Abstract
Shikonin, extracted from medicinal Chinese herb (Lithospermum erythrorhizo), was reported to exert anti-inflammatory and anti-cancer effects both in vitro and in vivo. We have found that proteasome was a molecular target of shikonin in tumor cells, but whether shikonin targets macrophage proteasome needs to be investigated. In the current study, we report that shikonin inhibited inflammation in mouse models as efficiently as dexamethasone. Shikonin at 4 μM reduced the Lipopolysaccharides (LPS)-mediated TNFα release in rat primary macrophage cultures, and blocked the translocation of p65-NF-κB from the cytoplasm to the nucleus, associated with decreased proteasomal activity. Consistently, shikonin accumulated IκB-α, an inhibitor of NF-κB, and ubiquitinated proteins in rat primary macrophage cultures, demonstrating that the proteasome is a target of shikonin under inflammatory conditions. Shikonin also induced macrophage cell apoptosis and cell death. These results demonstrate for the first time that proteasome inhibition by shikonin contributes to its anti-inflammatory effect. The novel finding about macrophage proteasome as a target of shikonin suggests that this medicinal compound has great potential to be developed into an anti-inflammatory agent.
Keywords: Shikonin, Proteasome inhibitor, Macrophage, Inflammation
1. Introduction
Dating back to ancient times, Zi Cao (purple gromwell) had been used for the treatment of throat sore, burns, cut and skin diseases such as macular eruption, measles and carbuncles in China. It is a commonly used herbal medicine in China and other countries (Chen et al., 2002, 2003). In recent years, compounds isolated from Zi Cao have been reported to confer many medicinal properties such as antibacterial, anti-inflammatory, anti-thrombotic and anti-tumor effects (Tanaka et al., 1986; Wang et al., 1995). Shikonin, one of the main active components of Zi Cao, has been shown to exert anti-inflammatory and anti-cancer effects both in vitro and in vivo (Wang et al., 1994; Yang et al., 2009a). Several molecular targets of shikonin have also been proposed (Wang et al., 1994; Staniforth et al., 2004; Nam et al., 2008; Su et al., 2008; Takano-Ohmuro et al., 2008; Yang et al., 2009a). We also reported that the proteasome is one of the important molecular targets of shikonin (Yang et al., 2009a).
The ubiquitin–proteasome system (UPS) is the major pathway for intracellular protein degradation (Ciechanover, 1998). Proteasomal degradation removes denatured, misfolded, or improperly translated proteins from cells and regulates the level of proteins such as cyclins and transcription factors. A growing body of evidence suggests that targeting the proteasomal pathway is an attractive approach to treat inflammatory and autoimmune diseases (Wang and Maldonado, 2006). The most important link between the UPS and inflammation is related to NF-κB, which is a master transcription regulator of many inflammatory cytokine genes (Orlowski and Baldwin, 2002). NF-κB is normally sequestered in the cytoplasm via association with its endogenous inhibitor IκB. Once stimulated, IκB-α is rapidly phosphorylated by kinase IKK. Phosphorylated IκB-α is specifically recognized by SCFβ-TrCP E3 ligase and transferred to the proteasome for degradation while NF-κB is allowed to be translocated to the nucleus and mediate transcription of many pro-inflammatory genes (Biswas and Iglehart, 2006; Solt and May, 2008). Accumulation of the IκB-α protein via proteasome inhibition therefore prevents the activation of the NF-κB and inflammation pathways.
NF-κB pathway has been an attractive target for the development of new anti-inflammatory drugs (Makarov et al., 1997). Even though it is well known that proteasome inhibition leads to blockage of NF-κB pathway, whether natural proteasome inhibitors extracted from medicinal Chinese herbs could inhibit macrophage proteasome function has never been demonstrated. Furthermore, the relationship between shikonin-mediated proteasome inhibition and its anti-inflammatory effect is unknown. Here we report for the first time that shikonin targets primary macrophage cellular proteasome which is either responsible for, or at least contributes to its anti-inflammatory effect.
2. Materials and methods
2.1. Materials
Purified shikonin (>98%) was purchased from the National Institute for the Control Pharmaceutical and Biological Products (Beijing, China) and was dissolved by DMSO at a stock concentration of 50 mM to different concentrations. NF-κB-p65 (Ab276) rabbit antibody was purchased from SAB biotechnology. Mouse monoclonal antibodies against Ubiquitin (P4D1) and rabbit polyclonal antibody against inhibitor of nuclear factor IκB-α (C-15) or GAPDH (FL-335) were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Apoptosis detection kit was from Nanjing Keygen Inc. (Nanjing, China). RPMI-1640, fetal bovine serum (FBS) and other materials required for culturing cells were purchased from Gibco Technology.
