Abstract
Aim
To describe the frequency of neovascular age-related macular degeneration (nAMD) in second eyes of patients undergoing ranibizumab therapy in their first eye and to evaluate the patterns of optical coherence tomography (OCT) abnormalities in fellow eyes before nAMD.
Method
Patients who developed choroidal neovascularization (CNV) in the second eye while on treatment for the first eye were identified. OCT scans of the second eyes, performed before the onset of CNV, were retrospectively examined and graded. Frequency of second eye involvement was estimated and patterns of progression of OCT abnormalities were described and classified.
Results
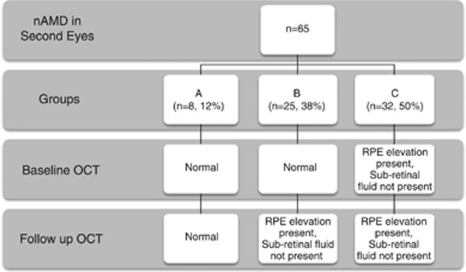
In all, 65 out of 749 consecutive patients required ranibizumab in their second eye for treatment-naïve nAMD over a 2-year period. The mean interval from commencement of ranibizumab in first eye to conversion in second eye was 12 months (2–35.5 months). There were three patterns of CNV development: group A (12%, n=8) had no OCT abnormalities in the second eye just before developing CNV; group B (38%, n=25) had no abnormalities at baseline but developed OCT changes more than one visit before conversion and group C (50%, n=32) had OCT changes from baseline, which did not progress until just before conversion.
Conclusion
Patients with retinal pigment epithelial elevation without sub-retinal fluid on OCT in their fellow eyes have a high risk of progression to require therapy within a 2-year period. An anticipatory approach may be warranted, but a small group with completely normal OCT appearances can still develop lesions between visits.
Keywords: wet AMD, fellow eye risk, second eye involvement, OCT appearance, OCT progression
Introduction
The development of neovascular age-related macular degeneration (nAMD) is a devastating event for elderly patients especially when both eyes are affected.1, 2 Although simultaneous, bilateral development is rare, sequential development of nAMD in one eye followed by the second, fellow eye after a variable time interval is unfortunately, quite common. From the data on patients enroled in large prospective clinical trials, it has been estimated, that 72.4% of patients have unilateral nAMD at study inception and still have good vision in their fellow eyes. In such patients who present with unilateral nAMD, the risk of second eye involvement in their good, fellow eyes has been estimated to be between 2 and 30% per year.1, 3, 4, 5, 6 This means that, in our clinical practice, a high proportion of patients present to us with unaffected, fellow eyes that are at a high risk of developing nAMD.
Recently, the visual prognosis for patients diagnosed with nAMD has been vastly improved by the development of intravitreal anti-vascular endothelial growth factor agents such as ranibizumab.7, 8, 9 Given that better visual outcomes are associated with better baseline visual acuities and that health-related quality of life scores are correlated to the better seeing eye,10, 11, 12 it is important to strive to diagnose nAMD early and initiate prompt treatment as any delay can result in poorer visual outcomes.13
Early diagnosis of nAMD remains a clinical challenge. A better understanding of the clinical markers of early choroidal neovascular membrane (CNV) formation should help to address this need. In a recent study looking at early markers of CNV formation in second eyes of patients undergoing regular follow-up, Cachulo et al14 found that abnormalities on indocyanine green and fluorescein angiography were detectable before the clinical diagnosis of active CNV. However, as the majority of patients who are at the highest risk of developing nAMD in their second eyes are undergoing regular first eye treatment and having their good, fellow eyes checked regularly by optical coherence tomography (OCT), it may be more pragmatic if OCT changes that precede the formation of active CNV can be recognised and used as markers of imminent CNV formation. To our knowledge there are no published studies that have looked at the OCT features of eyes that go on to develop CNV. The purpose of this study was therefore to try and evaluate the frequency of nAMD occurring in the fellow eyes of consecutive patients receiving intravitreal ranibizumab for their first eyes and also to describe the speed and patterns of any OCT changes before the development of nAMD with an intention to classify OCT features seen in fellow eyes before a decision to commence treatment.
