Abstract
Aim
To explore immunoregulatory and anti-inflammatory pathways specifically targeted by a subcutaneous anti-TNFαdrug—adalimumab—which might be relevant for controlling refractory uveitis.
Design
Non-randomized pilot intervention study on the effects of adalimumab on Treg populations and plasma VEGF levels in refractory uveitis patients. Inflammatory and immunological parameters were measured in 12 patients before therapy, and 1 and 6 months after therapy, and analyzed in the context of ophthalmological outcomes. The results were compared with those obtained in 10 systemic prednisone-treated uveitis patients.
Results
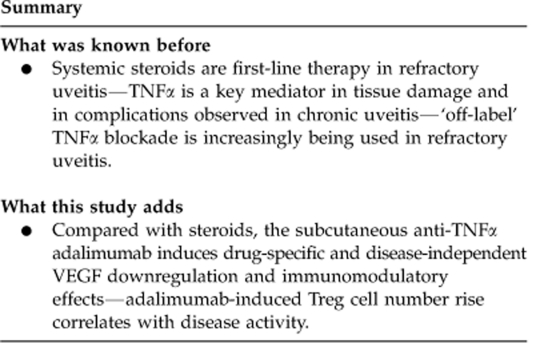
After 1 month of treatment, all patients responded, with 67% of adalimumab group and 80% of the corticosteroid group achieving inactivity (P=0.5). Unlike steroid-treated patients, a significant increase in T-regulatory CD4+ CD25high Foxp3+ CD127− cells was observed in adalimumab patients after 1 month of treatment, and maintained after 6 months (P=0.003). A significant adalimumab-specific drop in plasma VEGF was observed after 1 and 6 months of treatment (P=0.019). In every single patient, Tregs but not VEGF correlated with disease activity.
Conclusions
In refractory uveitis patients treated with adalimumab, clinical efficacy may be mediated through upregulation of Tregs in addition to modulation of VEGF-mediated inflammatory pathways. These biological properties, which were not observed in patients treated with corticosteroids, may reflect the specificity of TNF-αtargeting.
Keywords: uveitis, anti-TNFα, adalimumab, immunomodulation, Tregs, VEGF
Introduction
Whether isolated or associated to a systemic disease, noninfectious uveitic syndromes comprise a heterogeneous group of inflammatory conditions, accounting for about 10% of legal blindness.1, 2 From a pathogenic point of view, uveitis are thought to represent a continuum of immunological diseases, ranging from autoinflammatory conditions (ie, Blau syndrome and Crohn's associated uveitis) to the autoimmune uveitis observed within the monogenic APECED syndrome or the polygenic Vogt–Koyanagi–Harada syndrome (VKH).3
Tumor necrosis factor-α (TNFα) is a proinflammatory master cytokine produced by several cell types that can exert different effects depending on the tissue and the pathological conditions. In nonphysiological situations, TNFα has an important role in chronic inflammation and autoimmunity.4
Irrespective of whether innate or adaptive immune responses are prevalent, TNFα has been reported as an important mediator of the intraocular tissue damage observed in uveitis patients as well as in different models of experimental anterior and posterior uveitis.5, 6, 7, 8 It has also been associated with some of the most threatening uveitis complications, that is, cystoid macular edema,9 and choroidal and retinal neovascularization, probably through interaction with vascular endothelial growth factor (VEGF).10 However, because randomized-controlled trials in uveitis do not exist, anti-TNFα therapies have not yet been approved for any uveitis entity, except in Japan for refractory Behçet's uveitis. Notwithstanding, they are increasingly being used as off-label rescue therapies in some refractory uveitis (reviewed in Sharma et al11).
In systemic diseases in which anti-TNFα drugs are routinely used, anti-inflammatory clinical effects of anti-TNFα are usually accompanied by a reduction in the plasma levels of proinflammatory cytokines and chemokines, such as IL-1, IL-6, IL-8, and VEGF.12, 13 In uveitis patients, several papers have explored the diagnostic or pathogenic role of systemic and ocular levels of these and many other cytokines and chemokines,14, 15 but studies specifically dealing with the effect of anti-TNFα therapies on VEGF and other cytokines in different uveitis are scarce.
In addition to its anti-inflammatory properties, it has been reported that anti-TNFα therapies might also induce immunomodulatory effects on adaptive immune responses. Effects of anti-TNFα have already been described on CD4 cell numbers in sarcoidosis patients,16 on the expression of IL-10 by CD4 T cells in posterior uveitis patients,17 and on T-regulatory cells (Tregs) in RA and Crohn's patients.18, 19 This latter effect on Tregs might be of special clinical relevance in uveitis patients, as either a reduced frequency or impaired CD4+ Foxp3+ T-regulatory function has been described in noninfectious active uveitis,20 active Behçet's disease,21 and active VKH uveitis.22 However, some discrepant results have been published that might be probably related to different Treg identification strategies.23 In fact, identification of human Treg cells is confounded by multiple immunophenotypes reported in the literature, as well as the existence of several other T- and non-T-cell populations, exerting a regulatory function. However, it is widely accepted that Foxp3 regulatory T cells, either spontaneously arising from the thymus (nTreg) or peripherally-induced Tregs induced after infections, have the central role in controlling the immune activity against self-antigens.24
In the present work, we focus on the anti-inflammatory and immunomodulatory effects of a subcutaneous anti-TNFα drug (adalimumab) in a population of refractory active uveitis patients. By simultaneously measuring Treg cell numbers and plasma VEGF as surrogate end points, we wished to further understand the mechanisms of disease in ways which are not accessible from clinical observations or patient responses alone.