2.2. Rat primary macrophage preparation and culture
Sprague–Dawley (SD) rats were purchased from Sun-Yat Sen University laboratory animal center and peritoneal macrophages were separated as described previously (Zhang et al., 2008). Briefly, peritoneal lavage was harvested from SD rats by i.p. injection of 10 ml sterile ice cold phosphate-buffered saline (PBS). The cells were washed twice with PBS, resuspended in RPMI 1640 supplemented with 10% FBS, 2 mM glutamine, 100 unit/ml penicillin and 100 μg/ml streptomycin, and incubated in a humidified 5% CO2 incubator. Non-adherent cells were flushed out with RPMI 1640 after 3 h and the resulting adherent peritoneal cells were identified to be >95% as macrophages by morphology and nonspecific esterase staining.
2.3. Fluorescent immunostaining
Rat primary macrophage culture was performed on 8-well chamber slides (1.5×105/well) and macrophage cells were pretreated with different doses of shikonin or DMSO for 1.5 h prior to stimulation of LPS (1 μg/ml). After stimulation with LPS for 4 h, slides were washed with PBS, fixed in −20 °C for 5 min, washed again in 0.2% Triton X100/PBS for 3×5 min and permeabilized with 1% TritonX100/PBS for 5 min. Cells were blocked with 5% bovine serum albumin (BSA)/PBS for 1 h and incubated with 1:50 NF-κB-p65 (Ab276) rabbit antibody overnight at 4 °C in a humid chamber to prevent evaporation of the antibody solution. Slides were reprobed with 1:150 Rhodamine (TRITC)-Conjugated goat anti-rabbit IgG (Pierce Biotechnology) for 1 h at room temperature in a dark humid chamber. Cell nuclei were counterstained with Hoechst33342 for 10 min. Samples were analyzed using a confocal laser microscope (Zeiss LSM510 Meta). All images were acquired under identical settings with LSM Image browser software.
2.4. Western blotting
Western blotting was performed as previously reported (Liu et al., 2006). Briefly, rat macrophages were untreated or treated with indicated concentrations of shikonin for 1.5 h and/or stimulated with LPS (1 μg/ml) for 6 h in 6-cm dishes. After stimulation with LPS, cells were collected for Western blotting assay.
2.5. Cell death assay
Rat primary macrophage cells were pretreated with various concentrations of shikonin for 1.5 h, followed by stimulation of LPS (1 μg/ml)for 6 h. Macrophage cell death was measured by flow cytometry as previously described (Yang et al., 2009a).
2.6. Peptidase assay
Peptidase assay was performed as described previously (Yang et al., 2009a). 10 μg of protein lysate was incubated with different doses (1–40 μM) of shikonin in a Tris–HCL reaction system containing the synthetic fluorogenic peptide Suc-LLVY-aminomethylcoumarin (AMC) for the chymotrypsin-like activity, Z-LLE-AMC for the caspase-like activity, or Bz-VGR-AMC for the trypsin-like activity (25 μM, Calbiochem). The reaction mixture was then incubated at 37 °C for 90 min and analyzed for the fluorescence intensity of the free AMC using a luminescence microplate reader (Varioskan Flash 3001, Thermo, USA) at Ex360 nm/Em436 mm.
2.7. TNF-α ELISA assay
The TNF-α concentrations in the culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, rat macrophages were cultured at a density of 1×106/ml in 6-cm dishes and pretreated with of shikonin(1 μmol/L and 4 μmol/L)for 1.5 h. The production of TNF-α was stimulated with 1 μg/ml of LPS(E. coli O111: B4, Calbiochem)for another 2 h or 4 h and cell-free supernatants were collected for TNF-α determination by Rat TNF-α ELISA assay kit (Dakewe Biotechnology, Shenzhen, China) according to the manufacturer’s instructions.
2.8. In vivo models of inflammation
Kunming and NIH mice were purchased from Guangdong Animal Center and housed in accordance with protocols approved by the Guangdong Animal Center. Mouse’s auricle swelling was induced by xylene (Weirich et al., 1977). 60 Kunming mice were randomly divided into 6 groups (n=10 each group) for treatment of (i) DMSO, (ii) LPS (35 mg/kg), (iii) LPS (35 mg/kg)+shi (0.5 mg/kg), (iv) LPS (35 mg/kg) shi (1 mg/kg), (v) LPS (35 mg/kg)+shi (4 mg/kg), and (vi) LPS (35 mg/kg)+dexamethasone (2.5 mg/kg). Mice were pretreated with shikonin or dexamethasone for 30 min, then 50 μl of xylene was painted evenly to the same site of one ear for 30 min and a 9 mm ear pad biopsy was collected from the ear of each mouse with a coring tool and then weighted to determine the severity of edema. The weight difference of both parallel areas of ears was compared in different groups.
