Abstract
Multiple sclerosis (MS) is characterized by CNS demyelination and oligodendrocyte depletion, axonal loss, and reactive astrogliosis. Myelin loss causes conduction block, while remyelination is associated with recovery of conduction and return of function. Reactive astrocytes are a prominent feature of MS plaques, and have been implicated as producing factors regulating oligodendrocyte progenitor differentiation and myelin formation. Understanding their impact on these events may lead to new approaches for oligodendrocyte protection and/or remyelination in MS. Here, we outline protocols for the establishment and analysis of primary monocultures and cocultures of human astrocytes and oligodendrocytes. These approaches are designed to facilitate analysis of mechanisms underlying astrocytic regulation of progenitor survival and myelin repair.
Keywords: Astrocyte, Oligodendrocyte, Apoptosis, Proliferation, Differentiation
1. Introduction
The pathology of multiple sclerosis (MS) is characterized by CNS demyelination and oligodendrocyte depletion, progressive axonal loss, inflammation, and reactive astrogliosis (1). Loss of myelin causes conduction block in affected axons, a major cause of symptoms in early MS, while myelin repair is associated with recovery of conduction and return of function (2).
Genetically modified animal models have implicated reactive astrocytes as regulators of inflammation and repair following CNS insult (3, 4). Studies have also shown that these cells express factors that modulate oligodendrocyte progenitor survival and differentiation, and myelin formation (5–8). We have examined potential links between astrocyte reactivity and lesion formation and repair in MS, and this ongoing work has identified groups of genes potentially relevant to lesion pathogenesis or resolution (9–11). Each group has subsequently been explored using functional models, including cultures of human astrocytes and oligodendrocytes, and cocultures of both cell types (9, 12).
Here, we outline the protocols used for these primary culture models. These techniques are designed to facilitate identification of mechanisms underlying astrocytic regulation of oligodendrocyte survival, maturation, and myelin formation. Understanding these events may lead to new approaches for oligodendrocyte protection and/or myelin repair in MS.
2. Materials
2.1. Astrocyte Culture
Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% Fetal Calf Serum (FCS) and 1% Penicillin-Streptomycin-Fungizone solution (PFS).
10× Trypsin solution.
Sterile Dulbecco’s PBS without calcium or magnesium (dPBS).
DNAse type I (Sigma D-5025).
Sterile transfer pipettes (5 mL).
Sterile culture pipettes (10 mL).
50 mL sterile conical tubes (50 mL).
Sterile glass beakers (100 mL, autoclaved).
Sterile tweezers (autoclaved).
Sterile funnels with 250 and 130 µm size filter mesh.
Sterile Pasteur pipettes.
15 cm diameter culture dishes.
2.2. Oligodendrocyte Culture
Dulbecco’s Modified Eagle’s Medium (DMEM) 10% FCS 1% PFS.
Hanks’ Buffered Saline Solution (HBSS, Sigma) with 1% PFS.
DMEM/F12 medium.
T3 (tri-iodo-thyronine) stock solution (20 µg/mL). Make up a 1 mg/mL solution in 1 N NaOH, then further dilute with medium.
T4 (thyroxine) stock solution (396.3 µg/mL). Dissolve 2 mg T4 in 1 mL 1 N NaOH, then add 4.04 mL dH2O to final concentration.
1% BSA stock solution. Dissolve 50 mg BSA in 5 mL PBS, aliquot to 500 µL.
Oligodendrocyte Basic Medium (serum-free culture medium, (DMEM/F12/N2/T3/T4)). To make 50 mL add the following: 48 mL DMEM/F12, 500 µL N2 supplement (Sigma), 500 µL Penicillin-Streptomycin-Fungizone, 500 µL 1% BSA stock solution in PBS, 843 µL T3 stock solution, 50 µL stock solution T4.
Oligodendrocyte Enzyme solution #1. To make 100 mL add the following : 5 mL Trypsin (Sigma T-4549), 2 mg Collagenase type III (Sigma C-0255), 2.2 mg DNAse type I (Sigma D-5025), 40 U/mL (approx.60 mg) Papain (Sigma P-4762). Add MEM-Hepes to 100 mL, and aliquot into 4 mL lots.
