Abstract
Background
To determine the incidence of acute retinal necrosis (ARN) in the United Kingdom and to describe the demographics, management, and visual outcome in these patients.
Methods
This was a prospective study carried out by the British Ophthalmological Surveillance Unit (BOSU) between September 2007 and October 2008. Initial and 6-month questionnaires were sent to UK ophthalmologists who reported cases of ARN via the monthly BOSU report card system.
Results
In all, 45 confirmed cases (52 eyes) of ARN were reported in the 14-month study period, giving a minimum incidence of 0.63 cases per million population per year. There were 20 females and 25 males. Age ranged from 10 to 94 years. Eight patients had a history of herpetic CNS disease. Aqueous sampling was carried out in 13 patients, vitreous in 27, and cerebrospinal fluid (CSF) in 4. Varicella-zoster virus followed by herpes simplex were the most common causative agents. Treatment in 76% of the cases was with intravenous antivirals; however, 24% received only oral antivirals. In all, 47% of patients had intravitreal antiviral therapy. Visual outcome at 6 months was <6/60 in 48% of the affected eyes.
Conclusion
The minimum incidence of ARN in the UK is 0.63 cases per million. Patients with a history of herpetic CNS disease should be warned to immediately report any visual symptoms. There is increased use of oral and intravitreal antivirals in initial treatment.
Keywords: acute retinal necrosis, retinitis, uveitis, epidemiology, herpes viridae
Introduction
Acute retinal necrosis (ARN) is a rare but visually devastating condition. Standard diagnostic criteria from the American Uveitis Society consist of:1
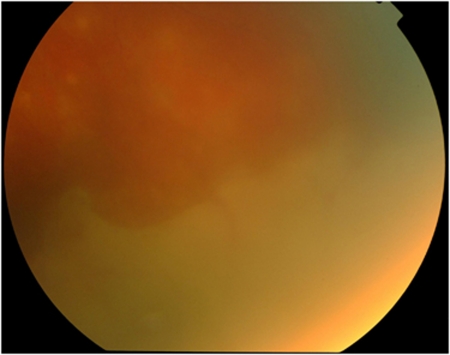
one or more foci of retinal necrosis with discrete borders in the retina (Figure 1);
progression of disease in the absence of treatment;
evidence of occlusive vasculopathy; and
an inflammatory reaction in the vitreous or anterior chamber.
Figure 1.
Acute retinal necrosis—peripheral retinal necrosis with discrete scalloped margin.
The diagnosis is therefore based not on aetiology but rather on clinical features. In recent literature most references to ARN assume a viral aetiology with other conditions that are subsequently identified as mimicking ARN. The human herpes viruses known to cause ARN are varicella-zoster virus (VZV), herpes simplex 1 and 2 (HSV1, HSV2), and, less commonly, cytomegalovirus (CMV) and Epstein–Barr virus (EBV).2,3 Progressive outer retinal necrosis (PORN) has been described as a similar syndrome with minimal inflammation in immunocompromised patients.
In this study we determine the minimum incidence of ARN in the United Kingdom and describe the demographic, clinical profile, investigations, management, and outcome of treatment in these patients.
Materials and methods
New cases of ARN or PORN were prospectively ascertained through the monthly active surveillance system of BOSU.4 At the end of each month the yellow BOSU report card was sent to all consultant and associate specialist ophthalmologists in the United Kingdom. (This list is maintained by BOSU, who monitor new appointments and conduct an annual telephone census.) The card contains up to 10 rare eye conditions of interest and respondents are asked to indicate all new cases of these conditions or confirm that they had no new cases to report. From 1 September 2007 to 31 0ctober 2008 ophthalmologists were asked to report any cases of ARN or PORN seen each month on the BOSU reporting card.
Once a case had been reported the ophthalmologist concerned was sent an initial and follow-up questionnaire 6 months later.
Visual acuity (VA) was recorded using Snellen acuity. For statistical analysis Snellen VA was converted to logarithm of the minimum angle of resolution (logMAR). Non-numerical acuities were converted using a previously described arbitrary scale:5 counting fingers 1.7, hand movements 2.0, perception of light 2.3, and no perception of light 3.0.
The results from the questionnaires were entered onto a database and analysed by the statistical department. Missing data and values were excluded from the analysis. Analysis was performed using SPSS Statistics, Rel. 17.0.1. 2008 (SPSS Inc., Chicago, IL, USA).
