Abstract
The most common functional motor goal of lower extremity rehabilitation is to improve walking ability. For reasons of feasibility, safety or intensity, devices are frequently used to facilitate or augment gait training. The objective of this study was to compare the muscle activity patterns of the quadriceps and hamstring muscles during four conditions: overground walking, treadmill walking, stationary cycling, and elliptical training. Ten healthy adults (6 male, 4 female; mean age 22.7 ± 2.9 yrs, range 20–29) participated. Surface electromyographic data were recorded from the rectus femoris and semitendinosus. Linear envelope curves were generated and time normalized from 0–100% cycle. The mean plus three standard deviations from a static trial was used as the threshold for muscle activity. Repeated measures analysis of variance procedures were used to detect differences between conditions. Elliptical training demonstrated greater quadriceps activity and greater quadriceps/hamstrings coactivation than all other conditions. Consistent with previous work, treadmill walking demonstrated greater quadriceps activity than overground walking. Minimal differences in hamstring activation were observed between conditions, limited to lower peak activity during cycling compared to treadmill walking. These results provide normative values for quadriceps and hamstring activation for different locomotor training methods and may assist in selecting the most appropriate training device for specific patients. Clinicians and researchers should also consider the kinematic and kinetic differences between tasks, which cannot necessarily be inferred from muscle activation patterns.
Keywords: gait, EMG, muscle activity, lower extremity
The most common functional motor goal of lower extremity rehabilitation is to improve walking ability. For reasons of feasibility, safety or intensity, training devices are often used to facilitate or augment gait training. The choice of a particular training device is influenced by the therapeutic goals (e.g. strengthen muscles, improve reciprocal muscle activation, or simulate muscle activity patterns during walking) and by the functional abilities of the patient. Muscle activation patterns during overground walking have been compared individually to walking on a treadmill1, 2 and to elliptical training,3 but not to stationary cycling, and not together in one study using the same participants in all conditions.
Stationary cycling has been investigated in rehabilitation programs4, 5 as a low-impact activity that requires similar reciprocal leg movement to walking while the majority of the patient’s body weight is supported by the seat. Elliptical training is another training alternative that may facilitate interlimb coordination but involves upright reciprocal activity, requiring the patient’s body weight to be largely supported by their legs with the use of upper extremity handlebars for balance support. Elliptical training generates lower pedal reaction forces than ground reaction forces during walking, but greater knee and hip moments than walking.6 Both stationary cycling and elliptical training have the potential to be performed in the home with minimal assistance from others, and can provide consistent practice environments that allow a high number of repetitions. Despite assumptions that these activities are similar to walking and, therefore, may offer similar benefits to gait training, electromyographic (EMG) patterns during cycling and elliptical training have not been systematically compared to walking.
The EMG analysis method most commonly employed clinically is the determination of the onset and offset of muscle activity during a particular movement. Determining the onset and offset is clinically useful to investigate differences in activation timing and to assess periods of coactivation of the agonist and antagonist muscles. While visual inspection of the raw EMG signals remains a popular method to determine onset and offset of muscle activation, systematic or non-systematic bias with this method and disagreement between experienced raters are known limitations.7 Several reliable automated methods have been proposed to facilitate the detection of muscle activity using a standard deviation amplitude threshold with respect to a resting baseline, eliminating subjective bias and reducing analysis time.7, 8 Additionally, for conditions with high levels of EMG activity that include higher activation bursts, comparison to a resting baseline trial is particularly useful to determine whether the relatively less active periods within those trials represent activation or not.
Absolute magnitude of muscle activation can only be approximated by comparing the EMG signal amplitudes during an activity to an isometric maximum voluntary contraction. However, this cannot account for changes in activation to torque relationships at different joint angles or for antagonistic or synergistic activity that may have occurred during the maximal test. Relative magnitude comparisons across a cycle or across conditions where the electrodes have not been moved can also be made, with the recognition that these have similar limitations such as being affected by differences in joint position and body orientation.
Quantifying the differences in muscle activity among these training activities may guide clinicians and researchers when choosing the most appropriate training protocol for specific patients according to the goals of the treatment. The objective of this study was to compare the muscle activity patterns of the quadriceps and hamstring muscles during four non-strenuous conditions: overground walking, treadmill walking, stationary cycling, and elliptical training.