Oligodendrocyte Enzyme Solution #2. To make 50 mL add the following: 3 mL Trypsin-EDTA (BD 15400–096), 1 mg Collagenase type III (Sigma C-0255), 1.1 mg DNAse type I (Sigma D-5025). Add MEM-Hepes to 50 mL and aliquot into 2 mL lots.
Oligodendrocyte Stop solution. To make 50 mL add the following: 110 µg DNAse I (Sigma D-5025), 83 mg BSA Fraction V (Sigma A-4503), 5 mL FBS. Add MEM-Hepes to 50 mL and aliquot into 2 mL lots.
2.3. Western Blotting
Loading buffer (1×): 50 mM Tris-HCl, pH 6.8, 100 mM Dithiothreitol, 2% Sodium dodecyl sulfate, 0.1% Bromophenol blue, 10% glycerol.
SDS-PAGE running buffer. To make 1 L, add 3 g Tris base, 14.3 g glycine, 10 mL of 20% SDS solution and make up to 1 L in ddH2O.
Transfer buffer. To make 1 L, add 3 g Tris base, 14.3 g glycine, 200 mL methanol and make up to 1 L in ddH2O.
Membrane washing buffer: 0.05% Tween 20 in 1× TBS (TTBS).
Blocking buffer: 5% Nonfat dry milk in TTBS.
SDS-PAGE fix solution. To make 500 mL, add 225 mL ddH2O, 225 mL Methanol, and 50 mL Acetic acid.
2.4. Immunocytochemistry
Glass confocal plates (Mat-Tek, Ashland, MA).
4% paraformaldehyde (PFS) in PBS.
0.1% glycine in PBS.
Astrocyte blocking/permeabilizing solution: 0.3% Triton X-100 in 10% goat serum in PBS.
Primary antibodies.
Secondary antibodies coupled to Alexa 488 or 594 (1:100; Molecular Probes, Eugene, OR).
Alexa 594-conjugated phalloidin (Molecular Probes).
Oligodendrocyte (nonpermeabilizing) blocking solution: 10% goat serum, 5% nonfat milk in PBS.
Antibody diluent: 5% nonfat milk, 1% goat serum.
PBS.
Confocal microscope.
Imaris software for 3-D rendering (Bitplane AG, Zurich, Switzerland).
3. Methods
3.1. Human Astrocyte Culture
This protocol is derived from that originally described by Lee and coworkers (13). It generates primary cultures of human astrocytes from 19 to 22-week fetal cerebral samples. These samples are obtained from the Human Fetal Tissue Repository (HFTR) at the Albert Einstein School of Medicine, Bronx, NY. Their collection and use are regulated at the institutional, state, and federal levels (see Note 1).
These protocols generate cells in large numbers (twenty 15 cm diameter culture dishes per tissue sample). Following initial plating, cultures consist of astrocytes, neurons, and microglia. The latter detach and are removed by aspiration at day 14 (see below), and can then be cultured separately. Following their removal, remaining cultures consist of slowly proliferating astrocytes and postmitotic neurons. Further passage leads to removal of the neurons, leaving the astrocytes as the remaining cell type.
Prepare autoclaved supplies (two funnels, tweezers, beaker, Pasteur pipettes).
Warm sterile PBS, trypsin, and DMEM 10% FCS 1% PFS to 37°C.
Obtain fetal cerebral tissue from HFTR. In Biosafety Level 2 flow hood, remove meninges from tissue with sterile tweezers. Transfer to sterile beaker, wash with PBS until erythrocytes are removed.
Bring total volume to 80 mL with sterile PBS. Add 8 mL 10× trypsin, and a pinch of DNAse. Use 10 mL pipette and pipette up and down to macerate for timed 5 min.
Use a sterile transfer pipette to macerate for a further timed 5 min to additionally dissociate the tissue.
Using the pipette, transfer the macerated tissue to two 50 mL conical tubes (40 mL each).
Bring to a 37°C shaker and leave for 50 min−1 h to complete the dissociation.
Add 10 mL DMEM 5% FCS to inactivate trypsin.
Uncap four 50 mL sterile conical tubes. Using an autoclaved large 250 µm mesh, filter 50 mL into each of two tubes. Then take a small 130 µm mesh and filter again.
