Abstract
Purpose
To assess the ability of patients to predict the need for retreatment with intravitreal ranibizumab in neovascular age-related macular degeneration (NVAMD) based on their perception of visual deterioration or distortion of objects in their everyday environment (environmental Amsler).
Methods
A questionnaire was given to 89 patients undergoing optical coherence tomography (OCT)-guided retreatment with intravitreal ranibizumab for NVAMD following an initial loading regimen of three injections and with at least 12-month follow-up. The patient's opinion on the need for an injection on the current visit, based on their perception of change in environmental Amsler, was recorded. This subjective measure was compared with the objective evaluation of retreatment, based on predefined retreatment criteria comprising of changes in visual acuity and morphological changes on OCT. The patients were then instructed on the technique of environmental Amsler, and this evaluation was repeated. The sensitivity and specificity of patient prediction were analyzed at baseline and after the training.
Results
The ability of patients to predict disease activity at baseline showed a sensitivity of 61% and specificity of 96.6%. The area under the receiver operating characteristic curve was 0.8. The presence of macular fluid correlated well with the patient's perception of an abnormal environmental Amsler. After training, the sensitivity and specificity improved to 87.5% and 98.5%, respectively.
Conclusion
In real life, most patients are able to predict the reactivation of the disease in NVAMD, after 12-month follow-up, after training to monitor their symptoms using the environmental Amsler test.
Keywords: neovascular AMD, environmental Amsler
Introduction
Current retreatment criteria for neovascular age-related macular degeneration (NVAMD), based on the PrONTO Study (Prospective Optical Coherence Tomography (OCT) Imaging of Patients with Neovascular Age-Related Macular Degeneration (AMD) Treated with intraOcular Ranibizumab),1 include a decrease in visual acuity (VA) of five or more ETDRS (Early Treatment Diabetic Retinopathy Study) letters with the presence of fluid in the macula on OCT, persistence or increase of intraretinal or subretinal fluid on OCT, occurrence of new macular haemorrhage or angiographic evidence of increase in lesion size. Most of the criteria, except decrease in VA, are based on objective measures of assessment of the morphological features on OCT, clinical assessment or rarely, fundus fluorescein angiography.
Various subjective tests are being evaluated to assess reactivation of the disease. The original or the modified Amsler grid, the preferential hyperacuity perimeter (PHP), entoptic perimetry, and various internet-based macular function tests are utilized for this purpose.2
In this study, we assessed the ability of the patients to predict the need for retreatment based on their perception of visual deterioration or distortion of objects in everyday life (environmental Amsler).
Materials and methods
This study was conducted in the age-related macular degeneration clinics in the Laser and Retinal Research Unit of King's College Hospital, where patients are being monitored for the need for retreatment with ranibizumab based on retreatment criteria defined by the PrONTO Study 1 and RCOphth guidelines.3 The study was approved by the Chair of the Institutional Review Board, and it adhered to the tenets of the declaration of Helsinki.
Inclusion criteria were patients over the age of 55 years diagnosed with NVAMD, with VA from 24 to 73 ETDRS letters, who had all undergone the loading dose of three monthly injections of ranibizumab and had completed at least 1-year follow-up. Patients who had not received any injections in the previous 6 month were excluded from this study, as they were defined as stable patients. Other exclusion criteria included patients who were on ranibizumab therapy with <1 year of treatment and patients with dementia or cognitive impairment; non-responders to treatment defined as persistent intraretinal or subretinal fluid despite two consecutive ranibizumab injections in addition to the loading dose and patients with VA of <15 letters after OCT-guided ranibizumab therapy for 12 months.