METHODS
Participants
Ten healthy young adults (6 male, 4 female) participated. The participants had a mean age of 22.7 ± 2.9 years (range 20–29 years), a mean height of 173.5 ± 9.8 cm (range 155–191 cm), and a mean mass of 69.5 ± 9.8 kg (range 49.5–79.1 kg). The protocol was approved by our institution’s Human Subjects Institutional Review Board. Written, informed consent was obtained from all individuals prior to testing.
Procedures
Data were collected in a motion analysis laboratory. Preamplified, single differential, surface EMG electrodes (Motion Lab Systems, Baton Rouge, LA) were placed on the rectus femoris and semitendinosus bilaterally (only right side reported here) according to SENIAM recommendations.9 The electrodes were medical grade stainless steel discs with a 12 mm diameter and 18 mm inter-electrode distance.
The skin areas were cleaned with alcohol and the electrodes were affixed to the skin with hypoallergenic tape. The electrodes were further secured to reduce motion artifact using flexible, non-adhesive wrap encircling the thighs (Coflex®, Andover Healthcare, Inc.). A volitional sub-maximal contraction of each muscle was elicited to verify suitable electrode placement, skin contact, and the absence of recording activity from the adjacent muscles (“crosstalk”). A self-adhesive reference electrode was placed on the skin over the olecranon process of one elbow. Raw EMG data were acquired at a rate of 960 Hz. A ten camera motion capture system (Vicon, Lake Forest, CA) captured concurrent 3D lower extremity kinematics at a rate of 120 Hz which were used here to determine cycle events for each condition.
A two-second standing static trial was collected prior to the test conditions for later determination of periods of muscle activation. Participants performed the overground walking task (W) at a self-selected speed, and were instructed to keep their heads up and walk at a “normal, comfortable pace” directly across the gait laboratory, approximately 8 meters in length. Four W trials were used to calculate an average velocity and cadence in real-time to pace the other conditions. A velocity indicator composed of two motion detection sensors determined walking speed in real-time. The number of frames between heel strikes on the ipsilateral foot were measured in Nexus, and then converted to time by dividing by the frame rate. Cadence was manually calculated by dividing two steps by the time required to perform those two steps. This method allowed for efficient determination of velocity and cadence without processing the entire W condition data using the motion analysis software, which would have extended data collection time.
Participants subsequently participated in treadmill walking (T), cycling (C), and elliptical training (E) in a randomized order. The average real-time W speed and cadence were used to pace the other training device conditions. T speed was matched to the W velocity. During C and E tasks, participants were asked to match the W cadence with the aid of a metronome.
A split-belt Bertec TM-06-B treadmill (Columbus, OH), Monark 818E stationary cycle (Vansbro, Sweden), and Nordic Track CX1000 elliptical trainer (Logan, UT) were used for the respective test conditions. The step length on the elliptical trainer and crank arm on the cycle were adjusted to a comfortable position for each individual. Participants wore their own sneakers while completing all tasks. They were given 1–2 minutes to familiarize themselves with each training device, but experience levels were not assessed, and no additional practice was given. After familiarization with the device and pace, participants performed 1–2 minutes in each condition while data were captured.
Data analysis
Five right-sided cycles were extracted for analysis from each condition using the time-synchronized marker data (initial foot contact for consecutive footfalls for walking trials, and the most anterior point of consecutive revolutions for elliptical and cycling trials3) collected by the motion analysis system. The hardware low pass filter was fixed at 450 Hz. EMG data were processed with Visual 3D software (C-Motion, Germantown, MD) using a 10 Hz high pass filter, full-wave rectification, a second-order low-pass Butterworth filter with phase correction and a cutoff frequency of 5 Hz, and averaged across cycles to generate linear envelope curves for each condition. All signals were time normalized to 101 data points, representing the cycle from 0 to 100%. Visual inspection of the standing static trials was conducted, to confirm that all participants had at least a two-second portion free of artifact or phasic EMG activity. The mean amplitude plus three standard deviations from the static EMG trial was used as the threshold above which defined muscle activity during all conditions.7
Data were analyzed for differences in muscle activation timing and amplitude across the four conditions. Activation and coactivation time were analyzed as a percent of the cycle. Coactivation was calculated as the total time both muscles were simultaneously active. Magnitude of muscle activity was analyzed by the peak amplitude that occurred during the cycle, and by calculating the total area of the linear envelope that exceeded the threshold. Cycle-to-cycle variability was measured by determining the coefficient of variation for the five individual cycles, averaged over the cycle as in Winter et al.10 A repeated measures analysis of variance (ANOVA) with repeated contrast for post-hoc comparisons was used to detect differences between conditions (p<0.05) using SPSS software (Chicago, IL).
