Abstract
Background
The P3b component of the event-related potential (ERP) has frequently been reported to be reduced in children and adolescents at high risk for developing alcoholism relative to control children and adolescents without familial loading for alcohol dependence. P300 amplitude changes during development for all children. Previously it has been shown that high-risk offspring display a pattern in which the amplitude is lower at age 8 with a smaller rate of change during adolescence.
Methods
Admixture analysis was applied to data obtained for those children and adolescents having five or more annual assessments of ERPs to determine if multiple P3b growth patterns exist. The P3b amplitude patterns obtained were related to risk status, concurrent presence of childhood psychopathology (internalizing or externalizing), and age of onset to develop a diagnosis.
Results
A pattern characterized by lower P3b amplitude at study entry and a slower rate of change during child and adolescent development (pattern 3) was most often associated with high-risk status in boys and high-risk status in combination with the presence of a childhood diagnosis in girls. Pattern 3 was significantly related to the overall presence of childhood psychopathology (internalizing or externalizing) and to the presence of an Axis I diagnosis at young adult follow-up.
Conclusions
The developmental pattern previously described for offspring at high risk for developing alcoholism because of their familial/genetic background was confirmed. Admixture analysis has refined this observation and suggests that among all children and adolescents tested, three developmental patterns can be identified, one of which is most often seen in association with male high-risk children and adolescents.
Keywords: Alcoholism, high-risk offspring, P300, development, K-SADS, childhood psychopathology
Introduction
There has been a long-standing interest in finding trait markers for psychiatric disorders (Zubin and Steinhauer 1981). Several promising neurobiological markers have been investigated including electroencephalographic and event-related potential characteristics, electrodermal response, eye tracking, heart rate, and pupillography (Venables 1991). The event-related potential (ERP) and the P300 component in particular, has been studied as a potential marker for development of psychiatric problems, including such diverse disorders as schizophrenia (Pfefferbaum et al 1989; Steinhauer et al 1991) and alcoholism (Pfefferbaum et al 1991; Porjesz et al 1987a, 1987b; Steinhauer et al 1987). The P300 is a scalp positive wave that occurs approximately 300 msec after an informative stimulus occurs (Sutton et al 1965) and is an index of an individual’s capacity to process stimulus information. Two variants of P300 have been described, P3a and P3b. The traditional P3b (Sutton et al 1965) that has been studied most extensively has a parietocentral distribution in comparison with the more anterior scalp distribution of the P3a (Courchesne et al 1975; Squires et al 1975). The two types of P300 also differ in the aspects of cognitive processing that they reflect. In contrast with the loading on novelty, or “surprise” in the words of Verleger et al (1994), associated with the more anterior P3a, P3b is associated with more of what Verleger et al (1994) termed “suspense” because it reflects the process whereby the participant awaits the next relevant stimulus. The P3a is elicited in response to deviant stimuli (rare target or rare nontarget). P3b is elicited usually by relevant target stimuli that are typically less frequent than the nontarget condition. A P3 in response to novelty has also been described (Comerchero and Polich 2000). A novelty P3 is elicited by unpredictable unique stimuli that have not been presented previously; however, recent evidence suggests that the P3a and the Novelty P3 are indistinguishable (Simons et al 2001). It is important to distinguish the varying types of P300 because the search for trait markers of psychiatric risk has often used the two variants interchangeably, leading to some confusion in the literature. Variations in trait characteristics among various psychiatric disorders can be expected between those in which reduced involuntary responding to novelty is the core trait and those for which the capacity to use attentional resources is the central feature.
Amplitude Versus Latency of P3b as a Trait Marker for Psychiatric Status
In contrast to the increased latency seen in association with environmental factors (e.g., solvent exposure, closed head injury) or organic disease states (e.g., Alzheimer’s disease; Morrow et al 1992; Polich 1991), decrements in P3b amplitude are more often associated with the presence of psychiatric disorders. Reduced amplitude has been reported for schizophrenic patients (Pfefferbaum et al 1989; Steinhauer and Zubin 1982; Steinhauer et al 1991) and for patients diagnosed with depression (Bruder et al 1995; Yanai et al 1997). Additionally, individuals without psychiatric illness but who carry an especially high loading for a particular psychiatric disorder, especially alcoholism, have been reported to show reduced amplitude when compared with control subjects (Begleiter et al 1984; Friedman et al 1995; Hill et al 1995a; Hill and Steinhauer 1993; Steinhauer and Hill 1993). Thus, it is possible that the amplitude of the P3b component may be an inherent characteristic of individuals before they develop psychiatric states that in turn may be related to their vulnerability for incurring these disorders (Hill 1994; Hill et al 1987). P3b amplitude appears to be superior to P3 latency as an endophenotypic marker for psychiatric risk because P3b amplitude shows greater heritability than P3b latency (Aston and Hill 1990; van Beijsterveldt 1996). Whether reduction in P3b amplitude shows specificity for particular disorders or is a marker of generalized susceptibility to psychiatric disorders is unknown.
Some investigators have argued that for P300 amplitude to be a valid biological marker of susceptibility to a psychiatric disorder it should be present in both child and adult high-risk relatives of psychiatrically diagnosed probands (Porjesz et al 1998); however, alcoholism is one condition in which study of adults may present a problem for understanding whether the P300 component is a trait marker. P3b reductions have been demonstrated to be larger in alcoholics with cortical atrophy than in alcoholics without neuropathological changes (Begleiter et al 1980). Although rigorous investigations have not found significant associations between P3b amplitude in adult alcoholics and lifetime alcohol consumption, conclusions must be tempered by the 20- to 30-year retrospective recall required for such investigations (Pfefferbaum et al 1991). Similarly, the presence of other psychopathology, which is common among alcoholics, may be responsible for the reduction in amplitude of P3b (Hill et al 1999b) and P3a (Costa et al 2000) in adult alcoholics. The presence of these contaminating factors may explain the variable results seen in adult alcoholics, which include reduction of P3b in adult male alcoholics by some (Pfefferbaum et al 1991; Porjesz et al 1987a, 1987b), but not all investigations (Hermanutz et al 1981; Hill et al 1999b; Hill et al 1995b; Lille et al 1987; Pfefferbaum et al 1979).
