Abbreviations: BCVA, best-corrected visual acuity; GLD, greatest linear dimension; ICGA, indocyanine green angiography; logMAR, logarithm of the minimum angle of resolution; PCV, polypoidal choroidal vasculopathy.
On the basis of student t-test.
On the basis of χ2-test.
Table 2. Mean number of injections.
| Baseline to 3 months | 6 months | 9 months | 12 months | |
|---|---|---|---|---|
| Bevacizumab (SD) | 3 (0.00) | 3.21 (0.75) | 3.88 (1.12) | 4.72 (1.84) |
| Ranibizumab (SD) | 3 (0.00) | 3.81 (0.94) | 4.61 (1.35) | 5.52 (1.54) |
| Pa | 0.36 | 0.05 | 0.02 |
On the basis of on student t-test.
Visual outcome
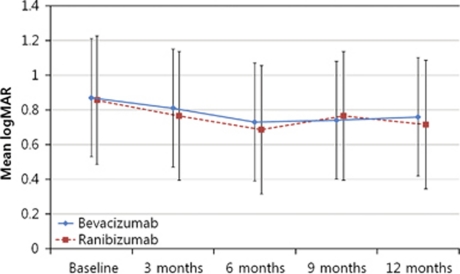
At baseline, the mean BCVA (±SD) in the bevacizumab and ranibizumab groups was 0.87. (±0.54; Snellen equivalent: 20/148) and 0.88 (± 0.57; Snellen equivalent: 20/151), respectively. With follow-up, the distribution of visual acuities improved for both groups (Figure 1). After 12 months treatment, both bevacizumab and ranibizumab significantly increased BCVA to 0.76 (±0.51; Snellen equivalent: 20/115; P=0.02) and 0.74 (±0.43; Snellen equivalent: 20/109; P=0.01), respectively.
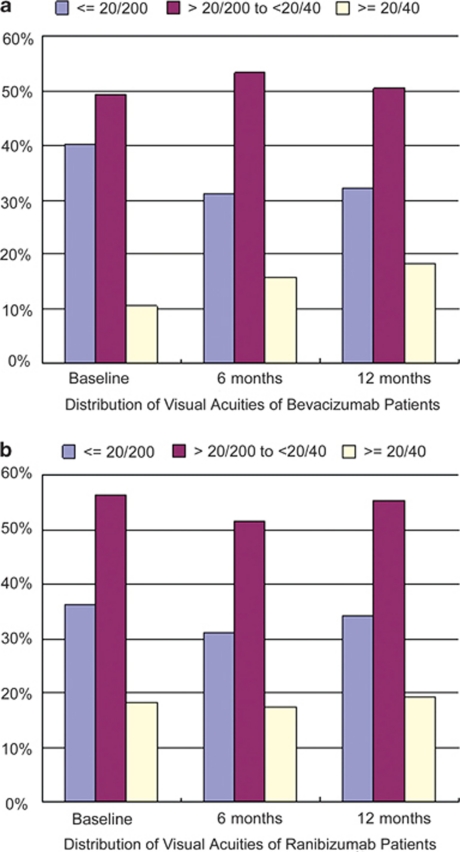
Figure 1.
Distribution of visual acuity at baseline and after initial injection. (a) Distribution for eyes treated with bevacizumab (n=66). (b) Distribution for eyes treated with ranibizumab (n=60).
Visual outcome was divided into three groups. Decrease of more than three lines was defined as ‘worse'. Increase of more than three lines was defined as ‘improved'. The rest were allocated to ‘stable'. At month 12, 19.7% (13/66) of bevacizumab group patients were in the worsened group, 62.1% (41/66) were in the stable group, and 18.2% (12/66) were in the improved group. In the ranibizumab group, the rates were 20.0% (12/60), 23.3% (14/60), and 56.7% (34/60), respectively. There was no statistical difference in proportion of stable or improved group (P=0.34).
There was no statistically significant difference in BCVA improvement achieved with these two agents (P=0.89). Both groups showed improvement from baseline and stability of vision over time (Figure 2). In all, 12 eyes (18.2%) out of 66 eyes in the bevacizumab group, and 14 eyes (23.3%) out of 60 eyes in the ranibizumab group showed gain of more than three lines of visual acuity. No significant difference in proportion of more than three lines gain of visual acuity was observed in either group (P=0.29; Table 3). There was also no significant difference in proportion of more than three lines loss in visual acuity in either group (P=0.82; Table 4).
Figure 2.
Graph showing serial changes in the mean logMAR visual acuity from baseline to month 12 after treatment. The differences in time course between two subgroups were not significant. There was a significant decrease in both groups.