Centrifuge at 300 g for 10 min.
Remove supernatant and resuspend the pellet (3 mL) in 30 mL DMEM 10% FCS 1% PFS for wash. Wash two additional times in the same way.
Place 5 mL medium with cells on each of twenty 15 cm diameter plates. Add 30 mL DMEM 10% FCS 1% PFS for a total of 35 mL/plate. Gently swirl to mix cells. Place in incubator. Leave undisturbed for several days to allow cultures to form.
At day 7, replace medium with 20 mL DMEM 5% FCS 1% PFS. Handle cultures very carefully to avoid dislodging cells.
On day 14, collect microglia. By day 14, cultures consist of a monolayer of astrocytes with neurons growing above them. Microglia detach from the adherent culture between day 7 and 14, and can be removed for separate culture by aspiration and centrifugation. Aspirate supernatant from all dishes into 50 mL conical tubes for microglial collection (see Note 2), and replace medium with DMEM 5% FCS 1% PFS.
The remaining cultures are comprised of neurons and astrocytes (G0 cultures, see Note 3). Neurons are postmitotic, whereas astrocytes are slowly proliferating. Passage of cultures to G1 will give lower density neurons on an astrocyte monolayer, subsequent passage to G2 and especially G3 and G4 will result in >90% GFAP+ astrocytes. Passage beyond G4, or use of cells after a total period in culture of 90 days, is not recommended, since cells become senescent (see Note 4).
For initial passage to G1 at day 28, wash cultures twice in 15 mL warm Dulbecco’s PBS, then add 15 mL 1× trypsin in dPBS and return cultures to incubator for 15 min, then collect cells into 50 mL conical tubes (two dishes/tube) and add 10 mL DMEM 5% FCS 1× PFS to each tube. Centrifuge at 300 g 10 min and wash twice with medium, then resuspend each tube in 40 mL of the same medium. Put 10 mL into each 15 cm diameter plate, add medium to 30 mL total.
Subsequent passages can be carried out at 7–14 days intervals. Cultures can also be left for up to 21 days at the same passage, with medium changed once per week (see Note 5).
Cells can also be cultured in smaller volume culture dishes or plates, or on glass confocal plates for immunocytochemistry, coated with poly-lysine or uncoated. Protocols for immunocytochemistry and imaging of astrocytes are outlined below (see Section 3.7).
3.2. Human Oligodendrocyte Culture
This culture system was originally described by Wilson et al. (14), and has also been used in our laboratory (12). Additional details can be found in both references. These protocols are designed to generate primary human oligodendrocyte-enriched cultures from samples of 19–24 week fetal spinal cord (see Note 6). These samples are obtained from the Human Fetal Tissue Repository (HFTR) at the Albert Einstein School of Medicine, Bronx, NY, as above.
Due to small numbers of starting cells, these cultures are generated via differential adhesion as opposed to immunopanning (which is used routinely for purification of rodent oligodendrocyte progenitors) (10). At plating, human cultures therefore contain Olig2+ oligodendrocyte lineage cells of all differentiation states, including A2B5+ PDGFRα+CNPase−MBP− progenitors and A2B5−PDGFR−CNPase+MBP+ mature cells (see Note 7 and below, and references (9, 14)). For this reason, the human culture system is usually employed in tandem with complementary studies in rodents using highly purified starting populations of oligodendrocyte progenitors.
Obtain fetal spinal cord tissue from HFTR. Prepare two sterile Petri dishes. In a Biosafety Level 2 flow hood, pull spinal cord into one Petri dish, tear off durameter and remove visible blood vessels.
Add 10 mL HBSS 1% PFS to another Petri dish. Transfer spinal cord to it. Cut tissue into small pieces using a #22 scalpel blade.
Transfer tissue to a 50 mL culture tube. Wash Petri dish with 10 mL HBSS 1% PSF and add washes to tissue suspension. Centrifuge tissue suspension at 200 g for 3 min.
Remove supernatant and add 2 mL Enzyme Solution 1 to digest tissue. Incubate in a 37°C shaker for 5–8 min. Centrifuge at 200 g for 3 min.
Repeat step 4 once.
Remove supernatant and add 2 mL Enzyme Solution 2. Incubate in a 37°C shaker for 5–8 min. Centrifuge at 200 g for 3 min.