Methods
The patient database of the nAMD treatment programme at our hospital was used to identify all patients who received bilateral injections of ranibizumab from September 2008 to September 2010. This criterion would have captured patients who developed nAMD in their second eyes while on treatment for unilateral nAMD. The case notes and all available OCT scans of these patients were retrospectively reviewed and data collected on the exact time point when intravitreal ranibizumab was commenced in second affected eyes of all patients. Because of the retrospective design it was not possible to adopt standard diagnostic imaging criteria of second eye CNV based on retinal imaging. Instead the time point of second eye involvement was taken as the visit at which the decision was made to commence intravitreal ranibizumab for the second eye. This time point or the conversion visit was easily obtainable by reviewing the case notes retrospectively. The time interval between baseline visit (visit at which the first eye received first injection of ranibizumab) and the conversion visit at which decision to commence second eye treatment was calculated in months (mean and range).
All OCTs scans performed on fellow eyes before the conversion visit were collated. For each patient the second eye OCTs on every visit from baseline to the visit at which decision to commence treatment were retrospectively analysed. These OCTs were qualitatively graded for changes in the retinal pigment epithelium (RPE) layer and the outer retinal layers from visit to visit before the conversion visit. In particular the scans were graded in terms of how early any RPE and outer layer changes appeared during the course of follow-up:
Just before the conversion visit.
On follow-up after the baseline visit.
Since the baseline visit.
This grading approach should have enabled an evaluation of the speed and pattern of OCT changes in second eyes over all visits before the conversion visit.
We also attempted to grade OCT scans for changes that occurred between the conversion visit and the visit just before it. Quantitative change in retinal thickness was calculated between these two visits just before commencement of therapy using the thickness measurement tool on the OCT machine software (Eye Explorer, Heidelberg, Germany). Qualitative changes in OCT scans occurring from the visits immediately before conversion and the conversion visits were categorised broadly according to the following categories:
No change.
New RPE elevation or worsening of existing RPE abnormality.
New or worsening of existing changes in outer neural retinal layers (thickening, sub- or intra-retinal fluid).
New or worsening of existing changes in both RPE layer and outer neural retinal layers.
Any angiogram performed at the conversion visit was also graded retrospectively for signs of active leakage. The frequency at which the progressive OCT signs were confirmed on angiogram was determined.
Results
Over the period of 2 years, 749 patients were commenced on ranibizumab monotherapy for the first time in at least one eye. Five patients who did not have CNV secondary to nAMD were excluded (three RAP only lesions, one myopia, one macular dystrophy). Of the remaining 744 patients, 671 patients received unilateral treatment to the same eye throughout and 73 patients received treatment to a second affected eye at some point on follow-up. Of these 73 patients (19 male, 54 female), 2 of them presented with bilateral CNV and received bilateral treatment from the outset and 6 of the second eyes had previous PDT. The remaining 65 (8.7%) patients who required commencement of injections to a treatment-naïve, second affected eye at some point during the follow-up period were included in the study. Their mean age was 79.3 (SD±8.4) years. The median time interval between OCT scans was 8 weeks (range: 2–14 weeks), indicating that the fellow eyes had OCT scans quite frequently. The time interval from baseline visit to second eye conversion visit was variable, ranging from 2 to 35.5 months (mean: 12 months). At the time of diagnosis of nAMD in second eyes, the mean acuity in those second eyes was 20/80 or +0.6 (SD±0.3) logMAR units.