Coupled with the fact that alcoholics tested in ERP paradigms usually have long drinking histories (usually more than 12 years) is the fact that they often are tested after very short periods of abstinence (usually only 2 weeks; Porjesz et al 1987a). Importantly, adult alcoholics tested after a period of 6 months abstinence do not differ in visual or auditory P3b amplitude from controls (Biggins et al 1995) though the alcoholics display longer latency characteristic of exposure to neurotoxic substances (Biggins et al 1995). The critical nosological question is whether P3b reduction, if seen, was present before the alcoholic individual began to drink. Current evidence based on children and adolescents suggests that this is the case.
Cross-Sectional Studies of P300 in Children
Developmental studies of normal children are infrequent. Fewer than 200 children and adolescents have been assessed across available studies using either visual or auditory ERPs (Courchesne 1977; Kurtzberg et al 1984; Polich et al 1990); however, these cross-sectional studies have demonstrated substantial differences between the ERP characteristics of adults and children (Kurtzberg et al 1984; Polich et al 1990). Using samples of minor children, differences in P3b amplitude are seen between children at high- and low-risk for developing alcohol dependence (Begleiter et al 1984; Berman et al 1993; Hill et al 1995a; Hill and Steinhauer 1993; Hill et al 1990; Steinhauer and Hill 1993; Whipple et al 1988), although no differences are found between high and low-risk children evaluated with a P3a paradigm (Holguin et al 1998). A meta-analysis performed by Polich et al (1994) suggests that the effect of age is critical in uncovering P3b amplitude differences by alcoholism risk status. The largest effect sizes observed were between high- and low-risk younger children, especially with more difficult ERP paradigms, with few differences being seen in young adulthood. Children at high risk for developing other psychiatric disorders have infrequently been studied; however, one study followed children at high risk for developing schizophrenia, finding lower P3b in childhood was associated with overall higher global impairment at age 18 (Friedman et al 1995).
Longitudinal Studies of P300 in Children
The presentation modality has been shown to affect the P3b amplitude trajectories of childhood and adolescence (Hill et al 1999c). Before that study, longitudinal data were not available to determine if the same children followed over time would have developmental trajectories that varied by modality (visual or auditory) or by risk group status (high or low risk for alcohol dependence). Growth curve modeling of 635 separate auditory and visual P3b assessments of children and adolescents between the ages of 8 and 18 revealed that auditory P3b showed an increase in amplitude with age followed by a leveling off by late adolescence. In contrast, visual P3b amplitude appears to be greatest at younger ages, declining until about young adulthood. Importantly, the developmental trajectories of P300 amplitude in high-risk offspring from high density for alcoholism families differed from that seen in low-risk offspring (Hill et al 1999c). Male high-risk offspring show a slower rate of change in P3b amplitude over the period of 8 to 18 years than do control children (Hill et al 1999c). Although similar trajectories were seen in high-risk girls, a comorbid childhood diagnosis was required for this pattern to be manifest. The high-risk group, as a whole, entered the study with lower visual P3b amplitudes than control subjects and showed a slower rate of change. Based on data acquired through late adolescence, the theoretical point of convergence when high- and low-risk groups would be indistinguishable was calculated to be at age 18 for the auditory condition and 22 for the visual (Hill et al 1999c). Empirical data (Hill et al, unpublished manuscript) confirm that, as with other developmental milestones that can be delayed during childhood (e.g., onset to walk or talk) but are clearly present by adulthood, the average P3b amplitude for the high- and low-risk groups are virtually identical by young adulthood as was predicted theoretically on the basis of longitudinal growth analyses projected to young adulthood (Hill et al 1999c).
Although the previous report had identified overall differences in the rates of change between high- and low-risk children and adolescents, visual inspection suggested the presence of multiple trajectory patterns. This report is based on an admixture analysis of subject data from the growth curve data set but restricted to participants for whom at least five repeated annual assessment waves of data were available. The goal of this analysis was to determine if risk status confers a different developmental pattern across childhood and adolescence. A secondary goal was to determine if pattern type would be related to the individual diagnoses of the offspring studied. To establish that P3b is a trait marker for psychiatric illness, it is important to rule out contributions from state variables such as concurrent psychiatric illness in the participants studied. It was expected that the results of these analyses might point the way to underlying neurobiological subtypes associated with risk for development of psychiatric illness that might be recognizable in childhood. Identification of developmental variants might potentially provide a means for identifying children at an especially high risk for developing adult psychopathology for targeted interventions.
Methods and Materials
Subjects
We followed annually 126 children between the ages of 8 and 18 who were offspring of parents enrolled in a large family study. The children were either at high or low risk for developing alcohol dependence based on differing familial loading for alcohol dependence. Some of these children and adolescents have matured into a second initiative designed to address prevalence of psychiatric disorders in young adults (ages 19–30) who vary by family history of alcohol dependence. Data for a subset of 85 children having at least five waves of data were analyzed to obtain developmental trajectories of P3b amplitude. These 85 children consisted of 49 high-risk and 36 low-risk individuals (45 male and 40 female subjects). Among this subset are 35 subjects who have been followed into young adulthood (18 high-risk and 17 low-risk). Among these were 21 men, and 14 are women. All subjects gave their informed consent to participate in the study on each visit to the laboratory. The protocol was approved by the institutional review board for the ethical treatment of human subjects.
The high-risk families, enrolled in the larger family study, were ascertained through a proband pair of male alcoholic siblings. The majority of adult family members of the pedigree were administered Diagnostic Interview Schedule (DIS) to determine the presence or absence of alcohol dependence and other Axis I diagnoses using both DSM-III (the criteria in use when the study originated) and Feighner criteria (Feighner et al 1972) and a best estimate diagnosis reached with another clinician (PhD or MD level) as previously described (Hill et al 1987). Control children and adolescents were also drawn from the larger family study. Low-risk control families were chosen for minimal Axis I DSM-III psychopathology, including alcohol dependence, by interviewing an index case who volunteered for the study and the majority of his first-degree relatives. Family history supplemented evaluations where first-degree relatives could not be directly interviewed.
Clinical Assessment of Children and Adolescents
Each child and his or her parent was separately and blindly administered the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS; Chambers et al 1985) by trained (master’s level) interviewers. Best estimate diagnoses were obtained by a second clinician (a third or fourth year resident in an integrated child/adult psychiatry program). Discrepancies were resolved in the presence of a third clinician. The presence or absence of any childhood diagnosis was used to classify subtypes of high- and low-risk children.
Clinical Assessment—Young Adults
Subjects over age 18 were interviewed by a trained master’s level clinician using the Composite International Diagnostic Interview (CIDI) to obtain a current diagnosis for the past year.