Patients and methods
Design
Non-randomized pilot intervention study on the effect of adalimumab as rescue therapy for active uveitis patients. Patients were clinically and immunologically evaluated before (t0) and 1 (t1) and 6 (t2) months after treatment.
Patients
A total of 12 patients (19 eyes) who had active chronic uveitis (lasting at least 6 months) refractory to systemic treatment were included. Data collected from patients before receiving treatment included demographic information (age and sex), diagnosis classified by anatomic location according to the Standardization of Uveitis Nomenclature criteria (SUN),25 laterality of disease, systemic disease activity, and previous systemic treatments (Table 1). Mean age was 36.16 years (range 14–58 years), and a variety of uveitis conditions were included: idiopathic panuveitis, VKH uveitis, Behçet's uveitis, juvenile idiopathic arthritis (JIA), SLE, AS, and psoriasis. Adalimumab (Humira, Abbott, Chicago, IL, USA), a fully human anti-TNFα monoclonal antibody, was chosen as rescue therapy for these patients because of failure with first-line systemic therapy. All of them received 40 mg of subcutaneous adalimumab every 14 days without modifications throughout the 6-month study period. None of them had received systemic and/or loco–regional corticosteroids within the last 30 days before starting adalimumab. Chest X-ray, Mantoux, and Quantiferon-TB Gold were performed in all patients before treatment. Adalimumab was the only immunomodulatory agent used in nine of them. In three patients (patients no. 3, 4, and 9), adalimumab was used alongside previous immunosuppressors, without any dosage modification throughout the study. All patients completed the study period without serious adverse effects.
Table 1. Characteristics of the patients before treatment with adalimumab or systemic steroids.
| ATG ID | Age | Sex | Diagnosis | Systemic disease activity | OCT/FA gradea | Previous systemic treatment |
|---|---|---|---|---|---|---|
| 1 | 58 | M | Idiopathic granulomatous panuveitis | NA | 1 | MM |
| 2 | 27 | F | Anterior non-granulomatous uveitis, AS | Active | 0 | SPN |
| 3 | 18 | M | Non-granulomatous panuveitis, VKH | Inactive | 2 | MTX |
| 4 | 34 | M | Non-granulomatous panuveitis, ABD | Active | 2 | CSA |
| 5 | 48 | M | Non-granulomatous panuveitis, ABD | Inactive | 2 | CSA |
| 6 | 58 | F | Non-granulomatous panuveitis, SLE | Inactive | 1+2 | MM |
| 7 | 36 | M | Anterior non-granulomatous uveitis, SpA | Inactive | 0 | PDN |
| 8 | 14 | F | Anterior non-granulomatous uveitis, JIA | Inactive | 0 | MTX |
| 9 | 31 | M | Anterior non-granulomatous uveitis, JIA | Active | 0 | MTX |
| 10 | 28 | F | Anterior non-granulomatous uveitis, JIA | Active | 1 | MTX, IBP |
| 11 | 49 | M | Intermediate non-granulomatous uveitis, AS | Inactive | 1+2 | MTX |
| 12 | 33 | M | Intermediate non-granulomatous uveitis, AS | Active | 2 | CSA |
|
STG ID |
Age |
Sex |
Diagnosis |
Systemic disease activity |
OCT/FA gradea |
Previous systemic treatment |
| 1 | 37 | M | JIA-associated anterior uveitis | Inactive | 0 | None |
| 2 | 46 | M | AS-associated intermediate uveitis | Active | 1 | None |
| 3 | 62 | M | Intermediate uveitis (RA patient) | Active | 1 | None |
| 4 | 38 | F | Idiopathic anterior uveitis | NA | 0 | None |
| 5 | 45 | F | RA-associated sclerouveitis | Active | 0 | None |
| 6 | 28 | M | HLA-B27+ anterior uveitis | NA | 0 | None |
| 7 | 39 | F | Sarcoidosis-associated anterior uveitis | NA | 0 | None |
| 8 | 80 | M | Idiopathic panuveitis | NA | 1 | None |
| 9 | 81 | M | Idiopathic intermediate uveitis | NA | 0 | None |
| 10 | 34 | M | Idiopathic anterior uveitis | NA | 0 | None |
Abbreviations: ABD, Adamantiades–Behçet's disease; AS, ankylosing spondylitis; ATG, adalimumab group; CSA, cyclosporine; FA, fluorescein angiography; IBP, Ibuprofen; JIA, juvenile idiopathic arthritis; MM, mycophenolate mofetil; MTX, methotrexate; NA, not applicable; OCT, optical coherence tomography; PDN, prednisone; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SpA, spondyloarthropathy; SPN, salazopyrin; STG, steroid group; VKH, Vogt–Koyanagi–Harada.
OCT/FA grading: 0=no signs of inflammation; 1=cystoid macular edema; 2=vasculitis; 1+2=CME+vasculitis.