Speed and pattern of OCT changes from baseline to conversion visit
The distribution of patients obtained by classifying second eye OCT changes according to the speed of onset of RPE and outer retinal changes before their conversion visits are shown in Figure 1. In group A (n=8, 12%), patients had no detectable OCT change in their fellow eye from baseline until the conversion visit at which active CNV was diagnosed, that is, very sudden onset (Figure 2a). In group B (n=25, 38%) there was a normal OCT appearance at baseline but the patients developed RPE elevation on subsequent visits, which slowly progressed to develop signs of leakage (Figure 2b). In group C (n=32, 50%), there was an abnormal OCT appearance at baseline, which was typically seen as an elevation of the RPE layer but without any sub- or intra-retinal fluid nor leakage on angiography to suggest active leakage. However, on follow-up, lesions in this group slowly developed signs of leakage to trigger the diagnosis of nAMD and commencement of therapy (Figure 2c).
Figure 1.
The different patterns of second eye SDOCT CNV development and how many patients there are per group, with a summary of the features present on OCT.
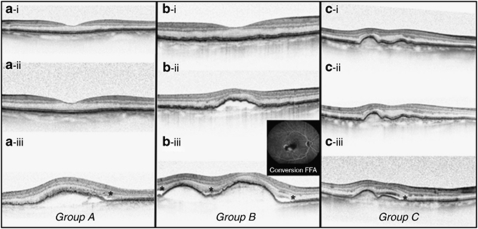
Figure 2.
Three different patterns of OCT changes before the development of wet AMD indicated by presence of sub-retinal fluid. All OCT scans were taken at the same point with a line scan going through the fovea. (a) (i) Baseline with normal looking RPE, (ii) month 11 showing normal appearance immediately before development of CNV and (iii) month 13 showing RPE elevation and sub-retinal fluid (*). (b) (i) Baseline with normal looking RPE, (ii) month 3 immediately before development of CNV, showing RPE detachment but no sub-retinal fluid, and (iii) month 4 showing progression of RPE elevation and sub-retinal fluid (*). (c) (i) Baseline-showing the abnormal appearance of an elevation of the RPE layer, but without any sub- or intra-retinal fluid, (ii) month 5- immediately before CNV development showing an abnormal RPE but no sub- or intra-retinal fluid, (iii) month 6 showing RPE elevation with sub-retinal fluid (*).
In summary, in terms of speed and pattern of second eye conversion 12% of patients had sudden and unpredictable onset of CNV even from the OCT scan performed very shortly before the conversion. However, in 57 out of 65 cases (88% groups B and C) active nAMD developed slowly in the second eyes by way of progression from wide based sub-RPE lesions, which appeared as elevations of the RPE band on OCT. These lesions were detectable easily on OCT scans but did not develop signs of leakage until a variable length of follow-up had passed.
Quantitative and qualitative changes in OCT scans occurring just before conversion visit
Overall, the average retinal thickness in the central subfield zone was greater at the conversion visit compared with the visit prior (Table 1). In group A, the central retinal thickness increased by 110 μm from a mean of 273 μm before conversion to 383 μm at conversion. In group B, the central retinal thickness increased by 9 μm from 351 to 359 μm. In group C, the retinal thickness increased by 56 μm from 348 to 404 μm. Using the descriptive criteria for comparing OCT scans the majority of patients had changes between the scan immediately before conversion visit and the visit at which conversion was diagnosed. The distribution of the types of recent changes is shown in Table 1. In group A, most of the changes involved changes in RPE layer. Detailed inspection of OCT scans performed just before the onset of nAMD in this group did not show any early OCT markers that could be reliable in predicting the imminent onset of nAMD (as shown in Figure 2a). In groups B and C where patients had pre-existing changes in RPE layer the majority of changes on conversion were associated with outer neural retinal layers. In total, 31 out of 65 patients (47%) had fluorescein angiography performed to confirm active leakage.
Table 1. The mean central retinal thickness just before conversion, and at the point of conversion to nAMD.
| Central retinal thickness | Group A | Group B | Group C |
|---|---|---|---|
| N=8 (12%) | N=25 (38%) | N=32 (50%) | |
| Mean CRT at visit immediately before nAMD conversion | 273 μm | 351 μm | 348 μm |
| Mean CRT at diagnosis of nAMD | 383 μm | 359 μm | 404 μm |
| Type of OCT change just before conversion | Number of patients | ||
| No change | 0 | 7 | 3 |
| RPE layer change only | 5 | 1 | 1 |
| Outer retinal layer change only | 2 | 6 | 16 |
| RPE layer and outer retinal layer change | 1 | 11 | 12 |
| Active leak confirmed on FFA | 5 | 10 | 16 |
The mean central retinal thickness just prior to conversion, and at the point of conversion to nAMD. The table also shows qualitative OCT differences between the conversion visit and the visit before conversion.