The model of increased capillary permeability in mice was induced by acetic acid (Blackham and Woods, 1986). Total 60 NIH mice were randomly divided into 6 groups: DMSO; LPS (35 mg/kg); LPS+shi (0.5 mg/kg); LPS+shi (1 mg/kg); LPS+shi (4 mg/kg) and LPS dexamethasone (2.5 mg/kg). NIH mice were pretreated with shikonin as indicated for 90 min. After i.p injection of LPS for 30 min, i.v injection of Evans blue and i.p injection of acetic acid was performed. 30 min later peritoneal fluid was collected for absorbance assay of Evans blue at OD590 nm.
2.9. Lactate dehydrogenase (LDH) activity assay
LDH assay was performed as reported (Huang et al., 2010). Briefly, 100 μl of reaction reagent was added to each well of a 96-well plate containing 100 μl of optimally titrated culture medium, and the mixture was incubated for 30 min at room temperature. Absorbance of the samples at 490 nm was measured on a microplate reader (Sunrise, Tecan, Austria). The reference wavelength was 620 nm. LDH activity was calculated according to the standard curve as μmol/L. Results from at least four separate experiments are presented.
2.10. Statistical analysis
Unpaired t test was used for determining the statistically significant differences between the values of various experimental groups. Data were expressed as means+S.D. and a P value <0.05 was considered statistically significant.
3. Results
3.1. Shikonin potently inhibits inflammation-associated auricle swelling and capillary permeability increase in mice
Xylene-induced auricle swelling and acetic acid-induced capillary permeability increase were two classical mouse models widely used in search for drugs with anti-inflammatory activity (Blackham and Woods, 1986; Weirich et al., 1977). Consistent to previous reports (Chen et al., 2002, 2003; Tanaka et al., 1986; Wang et al., 1995), shikonin efficiently inhibited auricle swelling (Fig. 1A) and capillary permeability increase (Fig. 2B) in a dose-dependent manner (p<0.05) in these mouse models. In fact, shikonin at 4 mg/kg yielded the same efficacy as dexamethasone at 2.5 mg/kg (Fig. 1).
Fig. 1. Shikonin inhibits LPS- and xylene-mediated inflammation in mouse models in vivo.
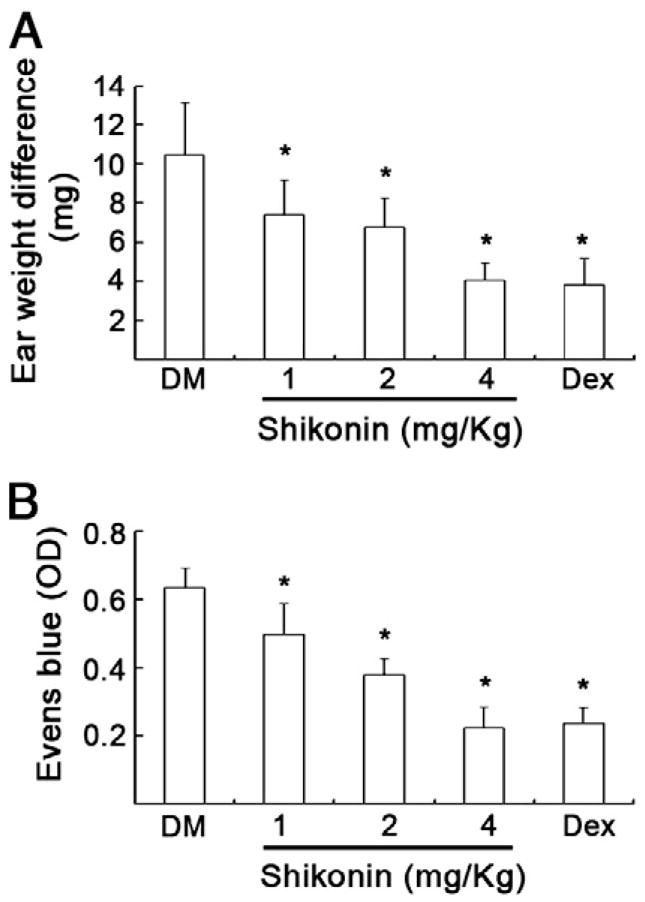
A. Kunming mouse model of auricle swelling: Mice were pretreated with various doses of shikonin (i.p injection of 0.5, 1, 4 mg/kg, respectively) or dexamethasone (2.5 mg/kg) for 30 min, then 50 μl of xylene was scraped evenly to the same site of one ear, and 30 min later the weight difference of both ears were compared in different groups. (Means+S.D., n=10). *P<0.05, compared with DM control group.