All patients underwent VA measurement with ETDRS charts at 2 m, in the day-to-day clinic setting. The pupils were dilated with 1% tropicamide and 2.5% phenylephrine. Spectralis OCT (HRA+OCT, Heidelberg Engineering, Heidelberg, Germany) scans were obtained using predefined protocols. The OCT volume scan was performed on a 20 × 20 degree cube with 49 raster lines, each containing 1064 pixels, separated by 125 μ. The patients then answered three questions on their perception of whether they required treatment on that visit and the reason for the same. The patients then underwent slit-lamp biomicroscopy and evidence of disease activity was noted. The decision to treat the patient was made by one of the authors (SS), who was masked of the patient's response. This was explained to the patients as well.
Questions:
Do you think you need an injection today? a Yes b No c Don't know
If answer is Yes, why do you need the injection? a Decrease in vision b Distortion of vision
What made you think you needed an injection? (free text)
Learning curve
The patients who had false-negative reports in the first visit were given a detailed explanation on the technique of monitoring for visual deterioration or distortion. They were advised vigilant observation of objects in their familiar environment for distortion, which included the edges of door frames, bathroom tiles, television screen, photo frames, and so on. All the patients in the study were retested with the same questions on a subsequent visit.
Statistical analyses
The patient's perception was divided into two groups based on their response to the first question on the questionnaire. The first group (group 1) consisted of patients who believed that they required treatment, and the second group (group 2) consisted of patients who did not consider that they required treatment. Group 2 also included patients who could not arrive at a decision as to whether they needed treatment or not. The presence of new or persistent fluid on OCT, drop in five ETDRS letters of VA with evidence of fluid or new macular haemorrhage were taken as objective measures for retreatment. The objective criteria were then compared with the subjective assessment of the need for retreatment by the patient.
Descriptive statistics of the patient characteristics was performed. Diagnostic accuracy of the patient's perception was determined using SPSS V.17.0, 2008 (SPSS Inc., Chicago, IL, USA). Sensitivity, specificity, positive and negative predictive values (PPV and NPV), and positive and negative likelihood ratios (LR+ and LR−) and the area under the receiver operating characteristics (ROC) curve, with 95% confidence intervals were calculated, using the physician's diagnosis as a reference standard. The true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN) were calculated. Sensitivity indicated the ability of patients not to miss reactivation of the disease (sensitivity=TP/TP+FN) and specificity, the ability to minimize false identification of activation (specificity=TN/TN+FP). PPV (PPV=TP/TP+FP) and NPV (NPV=TN/TN+FN) reflects the accuracy of the positive or a negative test, respectively. LR+ and LR reflect alterations in post-test probability when the test is positive or negative, respectively. The ROC curve is a plot of sensitivity vs 1 minus the specificity, and denotes the probability that the patient can correctly predict activation of the disease.
Clusters of functional findings and objective examination tests (as above), were also tabulated to determine the combinations that improved diagnostic accuracy. All possible combinations were examined to capture those factors that determined the strongest diagnostic accuracy. Post-test probability was calculated using LR+ and the prevalence of physician diagnosis of retreatment in the study sample (calculated as the number of participants who met the physicians' criteria for retreatment).
Results
Eight-nine patients (100 eyes) were included in the study. The patient characteristics in the groups 1 and 2 are shown in Table 1. There were 27 patients in group 1 and 73 in group 2, which included 3 patients who could not arrive at a decision on the need for retreatment. The presence of sub-retinal, intra-retinal or any fluid showed good correlation with the patient prediction. In group 1, 25 patients were TP and 2 were FP. In group 2, 57 were TN and 13 were FN. All three patients who were uncertain about the requirement for retreatment, required treatment and were included among the FN. The objective assessments of these patients are shown in Table 2. Table 3 shows the improvement in patient awareness on the subsequent visit.