Table 3. Improvement of visual acuity with follow-up.
| logMAR Gain of ≥ 0.3 | Baseline | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|
| Bevacizumab (%) | Reference | 9 (13.6%) | 11 (16.7%) | 13 (19.7%) | 12 (18.2%) |
| Ranibizumab (%) | Reference | 10 (16.7%) | 14 (23.3%) | 13 (21.7%) | 14 (23.3%) |
| Pa | 0.53 | 0.12 | 0.32 | 0.29 |
Abbreviation: logMAR, logarithm of the minimum angle of resolution.
On the basis of χ2 test.
Table 4. Loss of visual acuity with follow-up.
| logMAR loss of ≥ 0.3 | Baseline | 3 Months | 6 Months | 9 Months | 12 Months |
|---|---|---|---|---|---|
| Bevacizumab (%) | Reference | 8 (12.1%) | 10 (15.1%) | 11 (16.7%) | 13 (19.7%) |
| Ranibizumab (%) | Reference | 7 (10.6%) | 7 (10.6%) | 9 (19.5%) | 12 (20.0%) |
| Pa | 0.72 | 0.35 | 0.61 | 0.82 |
Abbreviation: logMAR, logarithm of the minimum angle of resolution.
On the basis of χ2-test.
FCT and angiographic findings
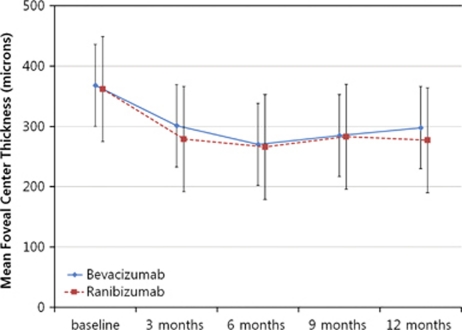
The mean (±SD) FCT at baseline in the bevacizumab and ranibizumab groups was 368 (± 62.48) and 371 (±50.79) μm, respectively. After 12 months treatment, both FCT of the bevacizumab and ranibizumab groups were significantly decreased to 298 (± 40.77) μm (P=0.01) and 286 (± 36.93) μm (P=0.01), respectively. Both groups showed improvement from baseline of FCT with follow-up (Figure 3). There was no statistically significant difference in reductions of FCT in either group (P=0.74). No significant difference in the proportion of increase of >10% from baseline FCT was observed in either group (P=0.34; Table 5).
Figure 3.
Graph showing serial changes in optical OCT mean FCT from baseline to month 12 after treatment. The differences in time course between the two subgroups were not significant. There was a significant decrease in both groups.
Table 5. Bevacizumab vs ranibizumab for PCV: 1-year result of treatment.
| Bevacizumab (66 eyes of 63 patients) | Ranibizumab (60 eyes of 58 patients) | P | |
|---|---|---|---|
| Mean logMAR | 0.76 | 0.74 | 0.82a |
| Mean logMAR change from baseline | −0.11 | −0.14 | 0.89a |
| BCVA changes | |||
| Gained ≥logMAR 0.3 | 18.2% | 23.3% | 0.65b |
| Loss ≥logMAR 0.3 | 19.7% | 20.0% | 0.85b |
| Mean FCT changes from baseline (μm) | −69 | −84 | 0.74a |
| FCT changes | |||
| Decreased by 10% or more | 43.4% | 51.2% | 0.34b |
| Increased by 10% or more | 13.8% | 12.2% | 0.91b |
| Polyp regression | |||
| Regression | 24.2% | 23.3% | 0.92b |
| Persistence | 75.8% | 76.7% | 0.88b |
Abbreviations: BCVA, best-corrected visual acuity; FCT, foveal center thickness; GLD, greatest linear dimension; ICGA, indocyanine green angiography, polypoidal choroidal vasculopathy; PCV, polypoidal choroidal vasculopathy.
On the basis of student t-test.
On the basis of χ2-test.
Polyp regression was found in 16 eyes out of 66 eyes (24.2%) in the bevacizumab group, and in 14 eyes out of 60 eyes (23.3%) in the ranibizumab group (Figure 4). No significant difference in polyp regression rate was observed in either group (P=0.92; Table 5).
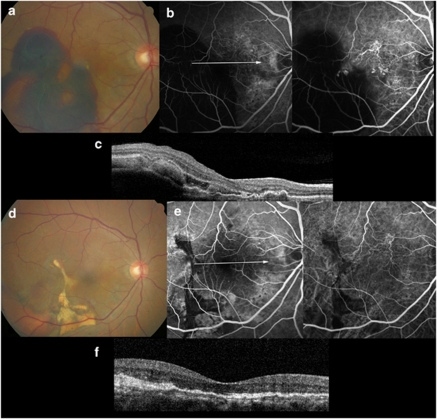
Figure 4.