Remove supernatant and add 2 mL Stop Solution. Triturate cell suspension three times each through 18′, 20′, and 22′ needle. Add 12 mL DMEM 10% FCS 1% PFS and centrifuge at 200 g for 10 min.
Remove supernatant and wash with 10 mL DMEM/F12. Centrifuge at 200 g for 5 min.
Remove supernatant and resuspend the pellet in 6 mL Basic Medium (see Note 8). Distribute cell suspension to a T-25 flask.
After 18–24 h incubation, collect cell suspension (oligodendrocyte enriched) from flask. Plate cells onto poly-l-lysine (5 µg/mL) coated confocal dishes (4–5 × 105/mL, 100 µL/dish). Flush dishes with 2 mL basic medium 2 h later.
3.3. Human Astrocyte–Oligodendrocyte Coculture
These cocultures are established from the astrocyte- and oligodendrocyte-enriched monocultures described above, and are designed for examination of the impact of astrocytic phenotypes on human oligodendrocyte viability, proliferation, and differentiation. They are suitable for examination of the effects of both contact-mediated signaling and astrocyte-derived soluble factors on oligodendrocyte lineage cells (see Fig. 1).
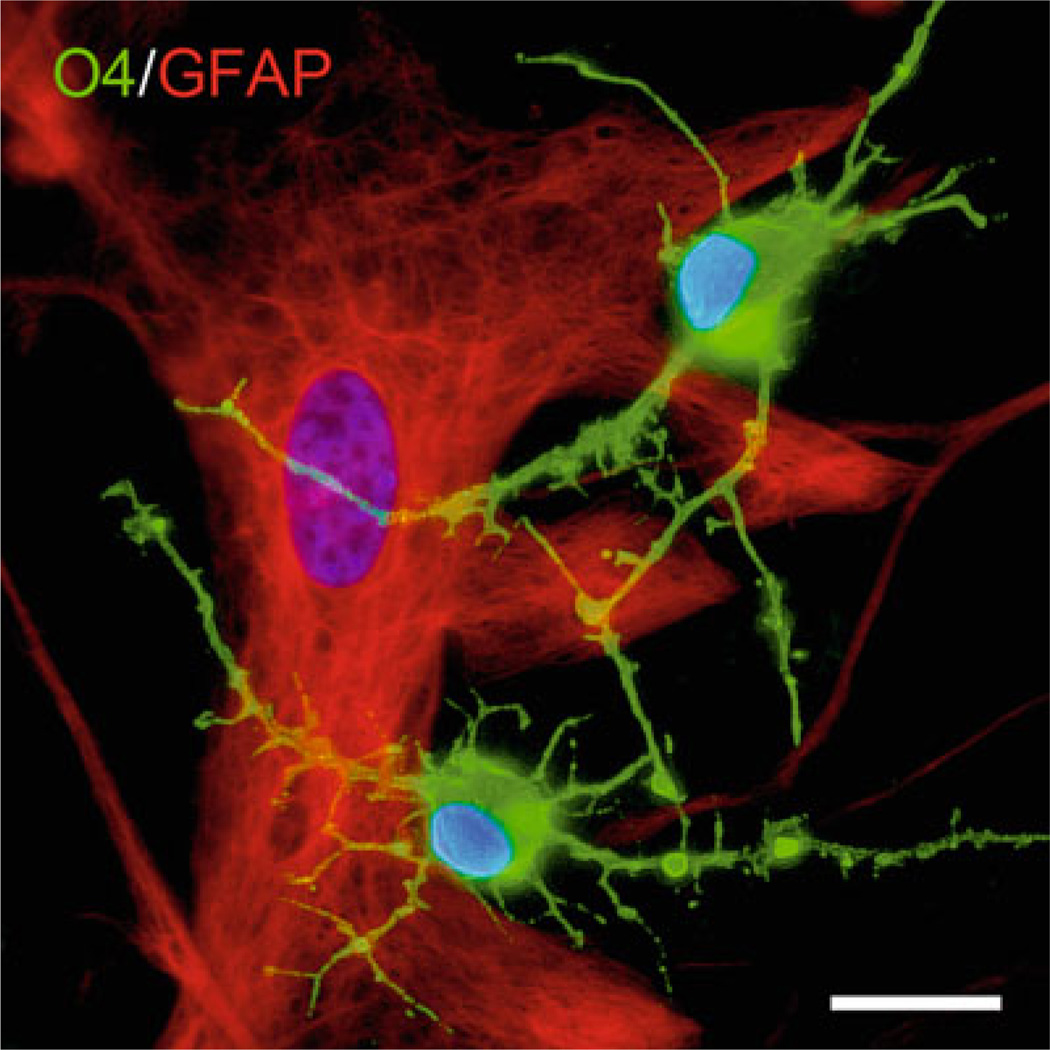
Fig. 1.