Table 1. Patient characteristics on the day of the interview.
| Group 1 (yes), n=27 | Group 2 (no + do not know), n=73 | P-value | |
|---|---|---|---|
| Age | 78.7±5.7 | 80.7±5 | 0.17 |
| Females (%) | 19/27 (70%) | 42/73 (56%) | 0.18 |
| VA at visit | 56.2±21.2 | 58.1±26.9 | 0.55 |
| VA of other eye | 42±26 | 45±29 | 0.35 |
| Change in VA | 1.2±4.9 | 1±8.5 | 0.11 |
| Number of injections to date | 10.3±6.4 | 8.3±7.8 | 0.05 |
| SRF (%) | 14/27 (52%) | 11/73 (15%) | 0.0003 |
| IRF (%) | 16/27 (59%) | 13/73 (17%) | 0.0001 |
| SRF+IRF (%) | 5/27 (19%) | 5/73 (7%) | 0.07 |
| Any fluid (%) | 25/27 (93%) | 16/73 (22%) | <0.0001 |
Abbreviations: IRF, intraretinal fluid; SRF, subretinal fluid; VA, visual acuity.
Group 1, those who predicted they needed an injection.
Group 2 included patients who predicted they did not need an injection and those who were uncertain on the need for re-treatment.
Table 2. The proportion of patients with and without macular fluid and their perception of fluid.
| Any retreatment criteria | New fluid | Fluid +loss of vision | No fluid | |
|---|---|---|---|---|
| Patient perception: yes (requires treatment), n=27 | 25 (93%) | 17 (63%) | 3 (11%) | 2 (7%) |
| Patient perception: no (does not require treatment), n=70 | 13 (19%) | 6 (9%) | 1 (1.4%) | 57 (81%) |
| Do not know, n=3 | 3 (100%) | 1 (33%) | 1 (33%) | 0 |
Table 3. The improvement in patient awareness on the second visit compared with the initial visit.
| First test | Second test | |||||
|---|---|---|---|---|---|---|
|
OCT fluid |
OCT fluid |
|||||
| Absent | Present | Total | Absent | Present | Total | |
| Positive prediction | 2 (FP) | 25 (TP) | 27 | 1 (FP) | 28 (TP) | 29 |
| Negative prediction | 57 (TN) | 16 (FN) | 73 | 67 (TN) | 4 (FN) | 71 |
| Total | 59 | 41 | 100 | 68 | 32 | 100 |
Abbreviations: FN, false negatives; FP, false positives; TN, True negatives; TP, true positives.
Patient prediction showed that 93% of patients could accurately predict the need for an injection (PPV), but only 78% were accurate when they predicted that they did not need an injection (NPV). The test had a sensitivity of 61% (95% CI: 45.5–75.5%) and specificity of 96.6% (95% CI: 88.3–99.6%) at the first visit. The LR+ was 12.5 (95% CI: 3.2–47.6) and LR− 0.28 (95% CI: 0.18–0.44). After patient education on the environmental Amsler technique, the sensitivity and specificity improved to 87.5% and 98.5%, respectively, and PPV and NPV to 96.5% and 94.3%, respectively (Table 4).
Table 4. The comparison of sensitivity, specificity, predictive values, and likelihood ratios over two consecutive visits.
|
Initial test |
Repeat test |
|||
|---|---|---|---|---|
| Value | Confidence intervals | Value | Confidence intervals | |
| Sensitivity (%) | 61 | 45.5–75.5 | 87.5 | 70–95.9 |
| Specificity (%) | 96.6 | 88.3–99.6 | 98.5 | 90–99.9 |
| Positive predictive value (%) | 92.6 | 75.7–99.1 | 96.5 | 80–99.8 |
| Negative predictive value (%) | 78 | 66.9–86.9 | 94.3 | 85.4–98.1 |
| Positive likelihood ratio | 12.5 | 3.2–47.6 | 28 | 4–30 |
| Negative likelihood ratio | 0.28 | 0.18–0.44 | 0.59 | 0.02–0.15 |
Out of the 16 patients who were false negative on the first visit, 12 could correctly predict the need for injections at the subsequent visit. The four patients who were persistently false negative/ wrong in their prediction had good vision in the other eye (75.7±4.5 ETDRS letters) compared with the treated eye (57.2±12 ETDRS letters) (P-value=0.01).