Discussion
The high risk of nAMD developing in the unaffected, fellow eyes of patients who have had CNV in their first affected eye has been well established at rates of about 12% per year.1, 3, 5 The lower rate of 8.7% of patients developing nAMD in second eyes found in our study was probably due to the variable length of follow-up, which was inevitable in such a retrospective study. Nevertheless, the quantification of the proportion of patients with bilateral involvement in this study serves to highlight not only the burden on health care providers, with about 1 in 10 patients needing intensive treatment and visual rehabilitation for bilateral visual loss at any one time in a service of this nature but also the impact of visual loss on a sizeable proportion of elderly individuals.14 Therefore, even though there is no proven value of a large-scale population screening programme for nAMD in patients with early age-related maculopathy15 the goal of early and accurate diagnosis of second eye conversion, using well-developed OCT criteria in very high-risk patients undergoing first eye treatment is intuitively of great public health importance.
From the analysis of the speed and pattern of OCT changes in our study, it appears that second eye conversion was unpredictable in only a small proportion of patients resembling those in group A (12%). However, in a large proportion of cases, there was RPE elevation or disturbance, without outer neural retinal changes evident in many visits before the diagnosis of second eye conversion. Also, in the analysis of the visits just before conversion, a large proportion of conversions were associated with changes in the RPE contour or height. This finding means that there could be some opportunity to diagnose second eye conversion in a large proportion of cases by looking for small progression in RPE contours or elevation rather than waiting for sub/intra-retinal fluid to be visible, which is the current practice among most clinicians.
In this retrospective study, second eye conversions were not confirmed with fluorescein angiography in about half of cases. In all the cases that had angiography, we were able to identify fluorescein leakage. The absence of fluorescein confirmation in a large proportion of cases would be unlikely to alter our conclusions on the OCT changes before and on conversion of second eyes, which was the primary aim of this study. It can only be assumed that in those cases without fluorescein angiograms, the clinician was confident in making the diagnosis of nAMD in the second eyes on OCT findings alone. This could be in keeping with recent studies suggesting that spectral domain OCT may be very reliable for CNV diagnosis.16, 17 In this regard, the findings from our study that most conversions involved changes in the RPE layer visible on OCT, the OCT may well prove to be of greater value than angiography in confirming very early neovascular change.
Our data also revealed another interesting observation. Almost all second eyes surveyed had sub-retinal fluid on OCT at the time the diagnosis of nAMD was made. This diagnostic behaviour is probably in keeping with common teaching and practice. Given that a high number of cases, such as those in group B and C exhibit progressive OCT changes before the appearance of sub-retinal fluid, it is reasonable to question whether this diagnostic behaviour ought to be challenged particularly when Shona et al12 have found that earlier treatment of CNV leads to improved visual outcome.
There are limitations with our study with its retrospective design. First, the lack of a strict follow-up protocol and not all patients had monthly visits. This may have affected our estimation of the average time to second eye conversion. In the absence of similar studies in the literature, we adopted a very basic grading system to evaluate OCT changes both in term of speed and the visit-to-visit changes before conversion. Future prospective studies may be able to improve on our basic grading criteria. This limitation probably did not affect our main observations as a majority of patients had some form of RPE change before conversion and could potentially be helped if, as a result of this initial study, we are able, in the future, to change our practice and diagnose and treat second eye nAMD earlier.