RESULTS
Speed and cadence
The average W velocity collected in real-time from the velocity indicator was 1.41 m/s (SD 0.19), which was therefore the average fixed T speed across the 10 participants. The actual mean W velocity of the selected gait cycles used for EMG analysis from all participants was 1.31 m/s (SD 0.18). The average self-selected W cadence was 107.9 steps per minute (SD 8.3), which was then used as the target pace for C and E trials. The actual cadence was 111.0 steps per minute (SD 15.5) for C, and 109.0 steps per minute for E (SD 12.6).
EMG activation time
See Figures 1 and 2 for representative data for the rectus femoris and medial hamstrings, respectively. The quadriceps were active for a significantly greater percent of the cycle during E than each of the other 3 conditions, and during T compared to W. There were no differences for hamstrings activation time (Fig. 3A). Coactivation time was also significantly greater during E than each of the other three conditions (Fig. 3B).
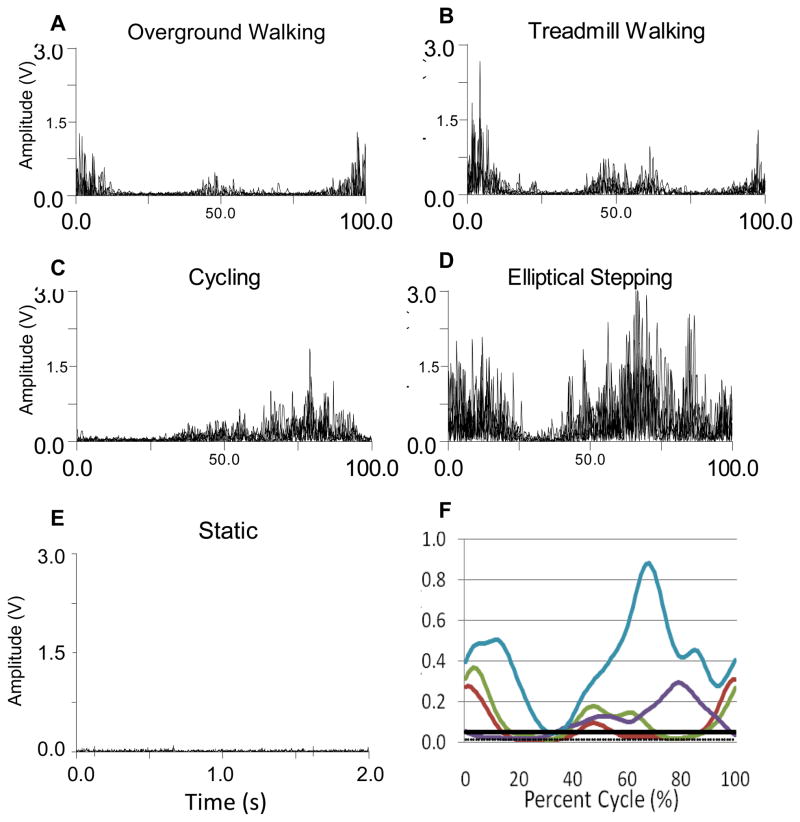
Figure 1.
Representative rectus femoris data for one participant for all conditions. A. Overground walking rectified EMG signals. B. Treadmill walking rectified EMG signals. C. Cycling rectified EMG signals. D. Elliptical training rectified EMG signals. E. Static rectified EMG signal. F. Linear envelope of EMG during all conditions (average of 5 cycles) with static mean and activation threshold. (W-red, T-green, C-purple, E-blue, threshold-solid black, static mean-dotted black).