Coculture of human astrocytes and human oligodendrocytes. Primary cultures of human fetal astrocytes were established from cortical samples as described in the text, and cells at passage G3 are allowed to reach 70% confluence. Primary human oligodendrocytes grown in parallel from fetal spinal cord samples were then plated onto the astrocyte culture, and resulting cocultures propagated in serum-free medium for 72 h. Cells were fixed with 4% paraformaldehyde, stained sequentially for the oligodendrocyte marker O4 and the astrocytic marker GFAP as described in the text, and counterstained with DAPI. Cocultures were imaged using confocal microscopy, and images captured at 63× magnification. The panel shows a large GFAP+ astrocyte (left) and two arborized O4+ oligodendrocytes (bottom and right). Scalebar, 10 µm.
Net effects of individual cytokines implicated in generation of reactive astrogliosis can be examined by treating astrocyte cultures with the relevant cytokine followed by washout prior to plating of oligodendrocytes (see for example reference (10), and see Note 9).
To define the contributions of individual factors to observed effects on oligodendrocytes, astrocytes can be transfected with relevant specific siRNA subsequent to cytokine treatment but prior to plating of oligodendrocytes (see below and reference (10)). Alternatively, effects of individual soluble astrocyte-derived factors can also be defined using application of astrocyte-conditioned medium to oligodendrocyte cultures, in preference to coculture of the two cell types. In studies using astrocyte-conditioned medium, the roles of individual factors can also be defined using specific blocking antibodies or peptides (see below and reference (11)).
To establish cocultures of human astrocytes and human oligodendrocytes, astrocyte cultures are initially seeded at passages G3 or 4 as above.
Once astrocytes are 70% confluent, medium is removed and replaced. If effects of specific cytokines on astrocytic phenotype are being examined, these are added to medium. At 6–48 h later (typically 24 h), cytokine is washed out and medium replaced.
If the experiment aims to define the role of a specific (perhaps cytokine-induced) astrocyte-produced factor on oligodendrocyte viability, proliferation and/or differentiation, astrocytes are transfected with relevant siRNA following cytokine treatment and prior to plating of oligodendrocytes. Cultures at 70% confluence are transfected with 5 nM specific siRNA (Dharmacon, Lafayette, CO) using Trans IT-TKO (Mirus, Madison, WI) according to the manufacturer’s instructions. Nontargeting siRNA, and sham transfection are included as controls.
At 6–48 h following transfection (typically 24 h), success and specificity of siRNA are confirmed by Western blotting, ELISA or immunocytochemistry (see below).
Astrocytes are then washed and primary human oligodendrocyte cultures plated onto astrocytes in basic medium as described above, to establish cocultures, at the same density as used in oligodendrocyte monocultures, or at 50% density if individual cell morphology/process extension is the focus of the study.
Cocultures are left to allow oligodendrocyte differentiation for 72–120 h, then are fixed and harvested for immunocytochemistry and confocal imaging, or Western blotting or quantitative PCR (QPCR, see below).
3.4. Astrocyte-Conditioned Medium
To establish whether soluble factors produced by reactive astrocytes alter oligodendrocyte proliferation, apoptosis and/or differentiation, human oligodendrocyte cultures can be exposed to conditioned medium from human astrocytes stimulated with cytokines known to be relevant to the induction of a reactive astrogliosis.
To define roles of specific astrocyte-produced soluble factors in these effects, astrocytes may be nucleofected with siRNA for the factor of interest, or nontargeting control, or sham transfection control, following cytokine treatment (see below). As an alternative to siRNA, blocking antibodies or peptides can be used to deplete conditioned medium of the factor of interest prior to application of the medium to oligodendrocyte cultures.
