Summary
Parry-Romberg syndrome (PRS) is a rare acquired syndrome consisting of progressive hemiatrophy of the face. We present a child with PRS and progressive neurological deficit caused by a giant intracranial aneurysm and reviewed the literature concerning all intracranial abnormalities in patients with PRS. A literature search identified 27 articles reporting on 88 patients with PRS and intracranial abnormalities. Ipsilateral brain calcification and hemiatrophy are the most prominent features on CT scan and hyperintense white matter lesions are most frequently seen on T2-weighted MRI. Although lacking precise prevalence data, intracranial abnormalities are not uncommon in patients with PRS. We found three other PRS patients with intracranial aneurysms. Our case and literature search suggests a possible association between PRS and intracranial aneurysms.
We consider this association important for clinical practice and recommend including intracranial vascular diseases in the differential diagnosis when dealing with a PRS patient with neurological symptoms.
Key words: Parry Romberg Syndrome, giant brain aneurysm, paediatric neuroradiology
Introduction
Parry-Romberg syndrome (PRS) is an acquired progressive hemiatrophy of the face first reported by Parry in 1825 and later described as a syndrome in 1846 by Romberg, naming it prophoneurosis. In 1871, Eulenburg was the first to use the more precise term progressive facial hemiatrophy. PRS is characterized by unilateral wasting of bone. There seems to be a predilection for the left side of the face. Onset is insidious, often affecting patients during the first or second decade of life, and is usually followed by progression over the first two to 20 years after which stabilization occurs. Patients with earlier onset of the disease are often more severely affected, with involvement of facial cartilage and bone. Later ages of onset rarely seem to occur (range 1-50 years) 1.
Neuropsychiatric problems are common in PRS patients with reported prevalence as high as 50%. In accordance with these findings, several neuroradiological abnormalities have been described in patients with PRS (e.g. cerebral hemiatrophy and calcifications) 2. By contrast, neurovascular abnormalities are rarely reported in PRS.
This paper describes a ten-year-old boy with progressive neurological symptoms attributed to a partially thrombosed giant intracranial aneurysm and two additional asymptomatic aneurysms.
To our knowledge this is the second report describing an association between PRS syndrome and intracranial aneurysms 3 in childhood. To facilitate the diagnostic work-up of PRS patients with neurological symptoms we reviewed the literature on the presence of intracranial abnormalities (vascular and non-vascular) on neuroimaging in these patients.
Case Report
A ten-year-old boy with Parry-Romberg syndrome was referred to our neurovascular unit for subacute aggravation of his chronic headaches (Figure 1). He was suffering from a progressive headache for over two years. His medical history described a left-sided hemifacial atrophy, first appearing shortly after birth, that seemed to have reached a stable phase over the last couple of years. On physical examination, hemifacial atrophy was noted involving the entire left side of his face, with wasting of subcutaneous tissues and thinning of the skin, which was more prominent over the forehead resulting in the typical `en coup de sabre` morphology. Hyperpigmentation was present in the region of the second and third division of the trigeminal nerve (Figures 1 and 2). Neurological examination showed a disorientated somnolent boy with left temporal visual field deficit and nugal rigidity. Ophthalmologic examination revealed bilateral optic disc pallor without edema indicating longstanding increased intracranial pressure.
Figure 1.
Ten-year-old boy with Parry-Romberg Syndrome. Note the left-sided hemifacial atrophy with wasting of subcutaneous tissues and thinning of the skin. This is more prominent over the forehead resulting in the typical `en coup de sabre` morphology. Hyperpigmentation is present in the region of the second and third division of the trigeminal nerve. (With permission from parents).
Figure 2.
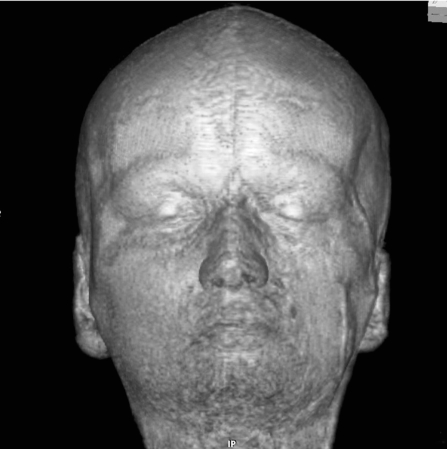
3D volume-rendered MR reconstruction of the face. Note the left-sided atrophy of the subcutaneous and osseous structures most obvious in the temporal fossa, but also visible in the asymmetry of the nostrils and around the orbit. The `en coup de sabre` can also be seen.