Event-Related Potentials—Visual Procedure
The visual event-related potential task employed was patterned after the procedure originally used by Begleiter et al (Begleiter et al 1984). Presentation conditions were identical to those previously reported (Hill et al 1999b, 1999c; Hill and Steinhauer 1993). Stimuli are presented at a duration of 33 msec (intertrial interval 2.25–4 sec). The nontarget stimulus is a simple circle to which the subject is instructed not to respond (blank condition). The target condition consisted of one of four possible views of a stick figure “head” with a “nose” and only one “ear.” The subject is instructed to press the button that corresponds to the depicted ear. Subjects are required to respond to a condition in which the nose is oriented upward and the ear (right or left) is on the same side as the button depressed, or a condition in which the ear is depicted on the opposite side of the head as the button pressed requiring spatial rotation of the “head.” The present analysis was based on the latter condition. Each subject was given 240 trials.
Recordings are obtained from electrodes placed at midline frontal, vertex, parietal, and occipital locations (Fz, Cz, Pz, Oz, respectively) as well as left and right parietal sites (P3, P4). All active electrodes were referred to linked ears, with a forehead ground. Eye movement and blink artifacts were recorded by an additional electrode located under the left eye, which also was referred to linked ears. Data were digitized for 1200 msec at 125 Hz, beginning 200 msec before the stimulus onset, and stored on magnetic media. Artifact-free trials (eye artifact trials exceeding approximately 50 µV were discarded) were averaged for each condition and electrode. In our laboratory, the P3b component is identified using an interactive computer algorithm that chooses the maximal amplitude for a given component (at Pz for P3b) within a predefined latency window (264–424 msec). This analysis was based on P3b amplitude recorded at the parietal electrode.
Statistical Analysis
LATENT VARIABLE GROWTH MIXTURE MODELING
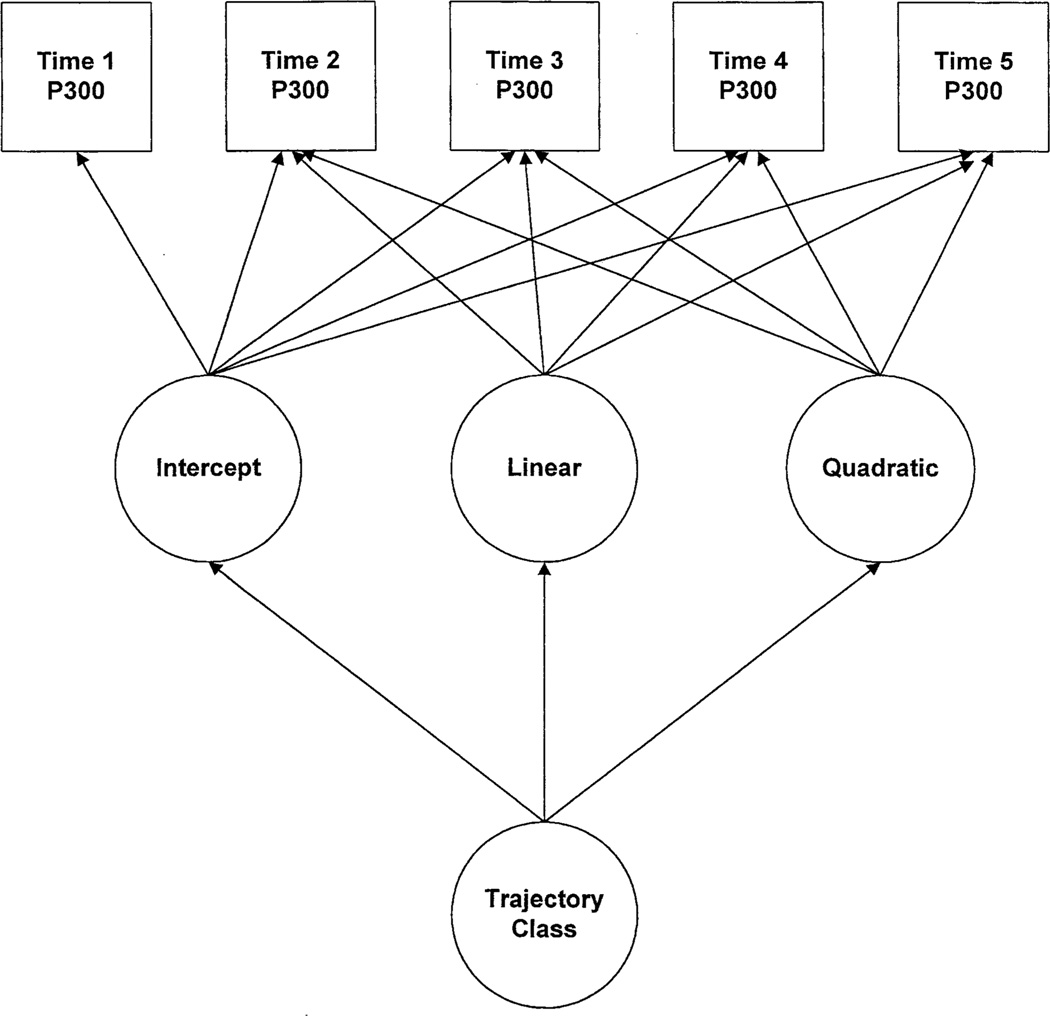
Growth mixture modeling is one approach for integrating variable-centered (relationship among variables) and person-centered (heterogeneous groups of individuals) analyses (Muthén and Muthén 2000). For this analysis, growth mixture modeling with latent trajectory classes (Muthén and Shedden 1999) was used to assess changes in the amplitude of the visual P3b amplitude recorded at the five time points (Figure 1), here the outcome variable in the latent mixture models tested. Three latent continuous variables were used: the intercept, linear, and quadratic growth factors.
Figure 1.
Visual P3b amplitude data were analyzed for children and adolescents for whom five consecutive repeated measures at approximately annual intervals were available. The model illustrates the structure of the latent variable growth mixture models tested.
Before applying growth mixture model analysis, data were inspected to determine the type and frequency of patterns obtained. Five patterns could be identified visually with varying frequency (33%, 29%, 22%, 14%, and 2%). Based on this frequency distribution, it was clear that more than one and less than five patterns would probably be the most appropriate solution. To more precisely determine appropriate class categorization, growth mixture models with varying numbers of classes were estimated using the Mplus program (Muthén and Muthén 1998). Model selection was implemented by comparing the Bayesian information criterion (BIC) derived from each model. The lower the BIC value, the better the model is considered to fit the data. In addition, the posterior probability that each individual belonged to a given class was estimated. Individuals were then classified into the appropriate trajectory class using the highest probability estimates for subsequent analyses.