Statistical analysis comparing means was performed using an independent samples t-test and associations between categorical variables were tested using Fisher's exact test. Statistical significance was defined as P<0.05.
The study had multi-domain research approval from the Oxfordshire ethics committee (REC reference number 07/Q1604/47).
Results
Cases
Seventy-seven cases of ARN were reported to BOSU between 1 September 2007 and 31 October 2008. Seventy (91%) initial questionnaires were returned and from those 45 new cases of ARN were identified and 25 were excluded. Of the 25 excluded cases, 3 were triplicate cases, 9 were duplicates, 4 were wrong diagnoses (2 were toxoplasmosis, 1 syphilitic retinitis, and 1 CMV retinitis), and 6 presented outside study dates.
Of the 45 cases of ARN, 2 were identified, by clinical characteristics, as being PORN. One patient died several weeks following ARN diagnosis; of the rest, 6-month follow-up questionnaires were returned for 37 cases (84.1%) with 42 affected eyes.
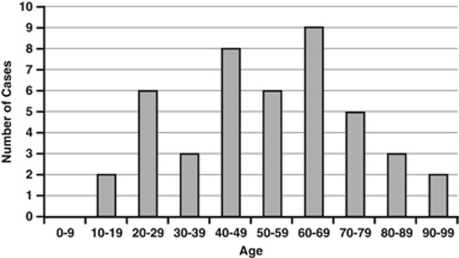
Forty-five new cases of ARN in the UK (population 60 975 0006) were identified over 14 months. This gives a minimum UK incidence of 0.63 cases per million population per year. Twenty-five (55.6%) ARN cases were male and 20 (44.4%) were female. Age at presentation ranged from 10 to 94 years (Figure 2). Thirty-eight (84.4%) cases were unilateral and 7 (15.6%) bilateral. In the seven bilateral cases five presented with both eyes affected simultaneously, and in two cases the fellow eye became involved 3 months after the first eye.
Figure 2.
Distribution of ages of patients with ARN.
Previous/concurrent herpetic infection
In all, 25 (55.6%) patients had a documented history of herpetic infection before presenting with ARN (Table 1). Two cases were diagnosed with ARN with concurrent herpetic illness (one herpes zoster ophthalmicus and one herpes simplex encephalitis). Two cases were diagnosed with chicken pox less than 6 weeks before developing ARN. Of the four cases of ARN with a previous history of fellow eye ARN, one was immunodeficient (HIV) and three had no risk factors for immunodeficiency.
Table 1. Recorded prevalence of previous herpetic illness in patients with ARN (n=45).
| Number of patients | Percentage (%) | Time between previous herpetic illness and ARN | |
|---|---|---|---|
| Chicken pox | 9 | 20.0 | Six childhood, one 10 years earlier, and two within 6 weeks |
| Anterior uveitis | 1 | 2.2 | 32 years |
| Herpes simplex keratitis | 2 | 4.4 | Recurrent |
| Encephalitis/meningitis | 7 | 15.6 | 22 years, 8 years, 9 months, 4 months, two at 4 weeks, and one at 3 weeks |
| Shingles/herpes zoster ophthalmicus | 6 | 13.3 | 10 years, two at 1 year, 2 months, 3 weeks, and 2 weeks |
| Fellow-eye ARN | 4 | 8.9 | 10 years, 7 years, 6 years, and 3 months |
Eight cases (17.8%) had a history of preceding (7), or concurrent (1), herpetic CNS disease. This was defined as herpetic disease affecting either the brain and its surrounding tissues or the spinal cord. Of those eight cases, four were immunodeficient (two were on long-term oral steroids, one was diabetic and one was HIV positive). Four of the nine patients with confirmed HSV ARN had a history of concurrent or preceding herpetic CNS disease. Of the 18 confirmed VZV patients, 2 had a history of concurrent or preceding herpetic CNS disease.
Immunodeficiency
In all, 13 cases (28.9%) had documented risk factors for immunodeficiency (malignancy, HIV, and iatrogenic immunosuppression). Of these 13 patients, final VA at 6 months was available for 15 eyes (mean VA 1.38 logMAR, median 1.70, range −0.1 to 3.0). There was no statistically significant difference in mean final VA between this group and the immunocompetent patients (mean VA 1.13 logMAR, n=28, P=0.403, median 1.00, range −0.1 to 3.0).