A total of 10 additional patients (15 eyes), with active recurrent uveitis and a median age of 49 years (range 28–81 years), who had not previously received any systemic treatment for their condition were selected for comparison and treated with systemic steroids (Table 1). All patients included in this group had had at least one previous episode of uveitis during the last 12 months. All of them received prednisone at an initial dosage of 1 mg/kg/day that was slowly tapered according to disease activity. Prednisone therapy was stopped at day 30 of treatment in eight patients. Patients 1 and 2 received small doses of prednisone (<10 mg) for two additional months. Topical steroids were permitted in these patients if required.
To control for variability and immunological differences within the normal non-uveitic population, 25 age-matched control healthy individuals were used for comparisons in VEGF and Treg analysis.
Informed consent was obtained from the participants. All the procedures in this study were specifically approved by the Hospital's Ethics Committee, and followed the tenets of the Declaration of Helsinki.
Clinical evaluation
Uveitis clinical evaluation and monthly follow-up included visual acuity (VA; best-corrected Snellen VA) and ophthalmic examination, establishing the presence or absence of anterior chamber and/or vitreous inflammation according to the SUN criteria.25 Vitreous haze was considered following the system for the evaluation of vitreal inflammatory activity previously reported.26 Optical coherence tomography (Stratus III, Carl Zeiss Meditec, Dublin, CA, USA) was used before and after treatment in both groups of patients to determine the presence of cystoid macular edema (CME). The 1-mm central retinal thickness was evaluated using the fast macula scan feature (Table 2). Fluorescein angiogram (FA) was also performed in all subjects before and after the treatment to assess the presence of retinal vasculitis.
Table 2. Tregs and plasmatic VEGF levels before and after adalimumab.
| Patient ID |
Pretreatment (t0) |
After 1 month of treatment (t1) |
After 6 months of treatment (t2) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC infl./Vit. infl. | OCT R/La | Tregsb | VEGFc | AC infl./ Vit. infl. | OCT R/La | Tregsb | VEGFc | AC infl./ Vit. iInfl. | OCT R/La | Tregsb | VEGFc | |
| 1 | Active/active | 290/309 | 1.5 (450) | 134.42 | Inactive/active | 300/270 | 1.7 (510) | 131.89 | Inactive/inactive | 220/214 | 3.5 (1050) | 20.95 |
| 2 | Active/inactive | 227/232 | 0.8 (240) | 57.69 | Inactive/inactive | 216/227 | 1.1 (330) | 44.89 | Inactive/inactive | 208/220 | 2.3 (690) | 50.29 |
| 3 | Active/active | 253/236 | 0.4 (120) | 85.63 | Inactive/active | 235/226 | 0.8 (240) | 46.68 | Inactive/inactive | 226/230 | 0.7 (219) | 39.61 |
| 4 | Active/active | 227/232 | 0.3 (90) | 158.21 | Inactive/inactive | 219/230 | 1.0 (300) | 104.31 | Active/active | 227/219 | 0.5 (150) | 82.47 |
| 5 | Inactive/active | 233/221 | 0.6 (180) | 46.68 | Inactive/inactive | 222/226 | 0.7 (210) | 30.16 | Inactive/inactive | 209/214 | 0.8 (240) | 27.66 |
| 6 | Active/active | 242/520 | 1.7 (510) | 34.44 | Inactive/active | 224/469 | 2.4 (720) | 46.68 | Inactive/inactive | 229/206 | 2.2 (660) | 96.45 |
| 7 | Active/inactive | 211/210 | 3.8 (1140) | 61.48 | Inactive/inactive | 200/207 | 4.0 (1200) | 37.88 | Inactive/inactive | 209/212 | 3.9 (1170) | 27.66 |
| 8 | Active/inactive | 209/220 | 0.5 (150) | 140.92 | Inactive/inactive | 214/210 | 1.5 (450) | 100.91 | Inactive/inactive | 211/223 | 1.3 (390) | 61.48 |
| 9 | Active/inactive | 217/211 | 0.8 (240) | 75.26 | Inactive/inactive | 221/219 | 2.2 (660) | 110.07 | Active/inactive | 217/222 | 1.4 (435) | 36.15 |
| 10 | Active/inactive | 299/321 | 2.0 (600) | 17.56 | Active/active | 242/300 | 2.1 (630) | 12.27 | Inactive/inactive | 227/242 | 2.1 (636) | 9 |
| 11 | Active/inactive | 305/219 | 2.0 (600) | 140.21 | Inactive/inactive | 277/221 | 2.1 (630) | 103.02 | Inactive/inactive | 233/230 | 2.1 (630) | 80.22 |
| 12 | Active/active | 221/228 | 0.38 (114) | 23.47 | Inactive/inactive | 220/225 | 0.7 (216) | 19.26 | Inactive/inactive | 224/227 | 1.4 (426) | 9 |
Abbreviations: AC infl., anterior chamber inflammation; L, left eye; OCT, optical coherence tomography; R, right eye; Vit. infl., vitreous inflammation.
OCT results (1-mm central retinal thickness) are in m.
Treg: results are % referred to CD4 T cells, and numbers in brackets are absolute Treg cell numbers in 30 000 CD4 T cells analyzed.
VEGF: results are in pg/ml.
We classified ‘inactive uveitis' as grade 0 cells in both anterior and/or posterior segment in addition to absence of other signs of intraocular inflammation (CME and vasculitis). Moreover, we defined ‘improved activity' as a two-step decrease in level of inflammation (eg, anterior chamber cells, vitreous haze) or decrease to grade 0.