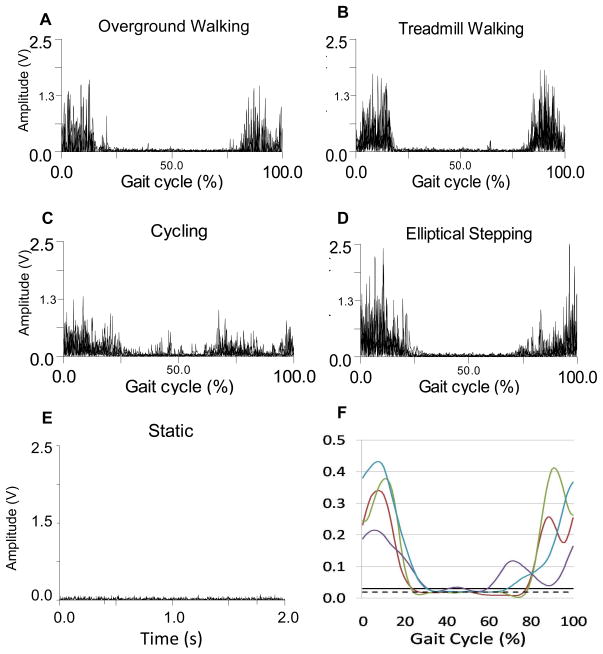
Figure 2.
Representative medial hamstrings data for one participant for all conditions. A. Overground walking rectified EMG signals. B. Treadmill walking rectified EMG signals. C. Cycling rectified EMG signals. D. Elliptical training rectified EMG signals. E. Static rectified EMG signal. F. Linear envelope of EMG during all conditions (average of 5 cycles) with static mean and activation threshold. (W-red, T-green, C-purple, E-blue, threshold-solid black, static mean-dotted black).
Figure 3.
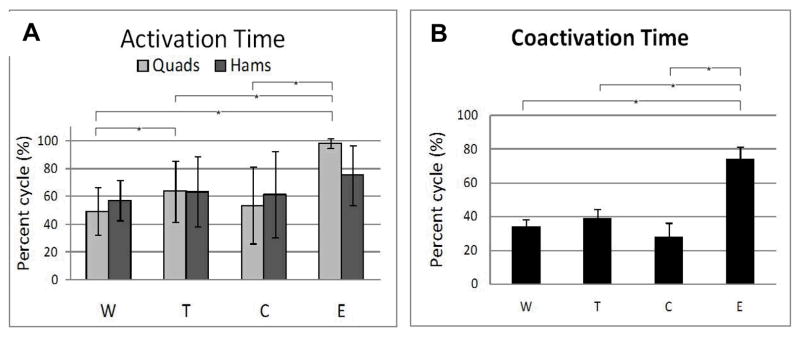
Activation time. A. Quadricpes and hamstrings activation time. Brackets indicate that Q was active for a significantly greater percent of the cycle during E than each of the other 3 conditions, and that activation time was greater in T compared to W. B. Q/H coactivation time. Brackets indicate that coactivation of Q/H during E occurred during a significantly greater percent of the cycle than each of the other 3 conditions.
EMG activation amplitude
The quadriceps demonstrated greater activation area and greater peak activity during E than each of the three other conditions, and during T compared to W. Hamstrings peak amplitude was less in C compared to T, with no differences for hamstrings activation area. Coactivation area was also greater during E than each of the three other conditions, and during T compared to C.
Cycle-to-cycle EMG variability
Mean coefficients of variation ranged from 0.29–0.36 for the quadriceps for the four conditions, with C demonstrating greater variability than W (p=0.024). There were no differences in hamstring activation variability among the four conditions, with mean coefficients of variation ranging from 0.31–0.37.