PCR analysis and viral characteristics
Vitreous biopsy was significantly more likely to yield viral DNA on PCR compared with aqueous tap (92.6%, n=27 vs 46.2%, n=13; P=0.002). Four cases underwent lumbar puncture for CSF analysis; of these, three yielded DNA.
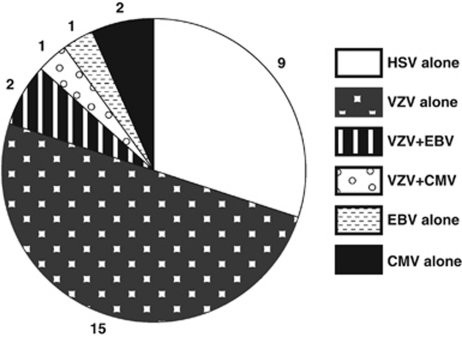
Ocular or CSF fluid analysis was performed in 34 cases (75.6%). Viral DNA was isolated from biopsy in 30 of those cases (88.2%): 6 from aqueous, 22 from vitreous, 3 from CSF, 1 from both aqueous and vitreous, 1 from both vitreous and CSF, and 1 from either aqueous or vitreous—data not recorded. Viral DNA findings are shown in Figure 3.
Figure 3.
Viral DNA isolated from aqueous, vitreous, or CSF in patients with ARN (n=30).
Of nine confirmed HSV cases, all were unilateral. Of 18 confirmed VZV cases, 13 were unilateral and 5 bilateral. Of nine cases with HSV-confirmed ARN, three were type I (aged 48, 54, and 75), five type II (mean age 27.4 years, range 15–41), and one not typed. For the 17 cases with VZV ARN, mean age was 57.4 years, range 24–88.
No statistically significant difference in final VA at 6 months was found between the affected eyes in the HSV group (n=9) and the VZV group (n=18). Nor was there a statistically significant difference between the groups in change in VA from presentation to final VA.
Treatment
In all, 11 (24.4%) patients were treated initially with oral antivirals (8/11 with Valaciclovir); the rest were initially treated with intravenous antivirals (median 8 days, range 3–24 days). Data on duration of antiviral therapy were available for 30 patients. Of those 15 received antiviral therapy for ≤3 months. Six were on treatment for more than 6 months.
Twenty-one (46.7%) cases received intravitreal antivirals (all Foscarnet). Of these, 16 received one injection only. Thirteen were injected within 48 h of commencing oral or intravenous antivirals.
No statistically significant difference was found in the mean VA at 6 months between the 33 affected eyes of patients initially treated with intravenous antivirals (mean VA 1.31 logMAR, median 1.70, range −0.1 to 3.0) and the 10 affected eyes of patients initially treated with oral antivirals (mean VA 0.89 logMAR, median 0.70, range 0.0 to 2.3, P=0.217). Oral steroids were used in 26 cases (57.8%). The mean VA at 6 months of eyes in cases treated with oral steroids (1.16 logMAR, n=27, median 1.00, range −0.1 to 3.0) was similar to that of eyes in cases who did not receive steroids (1.36 logMAR, n=13, median 1.00, range 0.0 to 3.0). The uveitis in three patients (four affected eyes) was treated with oral steroids before diagnosis of ARN. VA at presentation was 6/24 or better in all 4 eyes; VA at 6 months was 6/60 or worse in all 4 eyes. Aspirin use was documented in 14 of 43 (32.6%) cases.
Of 43 patients for whom the information was documented, 10 (23.2%) patients had prophylactic retinal laser to 12 eyes (for 11 of which we had 6-month data). Of these 11 lasered eyes, 2 went on to detach, whereas 14 of the 32 affected eyes documented as not receiving prophylactic laser (with 6-month data) went on to detach. This difference was not statistically significant (P=0.166).
There was no significant difference found in presenting visual acuity between patients who were later treated with retinal laser and those who were not.
Complications
Of 52 affected eyes, 16 (30.8%) eyes developed retinal detachment. Two detachments developed within 1 week of presentation; 12 detachments (75%) developed between 4 and 11 weeks after presentation.