Immunological work-up
Peripheral blood samples were obtained from 12 patients in the adalimumab group (ATG) and 10 patients from the systemic steroid group (STG). Three samples from each patient in the adalimumab group were studied, one obtained before the beginning of the treatment (t0), the second extracted after 1 month of treatment (t1), and the last after 6 months of treatment (t2). As all STG patients were inactive after 3 months, only the t0 and t1 samples were obtained from this group.
VEGF measurement. Aliquots of sera from patients were frozen at −70 °C until later use. To minimize for intra- and inter-experiment variations, one aliquot from each patient or control was run in duplicate within the same assay, and replicated in a different assay with the remaining aliquot. VEGF was measured by means of cytometric bead array technology (CBA; Becton Dickinson, Franklin Lakes, NJ, USA), acquired in a FACS Canto cytometer, and analyzed with FCAP Array software (Becton-Dickinson). Limit of detection for VEGF was 2.5 pg/ml.
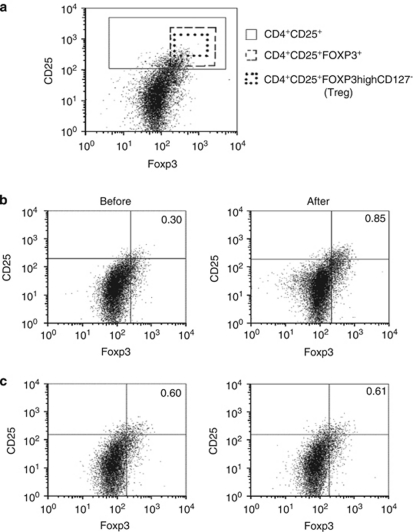
Peripheral blood and Treg immunophenotyping. Total T CD3, CD4, and CD8 lymphocytes were studied in whole peripheral blood samples at t0, t1 (STG and ATG), and t2 (only ATG) using flow cytometry. Absolute cell numbers were obtained by using Trucount tubes (Becton Dickinson). T-regulatory phenotyping was performed in peripheral blood mononuclear cells purified by Ficoll gradient. The following fluorochrome-labeled monoclonal antibodies were used for cell surface staining: CD3 (clone SK7) and CD25 (clone 2A3) from Becton Dickinson and CD4 (clone RPA-T4) and CD127 (clone M21) from Pharmingen (Franklin Lakes, NJ, USA). For intracellular staining, cells were fixed and permeabilized before adding antihuman Foxp3 (clone 259D/C7, from Pharmingen). Adequate isotype controls were always included in every experiment. Samples were run and analyzed in a two laser flow cytometer (FACS Canto, Becton Dickinson), and reanalyzed with FlowJo software (Tree Star, Ashland, OR, USA). Average number of CD3+ CD4+ cells analyzed from each sample was at least 30 000. The following populations were defined: total CD3 CD4 (CD3+ CD4+), total CD25 T cells (CD3+ CD4+ CD25+), total T cells co-expressing CD25 and FoxP3 (CD3+ CD4+ CD25+ FoxP3+), and T-regulatory cells (CD3+ CD4+ CD25high FoxP3+ CD127−; Figure 1a). Percentages and absolute numbers were referred to the total CD3+ CD4+ cells.
Figure 1.
Tregs in uveitis patients treated with adalimumab or systemic steroids. (a) Three different CD3+ CD4+ cell populations analyzed according to CD25, Foxp3, and CD127 degree of expression, after a previous selection as described in Patients and methods. (b) Treg percentages before (left) and after 1 month (right) of adalimumab are shown. (c) Treg analysis before (left) and after 1 month (right) of steroids. Results are in percent (%) referred to as % of CD3+ CD4+ total cells, and are representative of a typical patient from each group.
Statistical analysis
All the variables were analyzed with SPSS v 17.0 (SPSS, Chicago, IL, USA). Continuous variables were expressed as medians and SEM, unless otherwise specified. Differences between groups for quantitative variables were assessed by nonparametric Mann–Whitney U-test. The nonparametric Wilcoxon test was used when paired samples were compared. Dependency of variables was assessed by Spearman's Rho correlation test. A P-value <0.05 was selected to reject the null hypothesis.
Results
Clinical responses
All patients with refractory uveitis who received adalimumab as rescue therapy responded favorably after 1 month of treatment, with 8/12 patients showing fully inactive uveitis, 3/12 improved activity, and 1/12 (patient no. 10) remaining active at t1 (Table 2). By month 1, all subjects had VA improvement in at least one eye. Signs of active vasculitis on angiography (evidenced in six patients before treatment) were absent by month 1 in all patients, whereas OCT signs of macular edema (evidenced in four patients before treatment) were still present in three patients, but showed decreased central macular thickness (Table 2). After 3 months of treatment, all patients had their uveitis under control, with complete resolution of macular edema. However, relapses occurred in two patients later on, while still on adalimumab. Patient no. 4 with Behçet-associated panuveitis had a relapse after a 4-month period free of inflammation. Patient no. 9 had a relapse of his JIA-associated uveitis after cataract surgery. He had been under control for 3 months before surgery.