FACTORS ASSOCIATED WITH TRAJECTORY MEMBERSHIP
Associations between gender, risk status (high or low risk for developing alcohol dependence), childhood lifetime psychopathology (no disorder or any disorders), and visual P3b amplitude growth curve classes were assessed by using the χ2 statistic.
MODEL FIT FOR ELECTROPHYSIOLOGICAL DATA
Growth mixture models of varying number were applied to the P3b amplitude data to find the least number of classes that provided the best fit to the data based on the suggestions of Múthen and Múthen (2000). Three considerations were followed: 1) the BIC statistic was used to test the goodness of fit of the data to the models using maximum likelihood estimation while keeping models parsimonious (the lowest BIC value implied the best fit model); 2) classification quality was assessed by examining the posterior probability that each individual belonged to a specific class; and 3) utility of the latent class solution was evaluated by determining if individual trajectories of members within a class accurately reflected the class pattern.
Results
To determine the best-fit model, BIC values, classification quality, and utility of the latent class were examined. The BIC statistics were 3184.64 for the one-class model, 3097.95 for the two-class, 3109.25 for the three-class, 3104.50 for the four-class, 3118.45 for the five-class, and 3130.55 for the six-class solutions. BIC values for a single class or more than four were quite large and not further considered. The average estimated posterior probabilities, conditional on the class assignment for the two, three, and four-class solutions, revealed that the classification errors were similar and reasonably low. Therefore, based on the first two considerations, two, three, and four-class models were considered as optimal models for the data.
Because of an insufficient number of cases in two classes of the four-class model, this model was not considered further. Although the BIC value was slightly better for the two-class solution than the three-class solution, the three-class model was chosen because it most accurately reflected the number of classes found on visual inspection of all of the trajectory data. Moreover, individual class membership could be achieved with good confidence based on the average difference between likelihoods across pairs of comparisons. Specifically, each individual had a likelihood estimated for each of the three classes. Inspection of these likelihoods revealed that placement of a given subject in either of the two most extreme classes (e.g., disparity between likelihoods was greatest), resulted in a mean difference across all subjects of .85 ± .015. Even in the case where placement in either of two classes was more ambiguous (the smallest likelihood difference between any two classes for a given individual) when averaged across all subjects also gave reasonably large values (.71 ± .03), indicating that placement in a specific class could be done with minimal ambiguity for each individual.
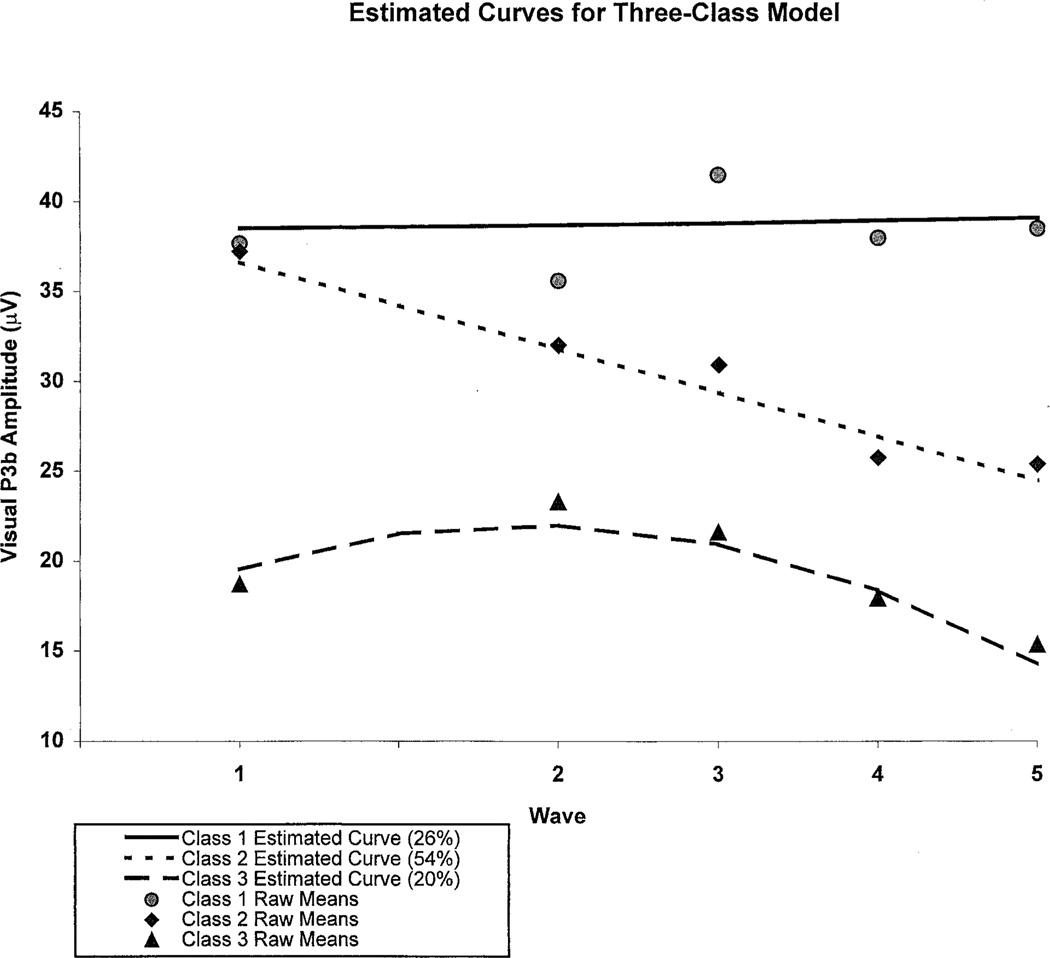
Figure 2 illustrates the three estimated developmental curves of P300 amplitude for the three-class solution: high intercept P3b—flat trajectory (Class 1), representing 26% of the participants; high intercept P3b—downward trajectory (Class 2), which included 54% of the subjects; and low intercept P3b—flat trajectory (Class 3), which represented only 20% of the children/adolescents studied. The mean ages and gender distribution for each class can be seen in Table 1.
Figure 2.
Estimated growth curves are illustrated for the three-class solution that provided the best fit to the visual P3b amplitude data analyzed obtained at the five annual assessments. Actual data are plotted for each curve. Note that 2 years separated waves 1 and 2.
Table 1.