B. NIH mouse model of LPS-mediated acute inflammation. NIH mice were pretreated with shikonin as indicated in A for 90 min. After i.p injection of LPS (35 mg/kg) for 30 min, i.v injection of Evans blue and i.p injection of acetic acid was performed. 30 min later peritoneal fluid was collected for absorbance assay of Evans blue. OD590 nm was expressed as means + S.D. (n =10). *P < 0.05, compared with DM control group.
Fig. 2.
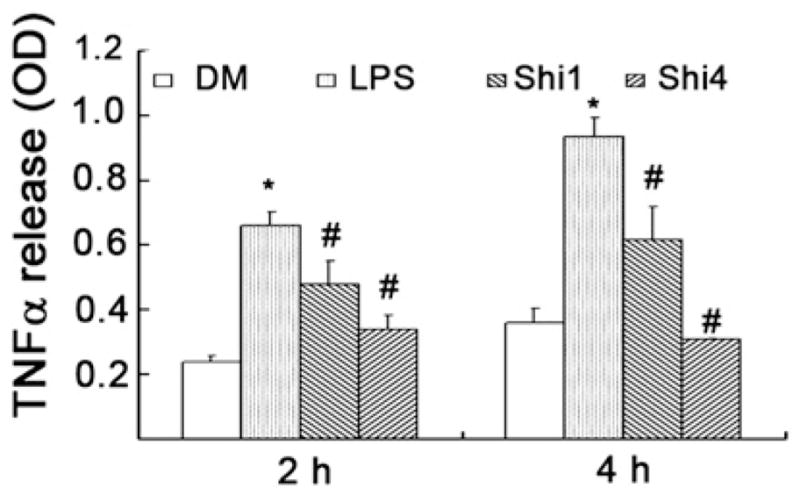
Shikonin inhibits LPS-induced TNFα production in rat primary macrophage cultures. Rat primary macrophage cultures were pretreated with either solvent DMSO (DM) or indicated concentrations of shikonin for 1.5 h and then exposed to LPS (1 μg/ml). At 2 and 4 h after LPS treatment, TNFα level in cultured supernatants was determined by ELISA kit. Values represent means ± S.D. (n=3). *P<0.05, compared with DM group; #P<0.05, compared with LPS group.
3.2. Shikonin inhibits TNF-α release in rat primary macrophage cultures
Toward the goal of understanding the molecular mechanism responsible for shikonin’s anti-inflammatory effect, we next determined the effects of shikonin on rat primary macrophage cultures. Macrophages are the most important inflammatory cells in the body (Qureshi et al., 2005). Among the pro-inflammatory cytokines secreted by macrophages, TNF-α plays an important role in immune and inflammatory responses. Inappropriate or overexpression of TNFα is a hallmark of a number of inflammatory and autoimmune diseases (Locksley et al., 2001). Treatment of rat macrophages with LPS for 2 to 4 h increased the level of released TNFα by ~3-fold (Fig. 2). However, addition of shikonin at 1 and 4 μM to the rat primary macrophage cultures pretreated by LPS for 2 h resulted in 46% and 77% inhibition of TNFα release, respectively (Fig. 2). When added to 4 h-pretreated macrophages by LPS, shikonin at 1 and 4 μM inhibited 56% and ~100% of TNFα release, respectively (Fig. 2).
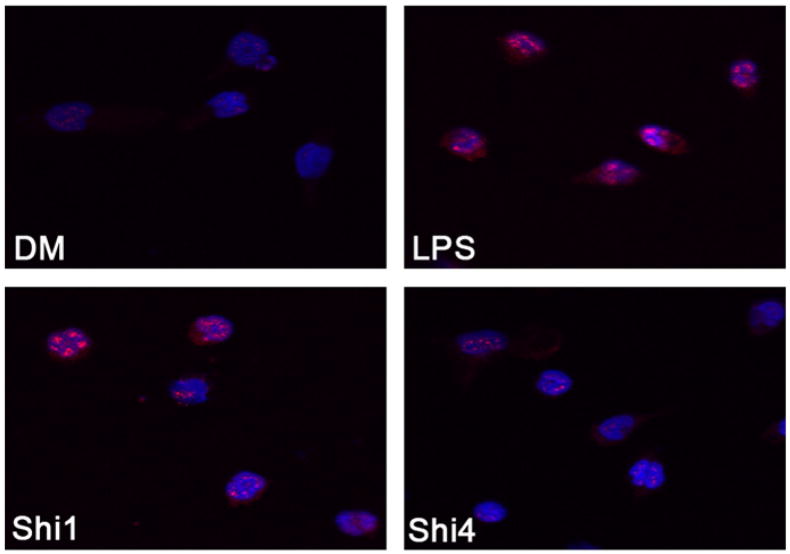
3.3. Shikonin inhibits NF-κB translocation from the cytoplasm to the nucleus in rat primary macrophage cultures
NF-κB translocation from the cytoplasm to the nucleus plays an important role in activating the NF-κB pathway. NF-κB signaling is the most important pathway in LPS-mediated inflammation (Baeuerle and Henkel, 1994). By binding to cell receptors (such as Toll-like receptors) and acting on the NF-κB pathway, LPS activates macrophages and induces pro-inflammatory cytokine release. As shown in Fig. 3, LPS treatment of rat primary macrophase cultures induced NF-κB translocation from the cytoplasm to the nucleus. However, shikonin at 1 and 4 μM inhibited the levels of nuclear NF-κB by ~50% and ~100%, respectively (Fig. 3), consistent with the dose-dependent inhibition of LPS-induced TNFα release in these primary macrophase cells (Fig. 2). Therefore, shikonin inhibits LPS-induced NF-κB activation and consequently inhibits TNFα release.