All steroid-treated patients but two (patients no. 2 and 3) had resolution of their ocular inflammation after 1 month of treatment, and systemic therapy was slowly discontinued. By month 6, intraocular inflammation was inactive in 9/10 patients (all patients except no. 3, who switched to mycophenolate), and remained asymptomatic until the end of the study (data not shown).
No major systemic adverse effects were observed. Only a local reaction (patient no. 2) and two minor upper respiratory infections (patients no. 4 and 9) were recorded in the adalimumab group.
VEGF measurement
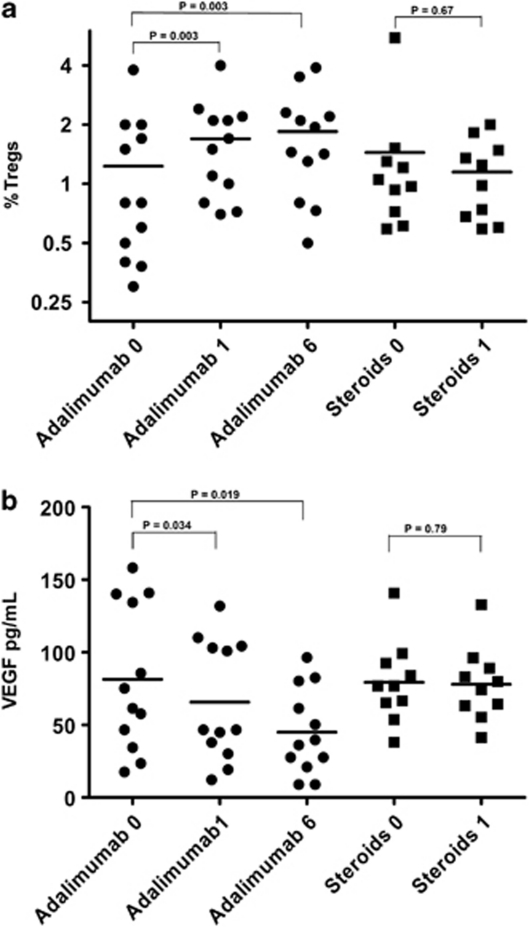
VEGF plasma levels were measured at t0, t1, and t2 in adalimumab patients, and t0 and t1 in patients treated with systemic steroids. As VEGF is not commonly measured and reference ranges do not exist, we used 25 healthy controls for comparisons. Median VEGF plasma levels in uveitis patients before treatment (n=22, t0) did not significantly differ from those observed in normal controls (78.18 and 102.45 pg/ml, respectively, P=0.077). Moreover, no significant differences in median basal VEGF levels were found at t0 between adalimumab- and steroid-treated patients (81.33 and 75.04 pg/ml, respectively, P=0.792). All adalimumab-treated patients but one (no. 6 with a diagnosis SLE) showed decreased VEGF plasma levels after treatment. As seen in Figure 2, VEGF levels significantly decreased after 1 month of adalimumab (median 65.68 pg/ml, P=0.034) and continued decreasing after 6 months (median 45.07 pg/ml, P=0.019). In all, 10 out 12 patients showed decreased VEGF at t1 and 11/12 at t2. This effect was not observed after treatment with steroids (P=0.79).
Figure 2.
Tregs and VEGF plasmatic levels at different time points in uveitis patients treated with adalimumab or systemic steroids. (a) Tregs (% of total CD4+ T lymphocytes) and (b) VEGF levels (pg/ml) from 12 adalimumab-treated and 10 steroid-treated patients are shown at different time points (0, 1, and 2 corresponding to pretreatment, after 1 month and 6 months of treatment). Individual patients and means are represented.
Adaptive immune-response parameters: total lymphocytes, total CD25 T lymphocytes, and Tregs
To look for changes in adaptive immune responses, peripheral blood lymphocyte phenotyping was performed at different time points. We did not find basal differences in T populations between uveitis patients and age-matched controls, or among adalimumab- and steroid-treated patients. No effect in absolute or relative T CD4 and CD8 subpopulations could be observed after either treatment (data not shown). A detailed analysis of three different populations of CD3+, CD4+, and CD25+ T lymphocytes was later performed (Figure 1a). Steroid-treated patients, but not adalimumab-treated patients, showed a significant median reduction in total CD25 T-cell numbers (CD3, CD4, CD25) after 1 month of treatment (P=0.028; Table 3). We next explored changes in CD25 T cells expressing Foxp3 (CD3, CD4, CD25, FoxP3), which include activated T and Treg cells. Both adalimumab and steroids induced a significant reduction of this population (Table 3).
Table 3. Changes in different CD4+CD25+ cell populations in uveitis patients treated with adalimumab or systemic steroids.
| Lymphocyte population |
ATG patients (n=12) |
STG patients (n=10) |
||||||
|---|---|---|---|---|---|---|---|---|
| t0 | t1 | t2 | P-value |
t0 | t1 | P-value | ||
| (t0–t1) | (t0–t2) | (t0–t1) | ||||||
| CD3 CD4 CD25 | 4.04 (2.48–8.48) | 2.73 (1.96–7.28) | 2.93 (1.12–14.64) | 0.239 | 0.108 | 4.03 (1.54–5.61) | 3.48 (1.21–4.88) | 0.028 |
| CD3 CD4 CD25 Foxp3 | 2.37 (0.80–4.36) | 1.98 (0.77–3.59) | 2.02 (0.85–3.42) | 0.084 | 0.023 | 2.7 (1.02–4.46) | 2.04 (1.12–3.52) | 0.032 |
| CD3 CD4 CD25high Foxp3 | 0.8 (0.38–3.80) | 1.6 (0.60–4.0) | 1.69 (0.50–3.90) | 0.003 | 0.003 | 1.01 (0.59–5.53) | 1.12 (0.59–2.0) | 0.799 |
Abbreviations: ATG, adalimumab-treated group; STG, steroid-treated group.