Human astrocyte cultures are established and propagated as above.
At passages G3–4, cultures at 70% confluence are treated with cytokines shown to be relevant to induction of a reactive astrogliosis, such as IL-1 β +/− IFNγ (10 ng/mL), or 10 ng/mL TGFβ1 (15). At 6–48 h later (typically 24 h), the cytokine is washed out and medium replaced.
If the experiment aims to define the role of a specific astrocyteproduced factor on oligodendrocyte viability, proliferation and/or differentiation, astrocytes are then transfected with relevant specific or control siRNA (see above).
At 16–24 h following transfection, astrocyte medium is removed and replaced by oligodendrocyte culture medium (typically basic medium). After 24–72 h, conditioned medium is then harvested from astrocyte cultures and stored at −80°C or used fresh. Success of siRNA treatment is confirmed by immunoblotting or sandwich ELISA.
If blocking antibodies or peptides are being used to deplete conditioned medium of the factor of interest as an alternative to siRNA, typically conditioned medium is treated with blocking peptide for 2 h at 4°C, then centrifuged to remove complexes prior to application to oligodendrocyte cultures.
Conditioned medium is added to oligodendrocyte cultures as above, and the impact of targeted inhibition of the factor of interest measured in terms of differentiation, proliferation, and/or apoptosis.
At timepoints specified (typically 24–120 h), oligodendrocytes are fixed and immunostained for lineage and differentiation markers, or protein harvested for Western blotting, or RNA harvested for QPCR (see below).
3.5. Western Blotting
Aspirate medium from astrocytes, oligodendrocytes, or cocultures. Wash once with PBS and remove.
Boil 1× SDS-PAGE loading buffer to 95°C, add 500 µL to each 10 cm diameter culture dish, rock the dish back and forth and pipette to collect cells.
Boil the sample at 95°C for 10 min, then sonicate for 5 seconds.
Centrifuge at 12,000 g, 10 min. Transfer the supernatant to a new tube and store at −20°C.
Run samples on a precast SDS-PAGE gel (10–12%, Bio-Rad) at 100 V in SDS-PAGE running buffer at RT.
Transfer to PVDF membrane (Bio-Rad) at 40 mA overnight in transfer buffer at 4°C.
To confirm even loading of gel, stain with biosafe Coomassie Blue solution (Bio-Rad) for 1 h then destain for 30 min, fix 30 min and dry.
-
To probe PVDF membrane:
Block in blocking buffer (see above) for 1 h, then incubate with primary antibody in blocking buffer for 1 h at RT.
Rinse twice with TTBS (see above), each 15 min, then incubate with HRP-conjugated secondary antibody for 2 h at RT.
Rinse once with TTBS, 10 min and once with TBS, 20 min.
Incubate with fluorescent HRP detection reagent (Pierce, 1:1 mixture) for 10 min, then place membrane in cassette immediately and develop using exposure time: from 10 sec−1 min.
3.6. Quantitative PCR
Cultures of primary human fetal astrocytes or oligodendrocytes, or cocultures, are treated with growth factors or conditioned medium.
At times specified (usually 6–72 h), RNA is harvested using an Absolutely-RNA RT-PCR Miniprep kit (Stratagene, La Jolla, CA) according to the manufacturer’s instructions.
CDNA is generated and real-time PCR performed, using a previously published protocol (9, 16). Each transcript in each sample is assayed in triplicate, and the mean detection threshold (CT) values used to calculate Fp values (fold-change ratios between experimental and control samples) for each gene.
Amplicon size and reaction specificity are confirmed by 1% agarose gel electrophoresis.
Data validity by modeling of reaction efficiency and analysis of measurement precision have been described previously (16).
3.7. Astrocyte Immunocytochemistry
Astrocyte cultures grown on glass confocal plates (Mat-Tek, Ashland, MA) and treated as described are fixed 15 min in PBS 4% paraformaldehyde, then rinsed twice in PBS and once in PBS 0.1% glycine
Cells are blocked in PBS 0.3% Triton X-100 10% goat serum 30 min and incubated with primary antibodies (1:100) in blocking buffer overnight at 4°C.