Means (SE) of Age (Years) by Gender and Class Membership
| Class 1 | Class 2 | Class 3 | ||||
|---|---|---|---|---|---|---|
| Male n = 9 |
Female n = 12 |
Male n = 24 |
Female n = 22 |
Male n = 12 |
Female n = 6 |
|
| First evaluation | 8.56 (.34) | 8.58 (.38) | 9.46 (.32) | 9.64 (.33) | 9.58 (.61) | 9.67 (.76) |
| Second evaluation | 10.44 (.53) | 10.25 (.52) | 11.71 (.38) | 11.77 (.38) | 11.75 (.57) | 11.17 (.75) |
| Third evaluation | 11.44 (.53) | 11.25 (.51) | 12.67 (.38) | 12.73 (.39) | 12.75 (.57) | 12.67 (.76) |
| Fourth evaluation | 12.44 (.53) | 12.17 (.52) | 13.79 (.37) | 13.82 (.38) | 13.75 (.57) | 13.67 (.76) |
| Fifth evaluation | 13.33 (.50) | 13.17 (.52) | 14.79 (.37) | 14.82 (.36) | 14.67 (.59) | 14.67 (.76) |
K-SADS Diagnoses
K-SADS diagnoses were obtained at approximately yearly intervals during the child–adolescent developmental period for which ERP was recorded. Among the 85 children and adolescents for whom P300 trajectories were classified, 37 reached an average age of 15.6 ± 2.1 years without having received a child or adolescent diagnosis (the age at the last interview). Analysis of the concurrent relationship between P3b Class membership and K-SADS diagnosis was based on only the first five waves of available data.
CIDI Diagnoses
The individuals followed into young adulthood were 20.1 ± 1.3 years of age at the time of their CIDI interview. The majority of the 35 cases (66%) did not meet criteria for having had a psychiatric illness within the past year; however, among the young adults receiving a diagnosis, the majority (76.9%) had a substance use disorder. The high rate of substance use disorders probably reflects the familial risk for alcohol dependence that the high-risk members of this cohort have. (Among the nine individuals with a substance use disorder, only two came from control families.)
Risk Status and Class Membership
As may be seen in Table 2, an approximately even proportion of high- and low-risk children, both boys and girls, were found in Class 1 and 2, whereas high-risk children outnumbered low-risk children by more than 2 to 1 in Class 3, although not significantly (χ2 = 3.75, df = 2, ns). The proportion of children classified as having one of the three patterns were, for Class 1, 2, and 3, respectively, as follows: high-risk: 26.5%, 46.9%, and 26.5%; low-risk: 25.0%, 63.9%, and 11.1%).
Table 2.
Percentage of Children Classified by Trajectory Pattern and Risk Group Status
| High-Risk Children | Low-Risk Children | |||||
|---|---|---|---|---|---|---|
| Class 1 26.5% (n = 13) |
Class 2 46.9% (n = 23) |
Class 3 26.5% (n = 13) |
Class 1 25.0% (n = 9) |
Class 2 63.9% (n = 23) |
Class 3 11.1% (n = 4) |
|
| Male | 23.1% | 60.9% | 69.2% | 77.8% | 43.5% | 50% |
| Female | 76.9% | 38.1% | 30.8% | 22.2% | 56.5% | 50% |
| No diagnosis | 61.5% | 52.2% | .00% | 55.6% | 60.9% | 75% |
| Any diagnosis | 38.5% | 47.8% | 100% | 44.4% | 39.1% | 25% |
Number of Children are in the parentheses—classification was based on five separate ERP assessments for each child. Data are presented for gender and diagnosis within the the trajectory classes.
Gender and Trajectory Patterns
Table 2 displays the observed frequencies of cases by gender, familial risk, and the presence of a child/adolescent lifetime diagnosis for each class. Although there was no evidence that the proportion of individuals classified into the three trajectory patterns differed by gender only (χ2 = 1.47, df = 2, ns), the interaction of risk and gender was further tested, based on previous findings (Hill et al 1999c).
Risk Status, Gender and Class Membership
Analysis by risk within each gender altered the results dramatically. The proportion of high- and low-risk boys in each of the classes differed significantly. More high-risk than low-risk boys fell in Class 3 (z = 2.06, p = .04), whereas fewer high-risk boys were in Class 1 (z = −2.12, p = .03). These findings are consistent with previous results showing significantly different growth curves for P300 amplitude when high- and low-risk male subjects were compared (Hill et al 1999c). Fewer high-risk than low-risk girls were found in Class 2 (z = −2.59, p = .01).
Childhood Diagnosis
The pattern of visual P3b amplitude across the child–adolescent developmental period studied was significantly related to the presence of childhood diagnosis (χ2 = 9.23, df = 2, p = .01). The proportion of children with a diagnosis, followed by those without in the three classes were as follows: Class 1: 20.9% and 31.0%; Class 2: 46.5% and 61.9%; Class 3: 32.6% and 7.1%, respectively. There were significantly more children and adolescents who had any lifetime disorder in Class 3 (z = 3.10, p = .002) compared with individuals who had no diagnosis. Previous reports have suggested that the presence of externalizing psychopathology (conduct disorder symptoms) is responsible for lower amplitude P3b in childhood and adolescence (Bauer and Hesselbrock 1999). Because of the small number of individuals in pattern 3, we could not determine if membership in this group was specifically associated with externalizing disorders or more generally associated with presence of a diagnosis; however, it may be noted that among the children in group 3 with a diagnosis, 23.5% had an externalizing disorder, 29.4% had an internalizing disorder, and 29.5% of the children had both an internalizing and an externalizing disorder. Therefore, it appears that this pattern is not associated with any particular type of psychopathology.
Risk and Childhood Diagnosis
Significant trajectory differences were seen when risk status was combined with the presence or absence of lifetime psychopathology (χ2 = 21.31, df = 6, p = .002). Accordingly, Class 3 contained more male and female high-risk children with any diagnosis by lifetime history than low-risk control subjects with any diagnosis (z = 3.27, p = .001). In addition, analysis within Class 3 showed that significantly more high-risk children with any lifetime diagnosis were included in this class than high-risk children with no diagnosis (z = 4.85, p < .001).