Patients who developed retinal detachment had a statistically significant poorer visual outcome at 6 months (mean VA 1.67 logMAR, n=15 (median 1.70, range 0.3 to 3.0) vs mean VA 0.95 logMAR, n=28 (median 0.65, range −0.1 to 3.0, P=0.0098)). In all, 60% (9/15) of eyes that detached had a final visual acuity of <6/60 Snellen, compared with 39.3% (11/28) of eyes that did not detach.
The rate of retinal detachment in eyes that underwent a vitreous tap (11/25 (44%)) was not significantly different from the rate of detachment in eyes that did not undergo vitreous tap (5/19 (26%), P=0.344).
The distribution of causal viruses across eyes that developed retinal detachment was similar to the distribution over all cases (4/16 (25.0%) HSV, 10/16 (62.5%) VZV, 1/16 CMV, and 1/16 not known).
Eyes that were treated with intravitreal foscarnet had a detachment rate similar to that of eyes that were not (7/24 (29.2%) treated eyes detached and 9/28 (32.1%) untreated eyes detached).
Two out of the 42 eyes (4.7%) with 6-month follow-up data became phthisical.
Visual outcome
In all, 21 of the 44 affected eyes with 6-month data (47.7%) had a final visual acuity of less than 6 / 60 Snellen.
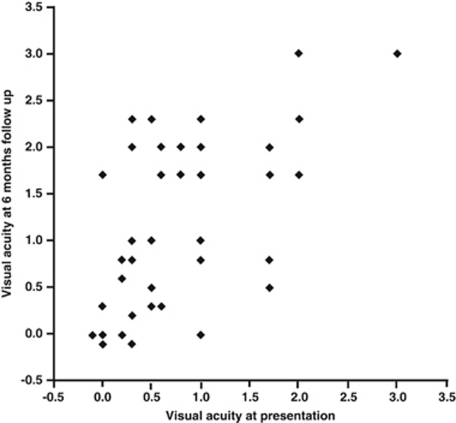
There was a significant correlation between presenting VA and final VA in affected eyes (Pearson's correlation coefficient 0.604, P<0.001).
Mean change in VA in affected eyes from presentation to final VA at 6 months was +0.321 logMAR (n=43). This ranged from a gain of six lines of Snellen acuity to a loss of seven lines (Figure 4).
Figure 4.
Visual acuity at presentation vs visual acuity at 6 months in eyes with ARN.
Discussion
The BOSU system allows the minimum incidence of a rare condition such as ARN to be determined in the UK population as underreporting is likely. In a similar study carried out between March 2001 and March 2002, the authors calculated an annual incidence of 0.5–0.63 cases per million population. In this study a minimum incidence of 0.63 cases per million was calculated, suggesting that there has not been any increase in the incidence of ARN over the preceding 6 years.7
This study confirms that ARN can affect all age groups, with HSV-2 being identified in younger patients and VZV and HSV-1 in older patients.2 Bilateral disease is less common than previously thought, probably because of early disease recognition and antiviral treatment.8,9,10
In recent years the introduction of the polymerase chain reaction (PCR) both qualitatively and quantitatively has revolutionized the diagnosis of necrotizing retinitis. In all, 75.6% of patients compared with 58.1% in the previous survey had ocular or CSF fluid analysis for DNA performed. A surprising finding in our study was that aqueous PCR was less likely to achieve a diagnosis than vitreous biopsy.
Aqueous biopsy is more easily obtained with less likelihood of complications compared with vitreous biopsy. In recent papers it has been suggested that aqueous sampling is highly sensitive, reporting a positive PCR in 86.4% of cases.11,12 Reasons for the discrepancy between this and our findings are unclear but may include inadequate size of sample and problems with processing. A sample taken early in the course of the disease may contain less viral DNA than vitreous for PCR amplification. A vitrectomy sample not only results in a larger volume of fluid for analysis by PCR, but also provides material for culture, cytology, and has a therapeutic role in removing dense vitreous opacities. Until studies are carried out comparing simultaneous vitreous and aqueous specimens the answer will remain unknown.
The results of this study confirm that VZV is the most common cause of ARN followed by HSV. In two cases EBV was isolated along with VZV. In one case EBV was isolated on its own. EBV is rarely implicated in ARN and in most instances is found coincidently on PCR analysis and not thought to be pathogenic.13
The seroprevalence of HSV-1 in the UK is ∼90% however, herpes simplex encephalitis rarely occurs. The annual incidence of herpes simplex encephalitis is between 1 and 2.2 per million of the population per year. In the UK this would give an annual incidence of ∼61–134 cases per year in a population of 61 million.14,15 These statistics combined with the fact that we identified five cases with herpes encephalitis in the year preceding ARN give an estimated incidence of ARN within 12 months of being diagnosed with herpetic encephalitis of between 4 and 8%. Doctors treating such patients should therefore warn them of the potential of sight-threatening visual complications and of the need to seek prompt medical attention if they develop visual symptoms.