After washing three times in PBS 0.3% Triton X-100, cells are incubated in relevant secondary antibodies conjugated to Alexa 488 and/or 594 (1:100; Molecular Probes, Eugene, OR) 1 h at room temperature (RT) in blocking buffer, and/or Alexa 594-conjugated phalloidin (Molecular Probes) at 1:30 in PBS for 5 min.
Samples are examined at RT using a Leica confocal microscope mounted on an inverted laser safe microscope with an infinity-corrected 60× objective.
Z-series stacks are taken from treated and control cultures using 0.2 µm on the Z axis.
3.8. Oligodendrocyte Immunocytochemistry
Some frequently-used oligodendrocyte antigens such as the progenitor marker A2B5 and the maturation marker O4 require staining in the absence of permeabilization. Permeabilization is used for most other antigens including the lineage marker Olig2, progenitor marker PDGFRα, and maturation markers CNPase and MBP.
Cultures or cocultures are grown on glass confocal plates (Mat-Tek). Wash cells twice with PBS @ RT for 5 min, then fix with 4% paraformaldehyde 10 min @ RT and wash again three times with PBS, 5 min each.
For O4 or A2B5, block 30 min @ RT with 10% goat serum, 5% nonfat milk in PBS. For other antigens (e.g., Olig2), include 0.3% Triton in blocking buffer.
Add primary antibody. For O4 or A2B5, 1:25 in 5% nonfat milk and 1% goat serum in PBS. For other antigens, primary antibody in blocking buffer in the presence of 0.3% Triton. Leave overnight at 4°C.
Wash three times with PBS @ RT, 5 min each. Add secondary antibody (as above in 5% nonfat milk and 1% goat serum in PBS, or with Triton), 1 h @ RT.
Wash three times with PBS @RT, 5 min each.
Mount, or if staining initially in the absence of permeabilization, can now use serial staining for additional antigens in the presence of permeabilization.
All samples are examined at RT using a Leica confocal microscope mounted on an inverted laser safe microscope with an infinity-corrected 60× objective, and Z-series stacks taken using 0.2 µm on the z axis.
Both astrocytes and oligodendrocytes have highly organized three-dimensional structures, and in some studies the details of their morphology can best be appreciated using three-dimensional reconstruction (17). Z-series stacks are collected from samples prepared and imaged as above, using 0.2 µm on the z axis between images. Stacks are then subjected to three-dimensional (3-D) rendering using Imaris software version (Bitplane AG, Zurich, Switzerland).
3.9. Time-Lapse Microscopy
Oligodendrocyte arborization and process extension, and cytokine-induced alterations in astrocyte morphology, can be followed in individual cells over time using time-lapse microscopy. Typically, oligodendrocyte cultures or astrocyte–oligodendrocyte cocultures established as above and treated as described are imaged by Kohler illumination at 37°C using a Cooke Sensicam cooled CCD camera (Auburn Hills, MI) mounted on an Olympus (Tokyo, Japan) IX70 microscope with environmental chamber, using a 60× objective. Images are then captured every 10 min using Scanalytics IPLab3.5 software (Fairfax, VA) and assembled using Apple Quicktime software.
Acknowledgments
We thank Dr. Bradford Poulos, Director of the Human Fetal Tissue Repository at the Albert Einstein College of Medicine, for tissue collection. Research in our laboratory is supported by USPHS Grants NINDS R01NS046620, R01NS062703 and R01NS056074, and ARRA administrative supplement R01NS056074-02S1. Additional support for members of the laboratory has come from USPHS training grant T32GM008553-13 (PI: Dr. Marek Mlodzik, MSSM), and for ATA from T32NS051147-03 (PI: Dr. Steven Levine, MSSM). Our work is also supported by National Multiple Sclerosis Society Research Grants RG3874 and RG4127 (to GRJ) and Postdoctoral Fellowship FG1739 (to YZ), and by the Jayne and Harvey Beker Foundation (to GRJ).
Footnotes
Note that use of human tissue samples carries potential health hazards and is subject to oversight by institutional safety authorities. Operators should maintain current status with regard to vaccination for tetanus and hepatitis.