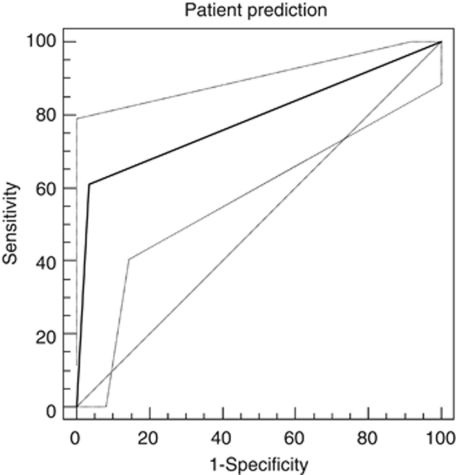
The ROC curve for initial patient prediction (Figure 1) showed the area under the curve (AUC) of 0.8 with a P-value of <0.0001.
Figure 1.
The ROC curve for initial patient prediction. The AUC is 0.8 with a P-value <0.0001.
The ROC curves for SRF and IRF had AUC of 0.67 and 0.69, respectively, with P-values 0.001 and 0.0001, respectively. A pairwise comparison of SRF and IRF showed a difference in AUC of 0.037 and a P-value of 0.67, indicating that fluid subtype did not determine the diagnostic accuracy of patient prediction. Seventy-eight percent of the patients reported visual distortion rather than visual deterioration as the criterion for retreatment.
Discussion
The ability of the patients to predict disease reactivation based on environmental Amsler test has a sensitivity of 61% and specificity of 96.6%. The area under the ROC curve was 0.8. The presence of intraretinal or subretinal fluid correlated well with the patient's perception. Training of patients on environmental Amsler technique helped to attain better predictive power.
The current treatment for NVAMD requires monthly monitoring of patients for objective evidence of reactivation of the disease in specialist retinal clinics. Most of the patients are able to perceive the improvement in vision and vision-related quality of life especially after the loading phase.4
The best-corrected visual acuity (BCVA) assesses the function in the central 1° to 2° of the visual field. However, BCVA is a poor indicator of macular function, as it does not accurately reflect difficulties with near vision tasks that require a wider, intact central visual field.
The Amsler grid measures the central 20° diameter of the visual field and is capable of capturing subtle changes in macular function.5 It is currently widely used for the self-monitoring of patients with early age-related maculopathy to detect conversion to the neovascular stage. However, the loss of fixation in patients with macular diseases and the phenomenon of cortical completion have been reported to adversely affect the reliability of this device.6, 7, 8 The test can also be challenging for some patients, as it involves an understanding of the test, perception of the visual defects while fixating at the centre, interpretation of their findings, and reporting the results.8 Hence, compliance with the test may be low.
Various other techniques such as the PHP (PreView PHP, Carl Zeiss Meditec, Dublin, CA, USA) have been reported to be useful in monitoring response to treatment in NVAMD by demonstrating statistically significant reductions in PHP contour areas between baseline and post treatment, which was shown to correlate well with OCT changes.9 However, this involves use of sophisticated tools either in a hospital setting or at home. A comparison of the PHP to the original Amsler grid showed that although the former had greater sensitivity than the Amsler grid in the detection of NV AMD, no difference in the specificity and accuracy of the two methods were observed in the diagnosis of NVAMD.10
Environmental Amsler is more practical and acceptable to patients. These functional vision tests include checking reading ability, monitoring straight edges for signs of distortion, overall image clarity, and colour intensity. Patients are advised to wear the appropriate glasses for distance or near vision, and check monocularly for any changes in vision function when viewing any familiar straight objects such as door frames, floor or wall tiles, lines of text on a page, and so on. They are also advised to become familiar with differences in clarity of vision between the eyes.11
The fact that most patients could be trained to detect reaccumulation of fluid in the macular area determining the need for retreatment, helps to extend the monitoring intervals in the AMD clinics and reduce the burden of the clinics. Patients who can reliably monitor their visual function with the environmental Amsler technique may be given longer intervals between their appointments, with the liberty of being seen urgently, when they detect a change in their visual function. However, this may not be applicable to all patients, especially those with early dementia and cognitive impairment.