EMG activation pattern
Compared to W, the quadriceps were active at a higher magnitude and for a longer time during T. This increased activation occurred largely in the stance phase during T. Three participants demonstrated activation throughout the stance phase of gait during T, with the other participants demonstrating shorter periods of no activation in the stance phase during T compared to W. All participants had a period of no activation during the stance phase during W. (Fig. 4)
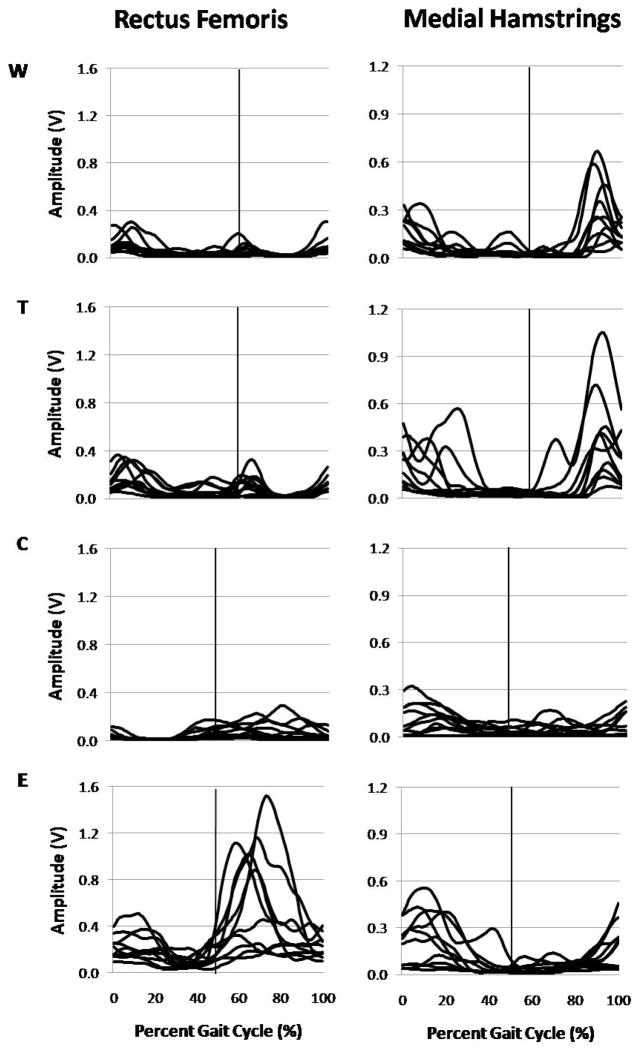
Figure 4.
Linear envelope curves for all participants during all conditions. Vertical lines represent the transition from stance phase to swing phase in the overground walking (W) and treadmill walking (T) conditions, and the transition from recovery phase to propulsive phase in the cycling (C) and elliptical training (E) conditions.
For C and E, the recovery phase of the cycle was defined as the period from the most anterior point to the most posterior point (as the foot moves through bottom dead center and toward the body), which was from 0–50% of the cycle. The propulsive phase of the cycle was defined as the period from the most posterior point to the most anterior point (as the foot moves through top dead center and away from the body), which was from 51–100% of the cycle. During both C and E, the quadriceps were active at a higher magnitude during the propulsive phase of the cycle compared to the recovery phase, with the onset of the activity beginning at the end of the recovery phase and ending at the beginning of the subsequent recovery phase. The hamstrings were active at a higher magnitude during the recovery phase, with activity beginning in the propulsive phase, although there was greater variability in patterns across individuals for the hamstrings compared to the quadriceps in both C and E. The activity in the hamstrings during the recovery phase suggests that this is an active phase in both C and E, rather than a passive phase created solely by the contralateral propulsive phase. (Fig. 4)
DISCUSSION
Using an automated threshold method to determine muscle activation avoids subjective bias in visual review of EMG data, which may be particularly important when analyzing EMG data in patients with neuromuscular conditions. Comparison of automated results to the EMG plots is essential to verify whether the threshold appears to accurately represent when muscles are or are not active. Timing measures are more sensitive than amplitude measures to the threshold level. If EMG magnitude is low, using an automated threshold may not be ideal as small changes in the threshold will significantly affect timing.
The results of this study provide normative values for quadriceps and hamstrings EMG activity during four training conditions and may assist in selecting the most appropriate training device for specific patients. During E, the quadriceps, but not the hamstrings, were active longer and at a higher intensity than in all other conditions. E might be a consideration if increasing quadriceps, but not hamstrings, strength, endurance, or power are treatment goals. If the goal is to restrict hamstrings activity to the greatest extent, cycling may be the best option. While there was no difference in hamstrings activity between W, T, and E, peak amplitude was lower in C than in T.
Reducing coactivation may be a goal for individuals with neurological injury resulting in excessive muscle activity, such as cerebral palsy, stroke, or brain injury. For these patients, C might be a preferred option, which demonstrated less coactivation compared to T and E. These differences may be a result of the reduced lower extremity weight bearing or limb-loading activity during stationary C, which has been reported previously in comparison to W and E.11 It did not appear that whether the foot was free and required controlled placement with each cycle (W and T) or relatively fixed on a foot plate (C and E) made significant differences in the results.