Fig. 3.
Shikonin inhibits NF-kB translocation from the cytoplasm to the nucleus in rat primary macrophage cultures. Rat primary macrophage cultures were plated on 8-well chamber slides and preincubated with vehicle (DM) or 1, 4 μmol/L of shikonin for 1.5 h, followed by stimulation with LPS (1 μg/ml) for 4 h. The cells were immunostained by NF-κBp65 antibody and the nuclear was stained with Hochest33342. Typical fluorescent images by Confocal microscopy were shown. Red signal stands for NF-κBp65 positive and Blue signal means nuclear. Shi1 and Shi4 indicates LPS + shikonin (1 μM) or LPS + shikonin (4 μM), respectively.
3.4. Shikonin treatment inhibits the proteasome activity in rat primary macrophage cultures
Because NF-κB activation is regulated by the ubiquitin/proteasome pathway, it is therefore possible that inhibition of NF-κB translocation in macrophages by shikonin is due to proteasome inhibition. Consistent with this hypothesis, our computational modeling predicts that the carbonyl carbons C1 and C4 of shikonin potentially interact with the catalytic site of β5 chymotryptic subunit of the proteasome (Yang et al., 2009a). To provide the direct evidence for shikonin inhibiting the macrophage proteasome, we performed a peptidase assay in vitro by using lysates of rat primary macrophage cultures as proteasome donor instead of purified 20S proteasome. As shown in Fig. 4A, shikonin preferentially inhibited the chymotrypsin-like activity over trypsin-like and caspase-like activities in the primary macrophage lysates.
Fig. 4. Shikonin accumulates IκB-α and ubiquitinated proteins in rat primary macrophage cultures and specifically inhibits the proteasomal chymotrypsin-like activity.
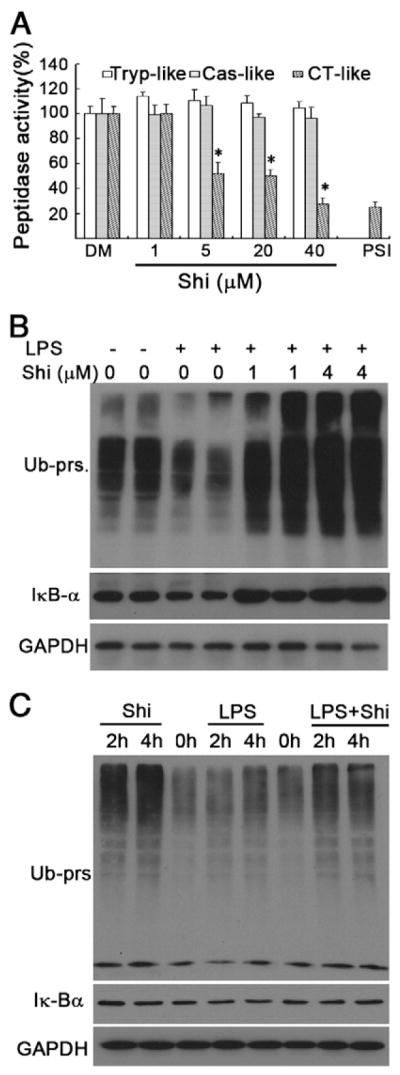
A. Cell lysate (10 μg protein) was incubated with indicated doses of shikonin in a Tris–HCL reaction system for 90 min, followed by measurement of peptidase assay with specific fluorescent proteasome substrates. Each column represents Means + S.D. of three repeats. PSI means MG132 (10 μM). Tryp-like means Trypsin-like, Cas-like means Caspase-like and CT-like means Chymotrypsin-like activity.
B. Rat macrophage cells were pretreated with 1, 4 μmol/L of shikonin for 1.5 h and subsequently treated with LPS (1 μg/ml) for 6 h. Protein levels of IκB-α and Ub-prs were determined by Western blotting. Typical images were shown. GAPDH was used as loading control.