The limitation of our study was that we excluded eyes that had not received injections in the past 6 months, and eyes with persistent fluid not responding to two injections. Therefore, we were unable to ascertain the use of environmental Amsler in these groups of patients. We also restricted our patient group to those with at least 12-month follow-up, but we are aware that some patients are able to detect a change in environmental Amsler as early as 6 months after initiation of therapy.
To conclude, the ‘environmental Amsler' technique seems to be a promising method in detecting the need for retreatment in NVAMD in a selected group of patients. There may be a short ‘learning curve' for some patients, but it can be easily mastered. However, we advocate further studies to determine the selection criteria for patients who can be relied upon to self-monitor their disease activity using the environmental Amsler technique.
Sobha Sivaprasad has received research grants, travel grants and speaker fees from Novartis, Pfizer, Bayer and Allergan.
References
- Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Trevino R. Recent progress in macular function self-assessment. Ophthalmic Physiolog Opt. 2008;28:183–192. doi: 10.1111/j.1475-1313.2008.00561.x. [DOI] [PubMed] [Google Scholar]
- Ranibizumab: The clinician's guide to commencing, continuing and discontinuing treatment. . http://www.rcophth.ac.uk . RCOphth Scientific Department - June 2008. [DOI] [PubMed]
- Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR. Improved vision-related function after ranibizumab treatment of neovascular AMD: results of a randomized clinical trial. Arch Ophthalmol. 2007;125 (11:1460–1469. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- Fletcher DC, Schuchard RA. Visual function in patients with choroidal neovascularization resulting from age-related macular degeneration: the importance of looking beyond visual acuity. Optom Vis Sci. 2006;83:178–189. doi: 10.1097/01.opx.0000204510.08026.7f. [DOI] [PubMed] [Google Scholar]
- Schuchard RA. Validity and interpretation of Amsler grid reports. Arch Ophthalmol. 1993;111 (6:776–780. doi: 10.1001/archopht.1993.01090060064024. [DOI] [PubMed] [Google Scholar]
- Fine AM, Elman MJ, Ebert JE, Prestia PA, Starr JS, Fine SL. Earliest symptoms caused by neovascular membranes in the macula. Arch Ophthalmol. 1986;104 (4:513–514. doi: 10.1001/archopht.1986.01050160069013. [DOI] [PubMed] [Google Scholar]
- Achard OA, Safran AB, Duret FC, Ragama E. Role of the completion phenomenon in the evaluation of Amsler grid results. Am J Ophthalmol. 1995;120 (3:322–329. doi: 10.1016/s0002-9394(14)72162-2. [DOI] [PubMed] [Google Scholar]
- Das R, Shi Y, Silvestri G, Chakravarty U. Distortion maps from preferential hyperacuity perimetry are helpful in monitoring functional response to Lucentis therapy. Retina. 2009;29:1013–1018. doi: 10.1097/IAE.0b013e3181a91dbf. [DOI] [PubMed] [Google Scholar]
- Isaac DLC, Avila MP, Cialdini AP. Comparison of the original Amsler grid with the preferential hyperacuity perimeter for detecting choroidal neovascularization in age-related macular degeneration. Arq Bras Oftalmol. 2007;70 (5:771–776. doi: 10.1590/s0004-27492007000500009. [DOI] [PubMed] [Google Scholar]
- Trevino R, Kynn MG. Macular function surveillance revisited. Optometry. 2008;7:397–403. doi: 10.1016/j.optm.2007.09.017. [DOI] [PubMed] [Google Scholar]