As expected, the pattern of muscle activity during T was the most similar to W (Fig. 4), and T may be desirable if replication of the walking pattern is critical. However, the quadriceps were still more active during T than W. More research needs to be conducted on transfer of training to determine how similar a specific training task needs to be to W in order for the training effects to impact functional W. It was surprising that during C, but not E, the participants demonstrated greater cycle-to-cycle variability compared to W. Generally, variability would be expected to increase with all less familiar or novel tasks. Again, future work should continue to explore the degree of variability or consistency within a training task that leads to the optimal transfer of ability to W.
This discussion on training device selection is based on the assumption that muscle activation patterns in those with neurological disorders are similar to those seen here, which is not necessarily the case.12 More work investigating muscle activation patterns in various patient groups needs to be done to allow more specific recommendations.
Our results from the walking conditions are consistent with those of Murray and colleagues who report increased EMG activity in the quadriceps, but not the hamstrings, during treadmill versus floor walking.2 Lee and Hidler also reported increased rectus femoris activity during T compared to W, but they also report differences in the hamstrings between the two conditions for four of seven distinct gait phases analyzed.1 Their analysis by phase compared to our analysis of the entire gait cycle may explain the differences observed.
The EMG patterns during cycling in this study are similar to those previously reported by Jorge and Hull13 and for the typical group reported by Lauer et al,14 with the exception of the rectus femoris demonstrating earlier onset and offset in Lauer’s study. The cycling position in Lauer’s study was recumbent, whereas in this study was upright, which may explain the need for earlier quadriceps activity to drive the pedal away from the body from the point where the pedal is closest to the body.
The increased peak and duration of quadriceps activity during E compared to W in this study are consistent with those reported by Burnfield and colleagues in a study of four different elliptical trainers.3 However, they found that medial hamstring activity was reduced compared to walking. The difference in hamstring activation from the present study could be attributed to the different method of EMG analysis used (normalized to an isometric maximum contraction with a 5% threshold), or to the older age of the participants in Burnfield’s study (mean age 48.5 years). It may be of interest in the future to also examine E in a reverse direction, which is feasible on most elliptical trainers, and may target different muscle activity patterns. A similar pattern might be observed with forward and backward E as has been reported in C, where the hamstrings were active for a longer duration during the recovery phase, and the rectus femoris was more active during the propulsive phase, of backward cycling compared to forward cycling.15
A limitation of this study is the speed discrepancy between W (1.31 m/s) and T (1.41 m/s). This difference was due to error in the real-time velocity indicator and the inclusion of more gait cycles in the real-time velocity measurement than the five cycles extracted for EMG analysis. The fixed T speed was determined by the velocity indicator while the W velocity and cadence results presented here are based on the analysis from the motion capture system using only the five selected cycles. However, in consideration of the results of van Hedel et al, a difference of 0.1 m/s likely has a negligible effect on the results of the EMG analysis.16 Clinicians and researchers should also consider the kinematic and kinetic differences between conditions before making a decision on a specific training device, which may reveal different results from the muscle activation patterns reported here.
CONCLUSION
This is the first study to compare muscle activation during stationary cycling and elliptical training to overground and treadmill walking. Elliptical training demonstrated greater quadriceps activity and greater quadriceps/hamstrings coactivation than all other conditions. Consistent with previous work, treadmill walking demonstrated greater quadriceps activity than overground walking. Minimal differences in hamstrings activation were observed between conditions, limited to lower peak activity during cycling compared to treadmill walking. These results may guide clinicians and researchers when designing rehabilitation programs in selecting the most appropriate training condition for specific patients. Clinicians and researchers should also consider the kinematic and kinetic differences between conditions, which cannot necessarily be inferred from muscle activation patterns.
Table 1.