Images from the right eye of a 63-year-old man with polypoidal choroidal vasculopathy treated with ranibizumab injection. (a) Fundus photograph obtained from at the initial visit showing diffuse subretinal hemorrhage; BCVA was 20/100. (b) FA and ICGA showing a large branching vascular network that terminates with multiple polypoidal lesions. (c) Sectional image obtained with OCT (with the arrow seen on FA) showing protrusions of RPE, which is reflective of the pigment epithelial detachment. (d) The patient was treated intravitreal ranibizumab injections (total injections; four times). Fundus photograph obtained at 12 months after initial visit showing no more subretinal hemorrhage; BCVA was 20/30. (e) FA and ICGA showing regression of the polyps. (f) Sectional image obtained with OCT (with the arrow seen on FA) showing much stabilized elevation of the RPE and no more subretinal hemorrhage.
Discussion
PCV is increasingly recognized as a major cause of vision loss throughout the world; however, the incidence is especially high in Asian countries and in Asian people living throughout the world.17 The most important causes of severe visual loss include repeated subretinal hemorrhage and leakage from PCV lesions,3 repeated injuries resulting in atrophy of RPE, or scar change. Although the pathophysiology of PCV is not completely understood, resolving subretinal hemorrhage and decreasing leakage from PCV lesions may be an important and reasonable approach to stabilization of visual acuity. Moreover, VEGF concentrations in the aqueous were found to be markedly increased in PCV eyes11 and strong expression of VEGF was observed in RPE cells of PCV specimens.12 These points of view support anti-VEGF treatment for PCV eyes.
Ranibizumab, which is specifically for intraocular use, has several theoretical advantages over bevacizumab. Ranibizumab is a humanized anti-VEGF antibody that inhibits all forms of biologically active VEGF-A;18 treatment with Ranibizumab appears to significantly decrease bleeding and exudation in PCV.10, 12 Bevacizumab (a humanized full-length anti-VEGF antibody) also appears to have a treatment effect in PCV eyes.8, 9 Considering the molecular weight (ranibizumab is a 48-kDa Fab fragment, whereas bevacizumab is a complete 149-kDa antibody), ranibizumab may be more effective in treatment of PCV because of its smaller molecular weight and possible deeper penetration to choroidal vascular abnormality lesions of PCV.17 In addition, ranibizumab is affinity matured and may provide better VEGF inhibition through stronger molecular binding, compared with bevacizumab. In comparison, penetration of the neural retina up to the choriocapillaries by intravitreal bevacizumab has been demonstrated.19 And the larger molecular weight of bevacizumab might result in a longer duration of action. Therefore, it has been advanced that the clinical efficacy of these two drugs might be different.20
However, in this study, there was no statistically significant difference in BCVA improvement achieved. Macular edema based on FCT significantly also improved in both groups and showed similar decrease in their mean FCT from baseline. Our results imply that there is no distinct difference in the biological activity between bevacizumab and ranibizumab for treatment of PCV. At 1 year, the number of injections was significantly varied between the two groups (4.72 vs 5.52 for bevacizumab vs ranibizumab). This difference may be due to in part to the belief that bevacizumab is a larger molecule and has a longer intraocular half-life.
The choroidal vascular branching network and polypoidal complexes have been resistant and poorly responsive to anti-VEGF therapy with bevacizumab or ranibizumab.8, 12 In the Gomi et al8, 17 study, choroidal vascular abnormalities remained in 10 of 11 eyes after one to three intermittent injections of bevacizumab. In the Kokame et al12 study, polypoidal complex decreased in 4 of 12 eyes (33%) after six continuous monthly ranibizumab injections (PEARL study). In this study, polypoidal lesions seem to be resistant to both anti-VEGF agents, and polypoidal complex showed a decrease in only 16 eyes of 66 (24.2%) in the bevacizumab group, and 13 eyes of 60 (21.2%) in the ranibizumab group. Even though ranibizumab has a theoretically better ability to penetrate through the retina and RPE to the choroidal vascular abnormalities of PCV,18, 20 there was no significant difference in polypoidal complex regression between the two groups. The location of the PCV vessels beneath the RPE may prevent sufficient penetration of anti-VEGF drugs to induce PCV regression. This result suggests that PCV may be a different inner choroidal vascular abnormality,21, 22 not just a variant of CNV.