C. Rat macrophage cells were treated with shikonin (2 μmol/L), LPS (1 μg/ml) alone or combination of the two agents for 2 h and 4 h, ubiquitinated-proteins. and IκB-α were detected by Western blotting. Typical images were shown. GAPDH was used as a loading control.
In inactivating cells, NF-κB is bound to IκB-α which directly controls its translocation and IκB-α level is regulated by the proteasome pathway (Baeuerle and Henkel, 1994). We then determined whether inhibition of proteasomal chymotrypsin-like activity by shikonin is associated with accumulation of IκB-α. In rat macrophages, consistent to other previous report (Qureshi et al., 2003), LPS enhanced the degradation of IκB-α and ubiquitinated proteins reflecting the activation of the ubiquitin–proteasome system (Fig. 4B and C), which was significantly inhibited by shikonin co-treatment (Fig. 4B–C). Therefore, inhibition of the UPS in rat primary macrophage cultures by shikonin leads to inactivation of NF-κB and TNF-α pathway.
3.5. Shikonin induces cell death in rat primary macrophage cultures
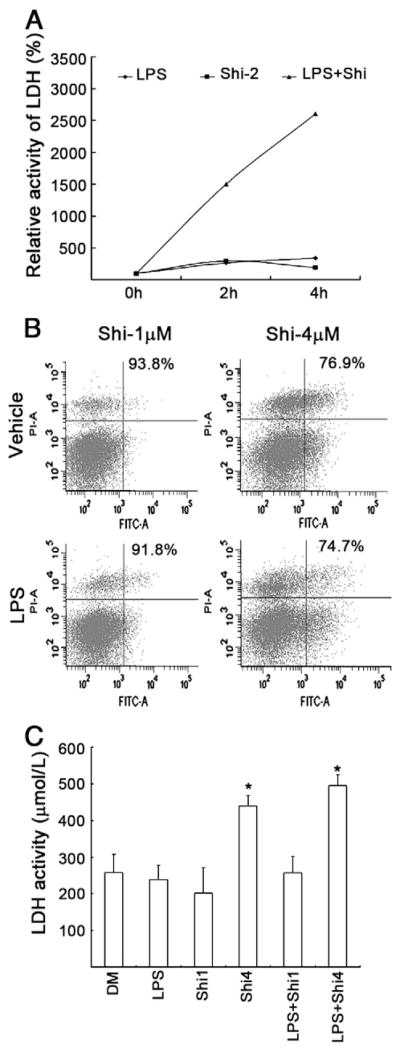
We and others have shown that inhibition of the proteasomal chymotrypsin-like, but not trypsin-like activity is associated with tumor cell apoptosis and growth arrest (An et al., 1998). Proteasomal inhibition-induced tumor cell death may be one of the major mechanisms responsible for the clinically used proteasome inhibitors for cancer therapy (Orlowski and Kuhn, 2008). We have already shown shikonin could reduce pro-inflammatory cytokine TNF-α release in vitro and inhibit inflammation in vivo (Figs. 1 and 2). We next determined whether shikonin could also induce rat macrophage apoptosis which would be helpful to control inflammation (Marriott et al., 2006). To do so, we treated rat primary macrophage cultures with shikonin in the absence or presence of LPS and determined the effects on apoptosis induction and cell death. As shown in Fig. 5A, shikonin (2 μmol/L) and LPS (1 μg/ml) per se did not affect LDH release at 2 h and 4 h points, reflecting no marked cell death, while combination of LPS and shikonin dramatically induced LDH release in the rat primary macrophage cultures. We further compared the dose effect of shikonin on cell death of the primary macrophage cells. As shown in Fig. 5B and C, 1 μmol/L of shikonin did not induce cell death up to 6 h but 4 μmol/L of shikonin dramatically affect cell survival. The cell death mode in the primary macrophage cells was similar to that observed in cancer cell lines (H22 and P388), as reported before (Yang et al., 2009a). This implies that shikonin induced both apoptotic and necrotic cell death in rat primary macrophage cultures.
Fig. 5. Shikonin induced cell death in rat primary macrophage cultures.
A. As in Fig. 4C, the supernatant was collected and LDH release was detected. The average is from the results of three repeats.
B. As in Fig. 4B, macrophages were collected and labelled with Annexin V and Propidium idodide (PI) for cell death assay by flow cytometry. Typical flow images were shown.
C. As described in Fig. 4B, the supernatants were collected for LDH assay. Each column represents Means + S.D. of three repeats.