Activation magnitude.
| AREA | PEAK | ||||
|---|---|---|---|---|---|
| Q | H | Coactivation | Q | H | |
| W | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) | 1.0 (0) |
| T | 2.3 (1.6)* | 1.7 (1.4) | 1.5 (0.9) | 1.7 (1.0)* | 1.5 (1.1) |
| C | 1.4 (1.3) | 0.7 (0.5) | 0.6 (0.7)^ | 0.9 (0.7) | 0.6 (0.4)*^ |
| E | 14.4 (10.1)*^# | 1.2 (0.8) | 5.3 (4.3)*^# | 6.9 (7.9)*^# | 0.8 (0.4) |
Values are means (standard deviation) normalized to the overground walking (W) condition. T=treadmill walking, C=cycling, E=elliptical training, Q=quadriceps, H=hamstrings.
significant difference from W,
significant difference from T,
significant difference from C (p<0.05).
Acknowledgments
This work was funded by the intramural research program at the NIH Clinical Center
References
- 1.Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J Appl Physiol. 2008;104(3):747–55. doi: 10.1152/japplphysiol.01380.2006. [DOI] [PubMed] [Google Scholar]
- 2.Murray MP, Spurr GB, Sepic SB, Gardner GM, Mollinger LA. Treadmill vs. floor walking: kinematics, electromyogram, and heart rate. J Appl Physiol. 1985;59(1):87–91. doi: 10.1152/jappl.1985.59.1.87. [DOI] [PubMed] [Google Scholar]
- 3.Burnfield JM, Shu Y, Buster T, Taylor A. Similarity of joint kinematics and muscle demands between elliptical training and walking: implications for practice. Phys Ther. 90(2):289–305. doi: 10.2522/ptj.20090033. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007;87(12):1580–602. doi: 10.2522/ptj.20060310. [DOI] [PubMed] [Google Scholar]
- 5.Williams H, Pountney T. Effects of a static bicycling programme on the functional ability of young people with cerebral palsy who are non-ambulant. DevMed Child Neurol. 2007;49(7):522–7. doi: 10.1111/j.1469-8749.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Lu TW, Chien HL, Chen HL. Joint loading in the lower extremities during elliptical exercise. Med Sci Sports Exerc. 2007;39(9):1651–8. doi: 10.1249/mss.0b013e3180dc9970. [DOI] [PubMed] [Google Scholar]
- 7.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalography and Clinical Neurophysiology. 1996;101(6):511–9. doi: 10.1016/s0013-4694(96)95190-5. [DOI] [PubMed] [Google Scholar]
- 8.Di Fabio RP. Reliability of computerized surface electromyography for determining the onset of muscle activity. Phys Ther. 1987;67(1):43–8. doi: 10.1093/ptj/67.1.43. [DOI] [PubMed] [Google Scholar]
- 9.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. 2000;10(5):361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 10.Winter DA, Yack HJ. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol. 1987;67(5):402–11. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 11.Burnfield JM, Jorde AG, Augustin TR, Augustin TA, Bashford GR. Variations in plantar pressure variables across five cardiovascular exercises. Med Sci Sports Exerc. 2007;39(11):2012–20. doi: 10.1249/mss.0b013e318148bdfa. [DOI] [PubMed] [Google Scholar]
- 12.Johnston TE, Barr AE, Lee SC. Biomechanics of submaximal recumbent cycling in adolescents with and without cerebral palsy. Physical Therapy. 2007;87(5):572–85. doi: 10.2522/ptj.20060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorge M, Hull ML. Analysis of EMG measurements during bicycle pedalling. J Biomech. 1986;19(9):683–94. doi: 10.1016/0021-9290(86)90192-2. [DOI] [PubMed] [Google Scholar]
- 14.Lauer RT, Johnston TE, Smith BT, Lee SC. Lower extremity muscle activity during cycling in adolescents with and without cerebral palsy. Clinical Biomechanics (Bristol, Avon) 2008;23(4):442–9. doi: 10.1016/j.clinbiomech.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisner WD, Bode SD, Nyland J, Caborn DN. Electromyographic timing analysis of forward and backward cycling. Med Sci Sports Exerc. 1999;31(3):449–55. doi: 10.1097/00005768-199903000-00015. [DOI] [PubMed] [Google Scholar]
- 16.van Hedel HJ, Tomatis L, Muller R. Modulation of leg muscle activity and gait kinematics by walking speed and bodyweight unloading. GaitPosture. 2006;24(1):35–45. doi: 10.1016/j.gaitpost.2005.06.015. [DOI] [PubMed] [Google Scholar]