Risk, Childhood Diagnosis, and Gender
Membership in the three classes was found to differ by risk status, the presence of any childhood diagnosis, and gender (χ2 = 35.12, df = 14, p = .001). The presence of a childhood diagnosis altered the likelihood of fitting a particular trajectory class even among control individuals. Comparing low-risk boys without a diagnosis to high-risk boys with a diagnosis, a significant difference was found (z = 2.80, p = .005). High-risk girls with one or more childhood diagnoses had P3b trajectories typical of the Class 3 pattern more often than did high-risk girls without a diagnosis (z = 2.58, p = .01). For high-risk boys, the presence of a lifetime diagnosis increased the likelihood that the high-risk boys would have a Class 3 pattern in comparison to high-risk boys without a diagnosis (z = 4.13, p < .001).
Level of Risk, Age of Onset to Develop Childhood Diagnoses and Class Membership
Age of onset to begin regular drinking has been shown to be related to the likelihood that an individual will develop alcohol dependence (Grant and Dawson 1997). High-risk offspring have an earlier onset to begin drinking than do low-risk control subjects (Hill et al 2000). The amplitude of P300 appears to be related to substance abuse outcome at 8-year follow-up (Hill et al 1995c). High-risk children also have a greater likelihood of having a psychiatric disorder (Hill et al 1999a). Therefore, data were analyzed to determine if membership in specific P300 trajectory categories would be related to age of onset to develop psychopathology. Knowing that a child or adolescent is at higher risk for developing psychiatric disorders in childhood, adolescence, or adulthood based on their familial and genetic loading is a useful prediction; however, it was hypothesized that those at highest risk might be identified by relating childhood diagnoses to the developmental P300 patterns identified. Accordingly, a survival analysis was performed using the earliest age of onset of any diagnosis based on the presence of a K-SADS diagnosis (e.g., depression, anxiety disorders, attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder). These diagnoses were obtained prospectively at approximately yearly intervals allowing for relatively precise ages of onset. Comparison of high-risk children in Class 1 and 2 versus those in Class 3 revealed a significant difference (Tarone-Ware statistic = 4.76, df = 1, p = .03). Clearly, those having a Class 3 pattern not only were more likely to have a diagnosis but also developed a disorder earlier than those having either a Class 1 or 2 pattern. Figure 3 illustrates the differing survival curves of high-risk individuals in Class 3 versus those in Classes 1 and 2.
Figure 3.
Survival curves for the age of onset at first childhood diagnosis among high-risk for alcoholism offspring. Membership in Class 3 P3b developmental trajectory pattern increases the likelihood that a child with a familial/genetic risk for alcohol dependence will exhibit a childhood diagnosis by a given age.
Young Adult Diagnosis and Class Membership
An analysis was performed contrasting young adults with any diagnosis with those without a diagnosis. A 2 × 3 chi square revealed a significant overall effect (χ2 = 6.65, df = 2, p = .04). Also, within Class 3 significantly more young adults with a diagnosis were found than those without (z = 3.30, p = .001).
Discussion
Our study demonstrated that varying patterns of developmental change in P3b amplitude occur among children. Using admixture latent growth variable modeling, three distinct patterns could be identified: high intercept P3b with a flat trajectory (Class 1); high intercept P3b with a downward trajectory (Class 2); and low intercept P3b with a flat trajectory (Class 3). Class 1 children displayed amplitudes that were higher than the mean for the entire group (over 35 µV) and remained at this level across the observation period. Class 2 was the most frequently occurring pattern and was characterized by higher amplitude (over 35 µV) at the youngest age, followed by a steady decline to approximately 25 µV at age 14. Class 3 membership occurred in approximately 20% of children evaluated and was characterized by lower P3b (approximately 20 µV) at the youngest ages studied with a slow rate of change from age 9 to 14. Results of this analysis suggest that the proportion of individuals having particular trajectories differed by gender, risk status, and presence or absence of childhood diagnoses.
A major finding of this study was that the developmental trajectories of P3b amplitude obtained over the age range studied (9–14 years) were related to the child’s familial risk for developing alcohol dependence in combination with the presence or absence of the child’s personal diagnosis. This diagnosis was based on the concurrent disorders of childhood and adolescence occurring between the ages of 9 and 14 and those obtained for a subset of individuals followed to young adulthood. For the concurrent results, it was shown that among high-risk children those with the highest risk for developing a childhood disorder could be predicted based on membership in the Class 3 P3b developmental trajectory pattern. Although the number of subjects available for the young-adult comparison is rather small, these findings do suggest the possible utility of the P3b developmental trajectory as an indicator of risk for developing a psychiatric disorder.
Our results confirm a previous growth curve analysis of the 8- to 18-year-old developmental period in which it was demonstrated that risk status confers a greater likelihood of having lower P3b at the youngest ages studied, and a slower rate of change in amplitude during childhood and adolescence, especially in high-risk male children (Hill et al 1999c). High-risk female children exhibit this pattern but only in the presence of a childhood diagnosis (Hill et al 1999c). Similarly, our admixture analysis confirms that both the presence of a childhood diagnosis and high-risk status are necessary in girls to increase the likelihood of having a Class 3 pattern characterized by lower P3b amplitude and a slower rate of change across adolescence. Thus, although gender differences do exist, it is reasonable to conclude that high-risk children overall can be distinguished from control children on the basis of the P3b trajectories that they display during childhood and adolescence.
Our findings have relevance to recent suggestions that reduced amplitude of P3b seen in association with familial risk for developing alcoholism is actually due to the greater likelihood that family history positive youth will have externalizing psychopathology, namely, conduct disorder (Bauer and Hesselbrock 1999). This suggestion is part of a continuing dialogue concerning the overlap between antisocial personality disorder, alcohol dependence, and P300 (Bauer and Hesselbrock 1999; Bauer et al 1994; Carlson et al 1999; Costa et al 2000). Based on cross-sectional data obtained from youth who were assessed for presence of conduct symptoms and family history of alcohol dependence, Bauer and Hesselbrock (1999) concluded that reduced amplitude of P300 was seen in association with conduct disorder symptoms (subjects did not meet DSM-IV criteria for conduct disorder) and that no evidence had been found that the family history variable was responsible for the reduction in amplitude; however, Bauer and Hesselbrock used the presence of a single alcoholic first-degree relative to define the family history positive group. Greater loading of alcohol dependence in our study may have made it possible to detect familial risk differences and the impact of this variable on P3b amplitude.