In conclusion, we found that bilateral ranibizumab therapy is commonly required for nAMD and a significant proportion of patients require ranibizumab therapy to the second eye within 2 years of their first injection. The presence of RPE elevation on OCT in the fellow eye should alert the clinician that there is a high chance that ranibizumab therapy would need to be initiated in the second eye within a 2-year period. Such patients will benefit from very regular OCT checks or may even benefit from ranibizumab therapy in the presence of progression of RPE elevation without sub-retinal fluid although this remains to be proven in controlled studies. This study could provide justification for future studies to evaluate the benefit of screening systems and OCT criteria for unaffected, fellow eyes in these, highly vulnerable group of patients.

The authors declare no conflict of interest.
Footnotes
This work was presented at the Royal College of Ophthalmologists Annual Congress 2011.
References
- Macular Photocoagulation Study Group Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol. 1993;111 (9:1189–1199. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- Connell PP, Keane PA, Oeill EC, Altaie RW, Loane E, Neelam K, et al. Risk factors for age-related maculopathy. J Ophthalmol. 2009;2009:360764. doi: 10.1155/2009/360764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Wong T, Chakravarthy U, Klein R, Mitchell P, Zlateva G, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115 (1:116–126. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Jefferys JL, Hawkins BS, Bressler NM, Bressler SB. Risk factors for second eye progression to advanced age-related macular degeneration: SST report no. 21 Submacular Surgery Trials Research Group. Retina (Philadelphia, Pa) 2009;29 (8:1080–1090. doi: 10.1097/IAE.0b013e3181b1baeb. [DOI] [PubMed] [Google Scholar]
- Barbazetto IA, Saroj N, Shapiro H, Wong P, Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. Am J Ophthalmol. 2010;149 (6:939.e1–946.e1. doi: 10.1016/j.ajo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112 (4:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355 (14:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Kaiser PK, Brown DM, Zhang K, Hudson HL, Holz FG, Shapiro H, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144 (6:850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145 (2:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Chang TS, Suñer IJ, Fine JT, Dolan CM, Ward J, et al. Vision-related function after ranibizumab treatment by better- or worse-seeing eye: clinical trial results from MARINA and ANCHOR. Ophthalmology. 2010;117 (4:747.e4–756.e4. doi: 10.1016/j.ophtha.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR, et al. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a Randomized Clinical Trial. Arch Ophthalmol. 2007;125 (11:1460–1469. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- Shona O, Gupta B, Vemala R, Sivaprasad S. Visual acuity outcomes in ranibizumab-treated neovascular age-related macular degeneration; stratified by baseline vision. Clin Exp Ophthalmol. 2011;39 (1:5–8. doi: 10.1111/j.1442-9071.2010.02424.x. [DOI] [PubMed] [Google Scholar]
- Muether PS, Hermann MM, Koch K, Fauser S. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefes Arch Clin Exp Ophthalmol. 2011;249 (5:633–637. doi: 10.1007/s00417-010-1520-9. [DOI] [PubMed] [Google Scholar]
- Cachulo L, Silva R, Fonseca P, Pires I, Carvajal-Gonzalez S, Bernardes R, et al. Early markers of choroidal neovascularization in the fellow eye of patients with unilateral exudative age-related macular degeneration. Ophthalmologica. 2011;225 (3:144–149. doi: 10.1159/000321064. [DOI] [PubMed] [Google Scholar]
- Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007–March 2008. Eye (Lond) 2010;24 (11:1692–1699. doi: 10.1038/eye.2010.122. [DOI] [PubMed] [Google Scholar]
- Malamos P, Sacu S, Georgopoulos M, Kiss C, Pruente C, Schmidt-Erfurth U. Correlation of high-definition optical coherence tomography and fluorescein angiography imaging in neovascular macular degeneration. Invest Ophthalmol Vis Sci. 2009;50 (10:4926–4933. doi: 10.1167/iovs.09-3610. [DOI] [PubMed] [Google Scholar]
- Khurana RN, Dupas B, Bressler NM. Agreement of time-domain and spectral-domain optical coherence tomography with fluorescein leakage from choroidal neovascularization. Ophthalmology. 2010;117 (7:1376–1380. doi: 10.1016/j.ophtha.2009.11.039. [DOI] [PubMed] [Google Scholar]