P-values are referred to differences between variation at the specified time points. t0=pretreatment; t1=after 1 month of treatment; t2=after 6 months of treatment. Results are median % values, and are referred to CD4.
Finally, we wished to specifically explore treatment-induced changes in the number of regulatory T cells exerting immunosuppressive functions (Tregs). For such a purpose, we restricted the analysis to CD4 T lymphocytes expressing the highest amount of surface CD25 that co-expressed Foxp3, and simultaneously lacking CD 127 (Figure 1a). Median baseline Treg values in refractory uveitis patients selected for adalimumab treatment did not differ from normal 25 non-uveitis controls (P=0.35), nor from steroid-treated patients (P=0,57). In all, 12 out 12 adalimumab-treated patients showed increased Treg cell numbers (Table 2). After 1 month on adalimumab (two infusions), median Treg cell numbers significantly increased above pretreatment values (P=0.003), and remained at the same increased values after 6 months of treatment (P=0.003; Figure 2). No effect on Tregs was observed in steroid-treated patients (P=0.67). Figure 1b shows a representative patient from the adalimumab group and from the steroid group (Figure 1c) before (left panel) and after (right panel) 1 month of either treatment.
Correlation between clinical activity, VEGF, and Tregs
First at all, we analyzed whether the relevant observed effects of adalimumab on VEGF and Tregs were related. In a paired analysis, no association was found between decreased plasma VEGF levels and increased Treg cell numbers (Spearman's ρ, P=0.396, 0.497, and 0.609 at t0, t1, and t2, respectively), ruling out a direct effect of Treg cell numbers on plasma VEGF levels.
Because changes in plasma VEGF and Tregs were apparently adalimumab specific, we next wished to explore whether they correlated with disease activity. An important variability in Treg cell numbers was observed among normal controls, and also among patients at t0. In an intraindividual analysis at different time points, increased Tregs mostly correlated with clinical remission, except in patients no. 1, 3, and 6, with a diagnosis of idiopathic panuveitis, VKH-, and SLE-associated uveitis, respectively. In these three patients, although Treg cell numbers had already raised and clinical improvement was evident by month 1, longer adalimumab treatment and steady Treg cell numbers were required for full clinical remission. Relapses in patients no. 4 and 9 were accompanied by a significant decline in Tregs (Table 2).
The same analysis on the entire active and inactive uveitis patients was performed with plasma VEGF levels. In an intra-patient analysis, despite falls in VEGF levels occurred in 8 out 12 patients, a correlation between falls in VEGF and clinical measures of activity was only observed in 6 out 12 adalimumab-treated patients (Table 2).
Discussion
In the present work, we wished to explore the effect of the subcutaneous TNFα blocker adalimumab on likely relevant immunological targets involved in refractory uveitis patients. In an attempt to investigate the mechanisms that might be associated with its reported clinical benefits in some uveitis patients, we focused on VEGF and Tregs, and compared the effects with those obtained with the current gold-standard therapy (ie, systemic steroids). We have shown that although both treatments were equally able to reduce clinical activity, only adalimumab induced increased Treg cell numbers and a reduction in plasma VEGF levels. A controlled trial—the ideal design for this purpose—could not be carried out because anti-TNFα in our and in most institutions is only allowed as rescue therapy for uveitis when other treatments have failed.
Inflammation and angiogenesis are tightly linked. TNFα is recognized as one of the inflammatory cytokines that upregulates VEGF production in choroidal endothelial cells, thus promoting ocular neoangiogenesis and cystoid macular edema.27, 28 In fact, treatment with anti-TNFα has been useful in regression of neovascular age-related macular degeneration,29 in an animal model of laser-induced neovascularization,30 and in cystoid macular edema.31 Although not yet described in uveitis patients, a reduction in plasma VEGF levels in infliximab-treated psoriasis, RA, and AS patients correlated with disease inactivity.12, 32, 33 Thus, we chose the presumptive pathogenically relevant proinflammatory and proangiogenic VEGF as a surrogate marker of the anti-inflammatory effect of adalimumab in our refractory uveitis patients. We observed a reduction of plasma VEGF in the adalimumab group, which was not observed in patients treated with steroids, suggesting a specific drug effect. Of note, VEGF consistently decreased in all but one patient with a previous diagnosis of SLE, a recognized anti-TNFα refractory disease. This finding extends to uveitis patients the previously reported effects of anti-TNFα therapies on systemic VEGF levels. It might be an additional interesting specific anti-inflammatory therapeutic effect that could be considered to prevent macular edema and neovascularization in certain chronic uveitis patients. However, because the small number of patients analyzed, larger and/or parallel studies on VEGF levels within ocular fluids are needed to address whether this adalimumab-specific effect might be relevant in uveitis' natural history.