The standard treatment for ARN is intravenous acyclovir for 5–10 days followed by 6–12 weeks of oral antiviral agents; however, no randomized controlled trials of this treatment have been conducted.8,16 The exception to this would be CMV ARN, which does not respond to acyclovir and requires intravenous ganciclovir or foscarnet.17 In recent literature the successful first-line use of the oral prodrugs valaciclovir, famciclovir, and valganciclovir has been reported.18,19 These drugs have high bioavailability, allowing them to achieve therapeutic serum levels when converted to their active forms. As there are no randomised controlled trials comparing oral and intravenous antivirals, it is still unclear whether oral valaciclovir or famciclovir is as rapid and effective as IV acyclovir for the initial treatment of ARN. Even though no significant visual acuity difference between the intravenous and orally treated patients were found in this study, clinicians must be cautious before adopting this approach.
In recent years the use of adjunctive intravitreal antivirals has gained popularity, as it means a high concentration of antiviral agent reaches where it is needed most. Intravitreal foscarnet was used in 46.7% of patients in this study. In recent studies the use of intravitreal foscarnet has been linked with a reduced rate of retinal detachment.20 This study found no significant difference in detachment rates between eyes treated with intravitreal foscarnet and those not.
The use of prophylactic confluent laser to prevent or localize any retinal detachment is controversial.21,22 In some papers on the topic it appears that those patients with severe disease and a poor view due to extensive vitritis tend not to undergo laser, whereas milder cases will be lasered.23,24 This can obviously lead to selection bias in the interpretation of results in that patients with clearer media are lasered. Such patients possibly have mild or earlier disease to begin with and are therefore less likely to detach. In this study no significant difference was found in initial visual acuity and whether or not laser was applied. Interestingly, the retinal detachment rates between eyes that had prophylactic laser and eyes that did not were not statistically significant. The study, however, may not have had sufficient power to detect a significant difference, though from a clinical point of view only 2/11 (18.2%) eyes lasered compared with 14/32 (43.7%) eyes not lasered went on to detach.
The strengths of this study are that cases were collected prospectively and completion of follow-up questionnaires allowed false positives to be identified. It must however be noted that questionnaires are completed in a retrospective manner and the potential for underreporting of cases, bias or loss of data must be taken into account when interpreting the results. This study was designed primarily as a descriptive study and the inevitable low numbers of patients result in an increased probability of significant differences between subgroups in multiple variables and an increased risk of type II error (false-negative results), but this limitation is to be expected given the rarity of ARN. The significance of prognostic factors and predictors of poor visual outcome should therefore be interpreted with caution.
In summary, ARN is a rare but potentially visually devastating condition whose incidence does not appear to have increased over the past 6 years. PCR on ocular fluids allows identification of viral DNA and appropriate treatment to be instituted. Oral antivirals and intravitreal injections are increasingly being used to treat this condition in the UK. It is still unclear from this study whether use of steroids or prophylactic laser is beneficial. Further large studies with longer follow-up are recommended to answer these questions.

Acknowledgments
We thank all the ophthalmologists throughout the UK who took time and effort to report the cases and fill out the questionnaires: Prof MR Stanford, Mr K Whittaker, Mr NP Jones, Prof IG Rennie, Prof A Dick, Prof S Lightman, Dr R Hamilton, Mr R Gray, Miss J Vodden, Mr GE Ozuzu, Mr YF Yang, Mr E Fraser, Miss R Rahman, Mr CRH James, Mr CH Hutchinson, Mr BJ McNeela, Dr PA Meyer, Mrs G Menon, Mr D Barr, Dr S Patterson-Brown, Mr R Johnston, Mr B Burton, Miss C Lim, Mr RHC Markham, Mr EG Davies, Dr S Hewick, Dr M Virdi, Mr B Burton, Mr D O'Neill, Miss F Bishop, Mr J Morgan, Dr RI Murray, Dr BW Fleck, Miss G Larkin, Mr E Herbert and Mr R Pandit. We would also like to thank the British Ophthalmological Surveillance Unit for facilitating the study and for the funding support of The Guide Dogs for The Blind Association and Fight for Sight.