4. Discussion
In the current study we report that shikonin inhibited inflammation in vivo and inhibited release of inflammatory mediator TNF-α in rat macrophage cells, as well as blocked LPS-mediated NF-κB translocation from the cytoplasm to the nucleus and induced cell death by inhibiting proteasome in macrophage cells.
Proteasome inhibitor bortezomib (Valcade or PS-341) has been approved by the United States FDA to treat multiple myeloma. Some other proteasome inhibitors are now under clinical trials for cancer therapy (Yang et al., 2009b). However, proteasome inhibitors have not been clinically used for inflammation therapy. Therefore, there is a need to search for novel natural anti-inflammation and anti-proteasome agents from medicinal plants.
Our data suggest that shikonin has the great potential to be developed into an anti-inflammation agent, as supported by the following lines of evidence. First, in vivo anti-inflammatory effect of shikonin (4 mg/kg) yielded the same efficacy as dexamethasone (2.5 mg/kg), the most effective anti-inflammation agent. Secondly, shikonin at 1 mg/kg and 4 mg/kg yielded ~45% and 65% inhibition, respectively, in two in vivo animal inflammation models (Yang et al., 2009a), which implies that it takes much lower dosage of shikonin in anti-inflammation therapy than in anti-cancer therapy. Thirdly, 4 μM of shikonin completely inhibited LPS-mediated TNFα release in primary macrophage cultures (Fig. 2). In addition, shikonin also partially induced apoptosis in macrophages. Induction of apoptosis is supposed to be a better strategy than induction of necrosis of inflammatory cells in anti-inflammatory therapy (Marriott et al., 2006; Haslett, 1999). As in tumor cells (Rajkumar et al., 2005), the ubiquitin–proteasome system is also activated in LPS-mediated inflammatory cells (Marriott et al., 2006). LPS-activated macrophages were sensitive to proteasome inhibition induced by shikonin. Shikonin at 1 μM induced several fold increase of ubiquitinated proteins reflecting the proteasome inhibition (Fig. 4A). LPS induced a translocation of NF-κB from the cytoplasm to the nucleus in macrophages and shikonin at 4 μM concentration almost completely inhibited this NF-κB translocation (Fig. 3). Consistently, shikonin accumulated high levels of IκB-α protein, a repressor of NF-κB translocation. Taken together, shikonin blocked NF-κB nucleus translocation via inhibition of proteasome-mediated IκB-α degradation. The anti-inflammatory effect of shikonin was, if not completely, at least partially attributable to proteasome inhibition in inflammatory cells. We also found that shikonin as a proteasome inhibitor induced cell death in a very narrow dose window and induced both apoptosis and necrotic cell death which is not helpful for anti-inflammatory therapy.
Several natural proteasome inhibitors extracted from medicinal Chinese herbs have been reported by our Lab recently. Curcumin is the yellow pigment derived from the rhizome of Curcuma longa which has been used in China Southeast Asia and India for centuries as food coloring and flavoring agent. Many studies have shown that curcumin targets the NF-κB pathway (Hussain et al., 2008; Shishodia et al., 2005). Celastrol is a quinone methide triterpene isolated from Lei Gong Teng and from Tripterygium regeli, a substitute of Lei Gong Teng found in northeast of China. It was also extracted from Nan She Teng which is widely distributed in China and has been used in the treatment of inflammation and detumescence (Yang et al., 2006). It was found that Celastrol could inhibit the TNF-induced activation of IκB-α kinase, IκB-α phosphorylation and degradation, p65 nuclear translocation and phosphorylation (Lee et al., 2006). All the novel natural proteasome inhibitors extracted from medicinal Chinese herbs were reported to suppress NF-κB activation at least partially via accumulation of IκB-α only in tumor cells. To our knowledge, the current report demonstrates, for the first time that macrophage proteasome is a potent molecular target of shikonin.
Acknowledgments
This work was supported by The National High Technology Research and Development Program of China, Grant number: 2006AA02Z4B5 (JL); Project 30770835 supported by National Natural Science Foundation of China (JL) and Project 7003073 supported by National Natural Science Foundation of Guangdong Province (LL); Karmanos Cancer Institute of Wayne State University (QPD), and National Cancer Institute grant 1R01CA120009 and 3R01CA120009-04S1 (QPD).
Contributor Information
Q. Ping Dou, Email: doup@karmanos.org.
Jinbao Liu, Email: liujinbao1@yahoo.com.cn.