When focusing on the immunomodulatory effect on the adaptive immune responses elicited by either treatment, we did not find any significant effect on T lymphocytes. However, both steroids and adalimumab equally reduced the number of CD4+ CD25+ Foxp3+ T lymphocytes, which besides Treg mostly include activated and memory T lymphocytes.34 It has to be stressed that some of these CD4+ CD25+ non-regulatory T cells can transiently co-express Foxp3 upon activation,35 rendering distinction between CD4 T-activated cells and Tregs complicated. This might explain several disparate reports of Treg quantification in disease settings.36 Interestingly, when we restricted the analysis to the CD25 brightest population that co-expressed Foxp3 and lacked CD127—which have been considered as more Treg-specific markers37—some important differences appeared. First of all, mean Treg cell numbers did not differ between uveitis patients and age-matched controls, apparently lessening its role at disease onset. There were, however, wide fluctuations among individuals, both controls and patients. Second, patients selected for adalimumab because steroid resistance did not exhibit lower basal Treg cell numbers than steroid-treated patients, suggesting that Tregs are not an appropriate biomarker for identifying refractory disease. Finally, adalimumab specifically induced a sustained increase in Tregs in 12/12 patients, which was not observed in any steroid-treated patient. This effect had been previously demonstrated in RA patients in whom infliximab—an intravenous anti-TNFα blocker—increased periphery-induced Treg cell numbers and function, which correlated with a reduction in C-reactive protein and inactive disease.38
Our finding of increased Treg, either natural or periphery induced, after treatment with adalimumab in all patients from a heterogeneous case series strongly suggests a drug-specific effect. Is this relevant for disease control? In the experimental autoimmune uveitis EAU model, it is thought that naturally occurring thymic nTregs control the threshold of susceptibility to uveitis, meanwhile periphery-induced Tregs are likely responsible of its termination.24 Interestingly, in a very recent paper, Sugita39 reported that patients with active Behçet's uveitis, but not patients with active VKH uveitis or active toxoplasmosis, showed decreased Treg cell numbers and function that specifically increased after infliximab therapy. In that paper, raised Treg was associated with remission and a lack of subsequent episodes of acute uveitis. Similar results were found here in both included Behçet's patients. In our heterogeneous case series, an association was observed between increased Treg cell numbers and reduction in disease activity at 6 months in each adalimumab-treated patient. This might point toward a role of Tregs in long-term control of relapses. However, the lack of a clinical correlation in some patients at 1 month clearly reflects that mechanisms other than Tregs—ie, other regulatory mechanisms—contribute to controlling uveitis flares, as observed in our steroid-treated uveitis patients.
Limitations in this study exist that include small sample size, heterogeneity of the uveitis, and a potential bias in patients selection, as adalimumab-treated patients only included those in whom steroids and other immunomodulatory drugs had previously failed. Moreover, a functional analysis of Tregs was not performed. Despite these concerns, we believe that the results herein presented suggest adalimumab-specific effects in uveitis patients irrespective of the underlying disease. If our findings are confirmed by other investigators, further studies should be carried out to assess whether earlier introduction of anti-TNFα drugs might modify the natural history of refractory uveitis syndromes.
Acknowledgments
We are grateful to the patients and controls for their participation in this study.
Dr JG Ruiz de Morales has received speaker honoraria from Abbott Laboratories (less than 10.000€). Dr Ruiz de Morales currently receives funding through a research grant (2010/A/531) from the Department of Health of the Junta de Castilla y León, Spain (SACYL).
References
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111 (3:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3 (8:e297. doi: 10.1371/journal.pmed.0030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinecker C, Redlich K, Smolen JS. Cytokines as therapeutic targets: advances and limitations. Immunity. 2008;28 (4:440–444. doi: 10.1016/j.immuni.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Dick AD, Duncan L, Hale G, Waldmann H, Isaacs J. Neutralizing TNF-alpha activity modulates T-cell phenotype and function in experimental autoimmune uveoretinitis. J Autoimmun. 1998;11 (3:255–264. doi: 10.1006/jaut.1998.0197. [DOI] [PubMed] [Google Scholar]
- Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Invest Ophthalmol Vis Sci. 1994;35 (3:924–930. [PubMed] [Google Scholar]
- Woon MD, Kaplan HJ, Bora NS. Kinetics of cytokine production in experimental autoimmune anterior uveitis (EAAU) Curr Eye Res. 1998;17 (10:955–961. doi: 10.1076/ceyr.17.10.955.5246. [DOI] [PubMed] [Google Scholar]
- Sartani G, Silver PB, Rizzo LV, Chan CC, Wiggert B, Mastorakos G, et al. Anti-tumor necrosis factor alpha therapy suppresses the induction of experimental autoimmune uveoretinitis in mice by inhibiting antigen priming. Invest Ophthalmol Vis Sci. 1996;37 (11:2211–2218. [PubMed] [Google Scholar]
- Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001;132 (5:794–796. doi: 10.1016/s0002-9394(01)01103-5. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Elner VM. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84 (5:812–822. doi: 10.1016/j.exer.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SM, Nestel AR, Lee RW, Dick AD. Clinical review: Anti-TNFalpha therapies in uveitis: perspective on 5 years of clinical experience. Ocul Immunol Inflamm. 2009;17 (6:403–414. doi: 10.3109/09273940903072443. [DOI] [PubMed] [Google Scholar]
- Macias I, Garcia-Perez S, Ruiz-Tudela M, Medina F, Chozas N, Giron-Gonzalez JA. Modification of pro- and antiinflammatory cytokines and vascular-related molecules by tumor necrosis factor-a blockade in patients with rheumatoid arthritis. J Rheumatol. 2005;32 (11:2102–2108. [PubMed] [Google Scholar]
- Visvanathan S, van der Heijde D, Deodhar A, Wagner C, Baker DG, Han J, et al. Effects of infliximab on markers of inflammation and bone turnover and associations with bone mineral density in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68 (2:175–182. doi: 10.1136/ard.2007.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok AM, Luyendijk L, Zaal MJ, Rothova A, Hack CE, Kijlstra A. Elevated serum IL-8 levels are associated with disease activity in idiopathic intermediate uveitis. Br J Ophthalmol. 1998;82 (8:871–874. doi: 10.1136/bjo.82.8.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Savant V, Scott RA, Curnow SJ, Wallace GR, Murray PI. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2007;48 (5:2203–2207. doi: 10.1167/iovs.06-1358. [DOI] [PubMed] [Google Scholar]
- Crouser ED, Lozanski G, Fox CC, Hauswirth DW, Raveendran R, Julian MW. The CD4+ lymphopenic sarcoidosis phenotype is highly responsive to anti-tumor necrosis factor-alpha therapy. Chest. 2010;137 (6:1432–1435. doi: 10.1378/chest.09-2576. [DOI] [PubMed] [Google Scholar]
- Greiner K, Murphy CC, Willermain F, Duncan L, Plskova J, Hale G, et al. Anti-TNFalpha therapy modulates the phenotype of peripheral blood CD4+ T cells in patients with posterior segment intraocular inflammation. Invest Ophthalmol Vis Sci. 2004;45 (1:170–176. doi: 10.1167/iovs.03-0659. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200 (3:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli I, Lindley KJ, Londei M, Quaratino S. Anti tumour necrosis-alpha therapy increases the number of FOXP3 regulatory T cells in children affected by Crohn's disease. Immunology. 2008;125 (2:178–183. doi: 10.1111/j.1365-2567.2008.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh S, Li Z, Forooghian F, Hwang FS, Cunningham MA, Pantanelli S, et al. CD4+Foxp3+ T-regulatory cells in noninfectious uveitis. Arch Ophthalmol. 2009;127 (4:407–413. doi: 10.1001/archophthalmol.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzaoui K, Hamzaoui A, Houman H. CD4+CD25+ regulatory T cells in patients with Behcet's disease. Clin Exp Rheumatol. 2006;24 (Suppl 42:S71–S78. [PubMed] [Google Scholar]
- Chen L, Yang P, Zhou H, He H, Ren X, Chi W, et al. Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci. 2008;49 (8:3475–3482. doi: 10.1167/iovs.08-1793. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10 (7:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120 (9:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140 (3:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, et al. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998;273 (34:22128–22135. doi: 10.1074/jbc.273.34.22128. [DOI] [PubMed] [Google Scholar]
- Hangai M, He S, Hoffmann S, Lim JI, Ryan SJ, Hinton DR. Sequential of angiogenic growth factors by TNF-α in choroidal endothelial cells. J Neuroimmunol. 2006;171:45–56. doi: 10.1016/j.jneuroim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Markomichelakis NN, Thedossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol. 2005;139:537–540. doi: 10.1016/j.ajo.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Lichtlen P, Lam TT, Nork TM, Streit T, Urech DM. Relative contribution of VEGF and TNF-α in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest Ophthalmol Vis Sci. 2010;51 (9:4738–4745. doi: 10.1167/iovs.09-4890. [DOI] [PubMed] [Google Scholar]
- Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP. Infliximab for chronic cystoid macular edema associated with uveítis. Am J Ophthalmol. 2004;138 (4:648–650. doi: 10.1016/j.ajo.2004.04.066. [DOI] [PubMed] [Google Scholar]
- Canete JD, Pablos JL, Sanmarti R, Mallofre C, Marsal S, Maymo J, et al. Antiangiogenic effects of anti-tumor necrosis factor alpha therapy with infliximab in psoriatic arthritis. Arthritis Rheum. 2004;50 (5:1636–1641. doi: 10.1002/art.20181. [DOI] [PubMed] [Google Scholar]
- Pedersen SJ, Hetland ML, Sorensen IJ, Ostergaard M, Nielsen HJ, Johansen JS. Circulating levels of interleukin-6, vascular endothelial growth factor, YKL-40, matrix metalloproteinase-3, and total aggrecan in spondyloarthritis patients during 3 years of treatment with TNFalpha inhibitors. Clin Rheumatol. 2010;29 (11:1301–1309. doi: 10.1007/s10067-010-1528-x. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203 (7:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103 (17:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37 (1:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30 (6:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204 (1:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Yamada Y, Kaneko S, Horie S, Mochizuki M. Induction of regulatory T cells by infliximab in Behcet's disease. Invest Ophthalmol Vis Sci. 2011;52 (1:476–484. doi: 10.1167/iovs.10-5916. [DOI] [PubMed] [Google Scholar]