Our analysis has demonstrated that the presence of any diagnosis and familial risk together increases the likelihood that an individual would have a Class 3 P3b developmental trajectory. High-risk boys and girls were more often in this category if they had a childhood diagnosis. Because persons in the any diagnosis category were as likely to have an internalizing as externalizing disorder, the presence of an externalizing disorder alone does not appear to explain the increased likelihood of membership in the lower P300 amplitude, slower developmental change group (Class 3). This seems to suggest that the hypothesis offered by Bauer and Hesselbrock (1999) that the presence of an externalizing disorder mediates the family history effect on reduced P3b amplitude may not be sufficiently comprehensive. First, the effect of diagnosis on reduced P3b seen in Class 3 suggests that the presence of any diagnosis, internalizing or externalizing, is associated with reduced amplitude. Second, for mediation to occur, one would need a sample in which an opportunity for revealing family history effects is possible. Moreover, statistical tests need to be performed to determine if externalizing psychopathology can substitute for the family history of alcohol dependence using guidelines such as those offered by Baron and Kenney (1986). This would require the use of oversampling of multiplex families, as was done in our study.
Developmental changes in the volume of limbic structures that have been observed in adolescents studied longitudinally (Giedd et al 1999; Jacobsen et al 1998; Jernigan and Sowell 1997; Pfefferbaum et al 1994) may be related to developmental changes in P3b amplitude that result in identifiable patterns in growth trajectories (Hill et al 1999c). Some of the regions that appear to increase in volume during childhood and adolescence (e.g., hippocampus, amygdala) also appear to be good candidates for the underlying source generators for P300 (Halgren et al 1980; Kirino et al 2000; Knight 1996) interacting with prefrontal and temporal–parietal areas (Kirino et al 2000; Comerchero and Polich 1999). Recently, right amygdala volume of high-risk male subjects was found to differ from age- and IQ-matched control subjects (Hill et al 2001). The right amygdala, in contrast with the left, shows increases in volume during adolescence (Jacobsen et al 1998), suggesting that high-risk offspring may show slower rates of change in this region than do control subjects.
In conclusion, a longitudinal study of children followed for up to 11 years has provided the opportunity to analyze a subset of these children for whom at least five waves of data exist. Admixture analysis has revealed three distinct patterns of development of visual P300 amplitude. One of the less frequently occurring patterns (Class 3) appears to be associated with greater likelihood that the individual comes from a high risk for alcoholism family and is associated with greater likelihood that the individual has a childhood disorder. This pattern appears to be equally associated with having an externalizing or internalizing disorder. Moreover, this pattern appears to predict the likelihood that a young adult will have a psychiatric disorder. Because the study design emphasizes the childhood and young adult psychiatric status of offspring of alcoholics from multigenerational families, the predominant disorder seen among these young adults to date appears to be substance abuse and dependence. Although the finding for the young adult sample is of a preliminary nature, follow-up is continuing, which will aid in confirming whether childhood and adolescent P3b patterns can be used as predictors of young adult psychiatric status. The relationship between childhood diagnoses, risk status, and P3b patterns was based on a larger number of assessments, a total of 425 in all, and confirms previous analyses suggesting differing growth curve trajectories for offspring at high and low risk for developing alcohol dependence. This analysis extends the previous work (Hill et al 1999c) by showing that the presence of a concurrent childhood diagnosis is associated with a greater likelihood of lower P3b at the younger ages studied, followed by a slower rate of change through late adolescence.
Acknowledgments
Supported by the National Institute on Alcohol Abuse and Alcoholism Grant Nos. AA05909, AA08082, and AA11304. We wish to thank all of the individuals involved in acquiring the ERP data, especially Jeannette Locke-Wellman, and Dr. Stuart Steinhauer for his consultation. We also thank the clinicians, especially Lisa Lowers, MA, for maintaining excellent retention in the follow-up. Also, we are grateful to the families who have participated multiple times.
References
- Aston CE, Hill SY. A segregation analysis of the P300 component of the event-related potential. Am J Hum Genet. 1990;47 (suppl):A127. [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenney DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;98(51):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biol Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcohol Clin Exp Res. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Tenner M. Neuroradiological and neurophysiological evidences of brain deficits in chronic alcoholics. Acta Psychiatr Scand Suppl. 1980;286:3–13. doi: 10.1111/j.1600-0447.1980.tb08050.x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance abuse. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- Biggins CA, MacKay S, Poole N, Fein G. Delayed P3A in abstinent elderly male chronic alcoholics. Alcohol Clin Exp Res. 1995;19:1032–1042. doi: 10.1111/j.1530-0277.1995.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, et al. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373, 381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small, average, or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–590. [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clin Neurophysiol. 1999;110:24–30. doi: 10.1016/s0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Comerchero M, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Cogn Brain Res. 2000;7:41–48. doi: 10.1016/s0926-6410(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, et al. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biol Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Event-related brain potentials: Comparison between children and adults. Science. 1977;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatry research. Arch Gen Psychiatry. 1972;28:238–243. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Friedman D, Squires-Wheeler E, Erlenmeyer-Dimling L. Subjects at risk for psychopathology from the New York high risk project: ERPs during adolescence and clinical outcomes in young adulthood. In: Karmos G, Molnar M, Csépe V, Czigler I, Desmedt JE, editors. Perspectives of Event-Related Potentials Research, Electroencephalogr Clin Neurophysiol, Suppl 44. New York, New York: Elsevier; 1995. pp. 379–386. [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Halgren E, Squires NK, Wilson C, Rohrbaugh J, Babb T, Crandall P. Endogenous potentials in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Hermanutz M, Cohen R, Sommer W. The effects of serial order in long sequences of auditory stimuli on event-related potentials. Psychophysiology. 1981;18:415–423. doi: 10.1111/j.1469-8986.1981.tb02473.x. [DOI] [PubMed] [Google Scholar]
- Hill SY. Etiology. In: Langenbucher J, McCrady B, Frankenstein W, Nathan P, editors. Annual Review of Addictions Research and Treatment. Vol. 3. Tarrytown, NY: Elsevier Science; 1994. pp. 127–148. [Google Scholar]