References
- An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol. 2006;209:645–652. doi: 10.1002/jcp.20785. [DOI] [PubMed] [Google Scholar]
- Blackham A, Woods FAM. Immune complex mediated inflammation in the mouse peritoneal cavity. J Phamacol Meth. 1986;15:77–85. doi: 10.1016/0160-5402(86)90007-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Oppenheim JJ, Howard OM. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2003;47:2810–2816. doi: 10.1128/AAC.47.9.2810-2816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin–proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang x, Li s, Liu N, Lian W, McDowell E, Zhou P, Zhao C, Guo H, Zhang C, Yang C, Wen G, Dong X, Lu L, Ma N, Dong W, Dou QP, Wang X, Liu J. Physiological levels of ATP negatively regulate proteasome function. Cell Res. 2010;20:1372–1385. doi: 10.1038/cr.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AR, Ahmed M, Al-Jomah NA, Khan AS, Manogaran P, Sultana M, Abubaker J, Platanias LC, Al-Kuraya KS, Uddin S. Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt’s lymphoma cell lines. Mol Cancer Ther. 2008;7:3318–3329. doi: 10.1158/1535-7163.MCT-08-0541. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, Hong YS, Lee JJ. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311–1321. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X. Impairment of the ubiquitin–proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–364. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Makarov SS, Johnston WN, Olsen JC, Watson JM, Mondal K, Rinehart C, Haskill JS. NF-kappa B as a target for anti-inflammatory gene therapy: suppression of inflammatory responses in monocytic and stromal cells by stable gene transfer of I kappa B alpha cDNA. Gene Ther. 1997;4:846–852. doi: 10.1038/sj.gt.3300461. [DOI] [PubMed] [Google Scholar]
- Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MK, Dockrell DH. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J Immunol. 2006;177:6480–6488. doi: 10.4049/jimmunol.177.9.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KN, Son MS, Park JH, Lee EH. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-kappaB: neuroprotective implications. Neuropharmacology. 2008;55:819–825. doi: 10.1016/j.neuropharm.2008.06.065. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- Qureshi N, Perera PY, Shen J, Zhang G, Lenschat A, Splitter G, Morrison DC, Vogel SN. The proteasome as a lipopolysaccharide-binding protein in macrophages: differential effects of proteasome inhibition on lipopolysaccharide-induced signaling events. J Immunol. 2003;171:1515–1525. doi: 10.4049/jimmunol.171.3.1515. [DOI] [PubMed] [Google Scholar]
- Qureshi N, Vogel SN, Van Way C, III, Papasian CJ, Qureshi AA, Morrison DC. The proteasome: a central regulator of inflammation and macrophage function. Immunol Res. 2005;31:243–260. doi: 10.1385/IR:31:3:243. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem. 2004;279:5877–5885. doi: 10.1074/jbc.M309185200. [DOI] [PubMed] [Google Scholar]
- Su PF, Staniforth V, Li CJ, Wang CY, Chiao MT, Wang SY, Shyur LF, Yang NS. Immunomodulatory effects of phytocompounds characterized by in vivo transgenic human GM-CSF promoter activity in skin tissues. J Biomed Sci. 2008;15:813–822. doi: 10.1007/s11373-008-9266-7. [DOI] [PubMed] [Google Scholar]
- Takano-Ohmuro H, Yoshida LS, Yuda Y, Morioka K, Kitani S. Shikonin inhibits IgE-mediated histamine release by human basophils and Syk kinase activity. Inflamm Res. 2008;57:484–488. doi: 10.1007/s00011-008-8067-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Tajima M, Tsukada M, Tabata M. A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod. 1986;49:466–469. doi: 10.1021/np50045a014. [DOI] [PubMed] [Google Scholar]
- Wang JP, Raung SL, Chang LC, Kuo SC. Inhibition of hind-paw edema and cutaneous vascular plasma extravasation in mice by acetylshikonin. Eur J Pharmacol. 1995;272:87–95. doi: 10.1016/0014-2999(94)00627-j. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Bai JY, Liu DP, Xue LM, Zhu XY. The antiinflammatory activity of shikonin and its inhibitory effect on leukotriene B4 biosynthesis. Yao Xue Xue Bao. 1994;29:161–165. [PubMed] [Google Scholar]
- Wang J, Maldonado MA. The ubiquitin–proteasome system and its role in inflammatory and autoimmune diseases. Cell Mol Immunol. 2006;3:255–261. [PubMed] [Google Scholar]
- Weirich EG, Longauer JK, Kirkwood AH. New experimental model for the primary evaluation of topical contra inflammatory agents. Arch Dem Med. 1977;259:141–149. doi: 10.1007/BF00557954. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhou P, Huang H, Chen D, Ma N, Cui QC, Shen S, Dong W, Zhang X, Lian W, Wang X, Dou QP, Liu J. Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int J Cancer. 2009a;124:2450–2459. doi: 10.1002/ijc.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zonder JA, Dou QP. Clinical development of novel proteasome inhibitors for cancer treatment. Expert Opin Investig Drugs. 2009b;18:957–971. doi: 10.1517/13543780903002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83:1–14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]