The authors declare no conflict of interest.
Footnotes
The results of this study were presented at the 2010 Annual Congress of the Royal College of Ophthalmologists in Liverpool.
References
- Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117:663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2001;129:166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- Kramer S, Brummer C, Zierhut M. Epstein-Barr virus associated acute retinal necrosis. Br J Ophthalmol. 2001;85:114. doi: 10.1136/bjo.85.1.110d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foot BG, Stanford MR, Rahi J, Thompson JR. The British ophthalmological surveillance unit: an evaluation of the first 3 years. Eye. 2003;17:9–15. doi: 10.1038/sj.eye.6700233. [DOI] [PubMed] [Google Scholar]
- Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal haemorrhage in age-related macular degeneration. Retina. 1996;16:183–189. doi: 10.1097/00006982-199616030-00001. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics. Population Estimates. Available at: http://www.statistics.gov.uk/cci/nugget.asp? ID Accessed May 15 2009.
- Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91:1452–1455. doi: 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35:327–343. doi: 10.1016/0039-6257(91)90183-g. [DOI] [PubMed] [Google Scholar]
- Young NJ, Bird AC. Bilateral acute retinal necrosis. Br J Ophthalmol. 1978;62:581–590. doi: 10.1136/bjo.62.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay DA, Sternberg P, Jr, Davis J, Lewis H, Holland GN, Mieler WF. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112:250–255. doi: 10.1016/s0002-9394(14)76725-x. [DOI] [PubMed] [Google Scholar]
- Tran THC, Rozenberg F, Cassoux N, Rao NA, LeHoang P, Bodaghi B. Polymerase chain reaction analysis of aqueous humour somples in necritising retinitis. BR J Ophthalmol. 2003;87:79–83. doi: 10.1136/bjo.87.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothova A, de Boer JH, Ten Dam-van Loon NH, Postma G, de Visser L, Zuurveen SJ, et al. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology. 2008;115:306–311. doi: 10.1016/j.ophtha.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Gan YJ, Sixbey JW. Detection of Epstein-Barr virus genome in ocular tissues. Ophthalmology. 1996;103 (4:687–690. doi: 10.1016/s0161-6420(96)30632-5. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Chaudhuri A. Herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 2002;73 (3:237–238. doi: 10.1136/jnnp.73.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson A, Blomqvist P, Sköldenberg B. Herpes simplex encephalitis in Sweden, 1990–2001: incidence, morbidity, and mortality. Clin Infect Dis. 2007;45 (7:875–880. doi: 10.1086/521262. [DOI] [PubMed] [Google Scholar]
- Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93:296–300. doi: 10.1016/s0161-6420(86)33740-0. [DOI] [PubMed] [Google Scholar]
- Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005;65:859–878. doi: 10.2165/00003495-200565060-00012. [DOI] [PubMed] [Google Scholar]
- Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114:307–312. doi: 10.1016/j.ophtha.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Smith JR, Wilson DJ, Rosenbaum JT, Flaxel CJ. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology. 2006;113:2259–2261. doi: 10.1016/j.ophtha.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Wong R, Pavesio CE, Laidlaw DA, Williamson TH, Graham EM, Stanford MR, et al. Acute retinal necrosis the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology. 2010;117:556–560. doi: 10.1016/j.ophtha.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Sternberg P, Han DP, Yeo JH, Barr CC, Lewis H, Williams GA, et al. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology. 1988;95 (10:1389–1393. doi: 10.1016/s0161-6420(88)32999-4. [DOI] [PubMed] [Google Scholar]
- Park JJ, Pavesio C. Prophylactic laser photocoagulation for acute retinal necrosis. Does it raise more questions than answers. Br J Ophthalmol. 2008;92:1161–1162. doi: 10.1136/bjo.2008.147181. [DOI] [PubMed] [Google Scholar]
- Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114:756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Crapotta JA, Freeman WR, Feldman RM, Lowder CY, Ambler JS, Parker CE, et al. Visual outcome in acute retinal necrosis. Retina. 1993;13:208–213. doi: 10.1097/00006982-199313030-00004. [DOI] [PubMed] [Google Scholar]