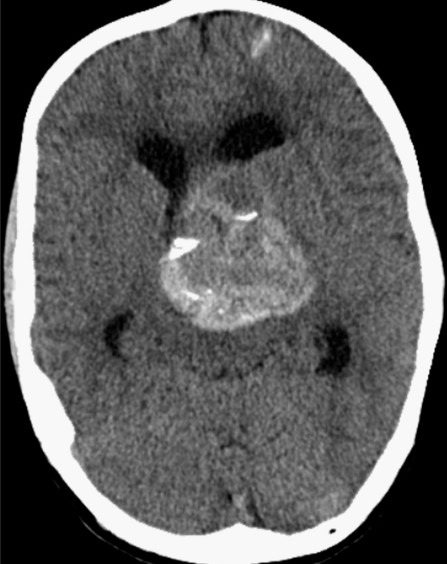
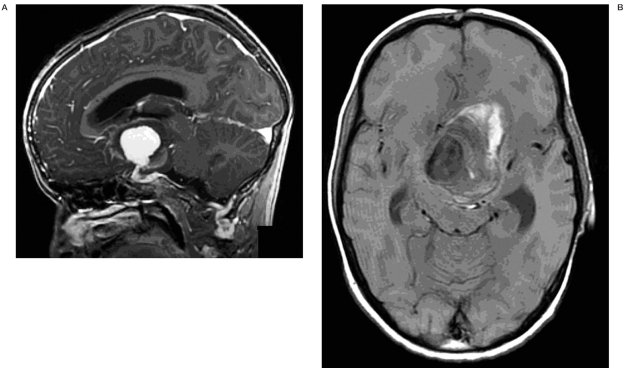
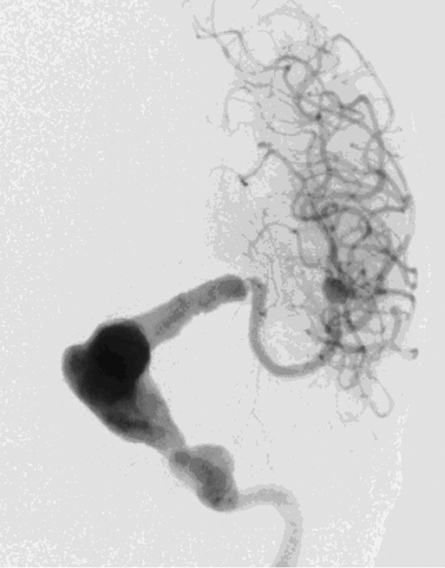
Brain computed tomography (CT) showed a large space-occupying lesion (5.9 x 4.2 cm) at the level of the basal ganglia. Heterogeneous densities and calcifications were observed in the wall of this lesion. Mass effect was evident with midline shift and brain stem compression resulting in an obstructive hydrocephalus. We also noted subcortical calcifications in the left frontal lobe. No signs of subarachnoid hemorrhage were present (Figure 2). Magnetic resonance imaging (MRI) showed a large aneurysm of the left distal internal carotid artery with mural thrombus and signs of an acute mural hemorrhage (Figure 3A,B). On digital subtraction angiography (DSA) distal carotid dolichoectasia was shown. Furthermore, two additional aneurysms were discovered. One located at the M1-M2 trifurcation and one of the cavernous portion of the ICA (Figure 4).
Figure 3.
Noncontrast axial CT showing a large space occupying lesion (5.9 x 4.2 cm) at the level of the basal ganglia with evident mass effect causing obstructive hydrocephalus. Different densities and calcifications are seen in the wall of the lesion. Note the subcortical calcification in the left frontal lobe.
Figure 4.
A) Sagittal contrast enhanced MRI shows a giant aneurysm of the left distal internal carotid artery. B) Axial unenhanced T1-weighted MRI showing mural thrombus and signs of acute mural hemorrhage.
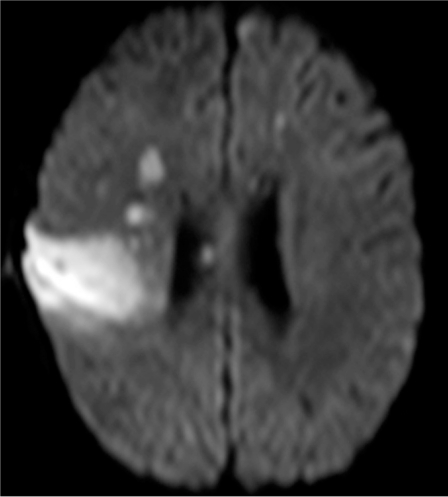
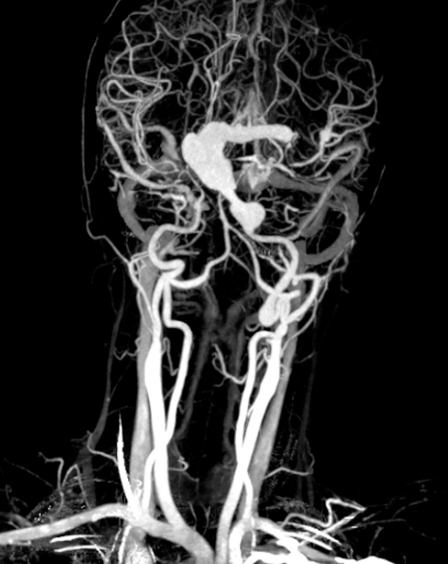
After multidisciplinary evaluation, it was decided to treat the giant aneurysm by placing an endovascular stent. Because of the unusual size of the aneurysm, the stent had to be custommade with a one month delivery time, consequently delaying treatment of the patient in this stage. One week after admission the patient developed acute deterioration of consciousness, epileptic seizures and paresis of the left leg and paralysis of the left arm. Brain CT showed increased enlargement of the ventricular system, configuration of the aneurysm was not altered. A ventriculoperitoneal drain was placed with subsequent gradual improvement of consciousness, while left hemiparesis persisted. On diffusion weighted MRI, increased signal intensity in the right middle cerebral artery territory, centrum semiovale on both sides, and in the dorsal part of the head of the left caudate nucleus was noted (Figure 5), indicating occurrence of recent ischemia in these areas. A subsequent CT angiography showed gradual narrowing of the right ICA which was most pronounced at the level of the siphon but also present in the distal ICA and M1 and proximal M2 branches (Figure 6). Gradual further deterioration of neurological deficit occurred with development of dysphasia.