For collection of human microglia, supernatants from G0 astrocyte cultures (Subheading 3.1) can be aspirated into 50mL conical tubes for microglial collection, and medium replaced with DMEM 5% FCS 1% PFS. Tubes are then centrifuged at 300 g 10 min. Pellets consist of CD11b+ microglia, which can be counted and cultured at 5 × 105/6 cm dish. Purity can be further enhanced using magnetic cell sorting.
Passage of cultures to G2 and especially later G3 and G4 will result in cultures composed of greater than 90% GFAP+ astrocytes. However, note that these cultures are not immunosorted or panned, and thus are not truly pure astrocyte cultures.
Astrocyte cultures established using these protocols have the advantage of being primary cells from our own species. However, they are fetal as opposed to adult, and data are subject to confirmation using immunohistochemistry and/or in situ hybridization of adult tissue sections.
Primary human astrocytes in DMEM, 5% FCS, 1% PFS divide slowly and do not have high metabolic requirements, thus they can be left in the same dishes for up to 21 days with medium changed once per week.
Spinal cord is used in preference to cerebral tissue since myelination begins caudally and proceeds rostrally.
Human oligodendrocyte cultures established using these protocols contain relatively small numbers of cells. They have the advantage of being primary cells from our own species, but are fetal, not adult. In addition, the restricted yield makes additional purification difficult. In contrast to rodent cultures, in which immunopanning is used to produce highly defined populations of progenitor cells, human cultures contain oligodendrocytes at all differentiation states including progenitors and mature cells, plus a small percentage of astrocytes. This can complicate analysis of changes in rates of differentiation in these cultures.
Oligodendrocyte basic medium is serum-free and contains thyroxine and tri-iodothyronine, and is designed to predispose progenitors toward differentiation.
Treatment of human astrocytes with some cytokines, for example IL-1β (especially in combination with IFNγ) can lead to significant morphological changes including process extension and stellation. The mechanisms and significance of these changes have been discussed (17). They may complicate attempts at establishment of cocultures with oligodendrocytes.
References
- 1.Raine CS, McFarland HF, Tourtellotte WW. Multiple sclerosis : clinical and pathogenic basis. 1st ed. London; New York: Chapman & Hall Medical; 1997. [Google Scholar]
- 2.Smith KJ, Blakemore WF, McDonald WI. The restoration of conduction by central remyelination. Brain. 1981;104:383–404. doi: 10.1093/brain/104.2.383. [DOI] [PubMed] [Google Scholar]
- 3.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- 6.Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the jimpy mutant. J Neurosci. 2000;20:2609–2617. doi: 10.1523/JNEUROSCI.20-07-02609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, Brosnan CF, John GR. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci. 2006;26:12174–12185. doi: 10.1523/JNEUROSCI.2289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, Lu QR, Rowitch DH, Raine CS, Brosnan CF, John GR. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci USA. 2009;106:19162–19167. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang J, Navrazhina K, Argaw AT, Zameer A, Gurfein BT, Brosnan CF, John GR. TGFbeta1 induces Jagged1 expression in astrocytes via ALK5 and Smad3 and regulates the balance between oligodendrocyte progenitor proliferation and differentiation. Glia. 58:964–974. doi: 10.1002/glia.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SC, Liu W, Brosnan CF, Dickson DW. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest. 1992;67:465–476. [PubMed] [Google Scholar]
- 14.Wilson HC, Onischke C, Raine CS. Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia. 2003;44:153–165. doi: 10.1002/glia.10280. [DOI] [PubMed] [Google Scholar]
- 15.John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 16.Yuen T, Zhang W, Ebersole BJ, Sealfon SC. Monitoring G-protein-coupled receptor signaling with DNA microarrays and real-time polymerase chain reaction. Methods Enzymol. 2002;345:556–569. doi: 10.1016/s0076-6879(02)45047-1. [DOI] [PubMed] [Google Scholar]
- 17.John GR, Chen L, Rivieccio MA, Melendez-Vasquez CV, Hartley A, Brosnan CF. Interleukin-1beta induces a reactive astroglial phenotype via deactivation of the Rho GTPase-Rock axis. J Neurosci. 2004;24:2837–2845. doi: 10.1523/JNEUROSCI.4789-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]