- Hill SY, DeBellis MD, Keshavan MS, Lowers L, Shen S, Hall J, et al. Right amygdala volume in adolescent/young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Lowers L, Connolly J. Psychopathology and achievement in children at high risk for developing alcoholism. J Am Acad Child Adolesc Psychiatry. 1999a;38:883–891. doi: 10.1097/00004583-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke J, Steinhauer S. Absence of visual and auditory P300 reduction in non-depressed male and female alcoholics. Biol Psychiatry. 1999b;46:982–989. doi: 10.1016/s0006-3223(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Hill SY, Locke-Wellman J, Steinhauer S, Shen S. Neurodevelopmental changes in P300 characteristics of offspring from female and male alcoholism families. Evidence for delay in association with risk. (unpublished manuscript) [Google Scholar]
- Hill SY, Muka D, Steinhauer S, Locke J. P300 amplitude decrements in children from families of alcoholic female probands. Biol Psychiatry. 1995a;38:622–632. doi: 10.1016/0006-3223(94)00384-7. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, et al. Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biol Psychiatry. 1999c;46:970–981. doi: 10.1016/s0006-3223(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J Stud Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Locke J. Event-related potentials in alcoholic men, their high-risk male relatives and low-risk male controls. Alcohol Clin Exp Res. 1995b;19:567–576. doi: 10.1111/j.1530-0277.1995.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Lowers L, Locke J. Eight year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biol Psychiatry. 1995c;37:823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR, Zubin J. Biological markers for alcoholism: A vulnerability model conceptualization (Nebraska Symposium on Motivation 1986). In: Rivers PC, editor. Alcohol and Addictive Behavior; Lincoln: University of Nebraska Press; 1987. pp. 207–256. [PubMed] [Google Scholar]
- Holguin SR, Corral M, Cadaveira F. Visual and auditory event-related potentials in young children of alcoholics from high- and low-density families. Alcohol Clin Exp Res. 1998;22:87–96. doi: 10.1111/j.1530-0277.1998.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis CA, Hamburger SD, Kumra S, et al. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry. 1998;155:678–685. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Sowell ER. Magnetic resonance imaging studies of developing brain. In: Keshavan MS, Murray RM, editors. Neurodevelopment and Adult Psychopathology. New York: Cambridge University Press; 1997. pp. 63–70. [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: An event related functional magnetic resonance imaging study. J Neurosci. 2000;20:6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Kurtzberg D, Vaughan HG, Courchesne E, Friedman D, Harter MR, Putnam LE. Developmental aspects of event-related potentials. Ann NY Acad Sci. 1984;425:300–318. doi: 10.1111/j.1749-6632.1984.tb23551.x. [DOI] [PubMed] [Google Scholar]
- Lille F, Hazemann P, El Massioui F, Lesevre N, Dally S. Effect of chronic alcohol intake and short-term abstinence on early sensory EPs and late ‘cognitive’ ERPs. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Current Trends in Event-Related Potential, Research. Electroencephalogr Clin Neurophysiol. Vol. 40. 1987. pp. 712–717. [PubMed] [Google Scholar]
- Morrow LA, Steinhauer SR, Hodgson MJ. Delay in P300 latency in patients with organic solvent exposure. Arch Neurol. 1992;49:315–320. doi: 10.1001/archneur.1992.00530270135031. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén B. Mplus user’s guide. Los Angeles: Muthén & Muthén; 1998. [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Roth WT. P3 in schizophrenia is affected by stimulus modality, response requirements, medication status, and negative symptoms. Arch Gen Psychiatry. 1989;46:1035–1044. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Horvath TB, Roth WT, Kopell BS. Event-related potential changes in chronic alcoholics. Electroencephalogr Clin Neurophysiol. 1979;47:637–647. doi: 10.1016/0013-4694(79)90292-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 in the evaluation of aging and dementia. Electroencephalogr Clin Neurophysiol Suppl. 1991;42:304–323. [PubMed] [Google Scholar]
- Polich J, Ladish C, Burns T. Normal variation of P300 in children: Age, memory span, and head size. Int J Psychophysiol. 1990;9:237–248. doi: 10.1016/0167-8760(90)90056-j. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987a;4:283–287. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. The N2 component of the event-related brain potential in abstinent alcoholics. Electroencephalogr Clin Neurophysiol. 1987b;66:121–131. doi: 10.1016/0013-4694(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, et al. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: Preliminary results from the COGA project. Alcohol Clin Exp Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Simons RF, Graham FK, Miles MA, Chen X. On the relationship of P3a and the Novelty-P3. Biol Psychiatry. 2001;56:207–218. doi: 10.1016/s0301-0511(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;39:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY. Auditory event-related potentials in children at high risk for alcoholism. J Stud Alcohol. 1993;54:408–421. doi: 10.15288/jsa.1993.54.408. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY, Zubin J. Event-related potentials in alcoholics and their first-degree relatives. Alcohol. 1987;4:307–314. doi: 10.1016/0741-8329(87)90028-0. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Zubin J. Vulnerability to schizophrenia: Information processing in the pupil and event-related potential. In: Usdin E, Hanin I, editors. Biological Markers in Psychiatry and Neurology. Oxford: Pergamon Press; 1982. pp. 371–385. [Google Scholar]
- Steinhauer SR, Zubin J, Condray R, Shaw DB, Peters JL, van Kammen DP. Electrophysiological and behavioral signs of attentional disturbance in schizophrenics and their siblings. In: Tamminga CA, Schulz CZ, editors. Advances in Neuropsychiatry and Psychopharmacology: Schizophrenia Research. New York: Raven Press; 1991. pp. 161–168. [Google Scholar]
- Sutton S, Barren M, Zubin J, John ER. Evoked potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt T. The Genetics of Electrophysiological Indices of Brain Activity: An EEG Study in Adolescent Twins. Amsterdam: Psychologie, University of Amsterdam; 1996. [Google Scholar]
- Venables PH. Overview of psychophysiology in relation to psychiatry with special reference to schizophrenia. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Neuropsychology, Psychophysiology, and Information Processing. Vol 5. New York, New York: Elsevier; 1991. pp. 3–37. [Google Scholar]
- Verleger R, Jaskowski P, Wauschkuhn B. Suspense and surprise: On the relationship between expectancies and P3. Psychophysiology. 1994;31:359–369. doi: 10.1111/j.1469-8986.1994.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Whipple S, Parker ES, Noble EP. An atypical neurocognitive profile in alcoholic fathers and their sons. J Stud Alcohol. 1988;49:240–244. doi: 10.15288/jsa.1988.49.240. [DOI] [PubMed] [Google Scholar]
- Yanai I, Fujikawa T, Osada M, Yamawaki S, Touhouda Y. Changes in auditory P300 in patients with major depression and silent cerebral infarction. J Affect Disord. 1997;46:263–271. doi: 10.1016/s0165-0327(97)00100-6. [DOI] [PubMed] [Google Scholar]
- Zubin J, Steinhauer S. How to break the logjam in schizophrenia. A look beyond genetics. J Nerv Ment Dis. 1981;169:477–492. doi: 10.1097/00005053-198108000-00002. [DOI] [PubMed] [Google Scholar]