PDT in recent studies showed good results in reduction of leakage and regression of polyps in PCV eyes.5, 6 Particularly, in the EVEREST study, the first randomized and prospective study, PDT combination with ranibizumab and PDT monotherapy showed a significantly higher proportion of patients with complete polyp regression at month 6 than in the ranibizumab monotherapy group. However, there was no significant difference in improvement of visual acuity from baseline between PDT combination with the ranibizumab group and the ranibizumab monotherapy group.23 In addition, severe visual loss due to extensive subretinal hemorrhage is not uncommon after PDT,24 and PDT itself can result in a temporary increase in VEGF.25 In the aspect of visual outcome, despite weakness in polyp regression, anti-VEGF monotherapy could be considered for PCV in cases with minimal polyp lesions or in cases with a branching vascular network only. We await long-term results of the EVEREST trial, which could confirm which modality has superiority for the treatment of PCV. More clinical and basic science studies are necessary to clarify the pathogenesis of PCV and therapeutic guidelines.
Because of the retrospective nature of this study, the inherent bias that exists in this study and the treatment choice was left to the discretion of the patient and treating physician, some potential for bias does exist. However, in our institute, the preferred PCV treatment with anti-VEGF (except PDT) shifted from bevacizumab to ranibizumab from 2008 to 2009. Almost all patients were treated with bevacizumab from 2008 to the first half of 2009, and with ranibizumab from the second half of 2009 to nowadays. As a result, this study could be more comparative. Moreover, the similarity in baseline characteristics between the two groups suggests that the groups were well balanced. Another limitation in this study was the absence of a strict protocol for measurement of visual acuity, which led to some of the variances in visual acuity that were noted in the two groups and may limit interpretation of these visual acuity results. This fact likely led to some of the variances in visual acuity that were noted in the two groups and may limit interpretation of these visual acuity results. However, we could identify similar effectiveness of both anti-VEGF agents not only in visual acuity but also in FCT and polyp regression. Planned randomized, controlled study would be necessary for a more precise determination of the differences between these two treatments.
In summary, bevacizumab and ranibizumab have similar effects in stabilization of visual acuity and macular edema with PCV eyes. This result could be considered clinically when performing combination therapy with PDT or deciding on selection of anti-VEGF agent for treatment of PCV eyes.
The authors declare no conflict of interest.
References
- Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 1997;115:478–485. doi: 10.1001/archopht.1997.01100150480005. [DOI] [PubMed] [Google Scholar]
- Sho K, Takahashi K, Yamada H, Wada M, Nagai Y, Otsuji T, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003;121:1392–1396. doi: 10.1001/archopht.121.10.1392. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–110. doi: 10.1097/00006982-199515020-00003. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004;111:1576–1584. doi: 10.1016/j.ophtha.2003.12.056. [DOI] [PubMed] [Google Scholar]
- Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmololgy. 2008;115:141–146. doi: 10.1016/j.ophtha.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R. Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2007;51:270–277. doi: 10.1007/s10384-007-0452-3. [DOI] [PubMed] [Google Scholar]
- Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M, et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008;92:70–73. doi: 10.1136/bjo.2007.122283. [DOI] [PubMed] [Google Scholar]
- Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2008;92:661–666. doi: 10.1136/bjo.2007.135103. [DOI] [PubMed] [Google Scholar]
- Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009;148:70–78. doi: 10.1016/j.ajo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456–462. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88:809–815. doi: 10.1136/bjo.2003.032466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame GT, Yeung L, Lai JC. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy (PEARL study): 6-month results. Br J Ophthalmol. 2010;94:297–301. doi: 10.1136/bjo.2008.150029. [DOI] [PubMed] [Google Scholar]
- CATT research group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–391. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series. Ophthalmology. 2004;111:1576–1584. doi: 10.1016/j.ophtha.2003.12.056. [DOI] [PubMed] [Google Scholar]
- Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008;19:208–212. doi: 10.1097/ICU.0b013e3282fb7c33. [DOI] [PubMed] [Google Scholar]
- Lantry LE. Ranibizumab, a mAb against VEGF-A for the potential treatment of age-related macular degeneration and other ocular complications. Curr Opin Mol Ther. 2007;9:592–602. [PubMed] [Google Scholar]
- Shahar J, Avery RL, Heilweil G, Barak A, Zemel E, Lewis GP, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–269. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- Kuroiwa S, Tateiwa H, Hisatomi T, Ishibashi T, Yoshimura N. Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Experiment Ophthalmol. 2004;32:297–302. doi: 10.1111/j.1442-9071.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002;86:1093–1098. doi: 10.1136/bjo.86.10.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TY.Verteporfin PDT and ranibizumab combination therapy for symptomatic macular polypoidal choroidal vasculopathy: EVEREST result Invest Ophthalmol Vis Sci 201051E-abstract 2228. [Google Scholar]
- Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27:335–341. doi: 10.1097/01.iae.0000233647.78726.46. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K, Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol. 2004;88:809–815. doi: 10.1136/bjo.2003.032466. [DOI] [PMC free article] [PubMed] [Google Scholar]