Figure 5.
Digital subtraction angiography showing dolichoectasia of the distal carotid artery and a severe junctional stenosis. In addition, aneurysmal dilation of the cavernous portion of the ICA and an aneurysm on the M1-M2 trifurcation were seen.
Figure 6.
On diffusion weighted MRI, increased signal intensity in the right middle cerebral artery territory, centrum semiovale, and in the dorsal part of the head of the left caudate nucleus were noted suggestive of infarction in the right MCA territory.
Figure 7.
CT-angiography showing narrowing of the right carotid artery most pronounced at the level of the siphon but also present in the distal carotid artery, M1 and proximal M2 branches.
Endovascular treatment options were not available at that time, so the patient was transferred to a neurosurgical centre with expertise in performing high-flow bypasses. A high-flow bypass from the external carotid artery to an M3 branch of the middle cerebral artery (MCA) was performed, with ligation of the MCA just after the bifurcation. The aneurysm was debulked to diminish mass effect. After surgery, with a follow-up of six months, the patient almost completely recovered from his neurological deficit.
Search Strategy of Systematic Review of the literature
To identify cases with PRS associated with intracranial abnormalities we performed a literature search using PubMed, Embase and Web of Science (from 1988 up to September 2008) with the following key words: [hemifacial atrophy [OR] Parry-Romberg syndrome] [AND] [intracranial aneurysm [OR] cerebrum [OR] intracranial arterial diseases [OR] neuroimaging [OR] intracranial abnormalities]. Additional articles were found by searching the reference lists of relevant articles. We included all articles reporting patients diagnosed with Parry-Romberg disease and intracranial abnormalities discovered by neuroimaging. Articles in languages other than English were excluded. Two authors (T.B., J.v.B.) independently reviewed the included articles.
Results
We identified 27 articles reporting on intracranial abnormalities in 88 patients with PRS (see Table 1). These articles were mostly case reports and some case series, with ages of onset ranging from neonatal onset up to 50 years. All patients underwent neuroimaging, except for the patients in the study by Tollefson et Al4 who included both patients with `en coup de sabre` morphea and PRS in their study. Different abnormalities are described by the various authors. Hemispheric atrophy was described in 11 out of 88 patients. On CT ipsilateral calcifications were the most prominent anomaly, described in nine out of 88 patients, including: basal ganglia, cingulated gyrus and frontoparietal parenchyma.
Table 1.
Overview of the literature on intracranial abnormalities in patients with PRS between 1988 and 2008.
| Authors | Date | N | Imaging | Abnormalities |
|---|---|---|---|---|
| Hirata et Al (7) | 1988 | 1 | CT CCA |
Ipsilateral AVM with calcification preand postcentral gyrus Ipsilateral AVM |
| Fry et Al (18) | 1992 | 6 | CT MRI |
Cerebral calcifications (N=3) Ipsilateral white matter lesions on T2 (N=5) |
| Leao et Al (19) | 1994 | 1 | CT MRI |
Asymmetrical lateral ventricles Ipsilateral atrophy and agenesis head of caudate nucleus |
| Terstegge et Al (20) | 1994 | 3 | MRI | Ipsilateral monoventricular enlargement Meningocortical dysmorphia. White-matter changes |
| Derex et Al (21) | 1995 | 1 | CT + MRI | Ipsilateral porencephaly and cerebral calcifications |
| Schievink et Al (8) | 1995 | 1 | CT MRI CCA |
Bilateral middle fossa arachnoid cysts and cerebral atrophy Bilateral atrophy and ipsilateral middle fossa arachnoid cyst Ipsilateral carotid-jugular fistula and carotid dissection |
| Schievink et Al (3) | 1996 | 1 | CT CCA CCA-FU1 CCA-FU2 |
Ipsilateral calcifications (basal ganglia, wall of the ventricle, parietal lobe) atrophy and giant aneurysm Ipsilateral cavernous giant aneurysm Contralateral cavernous giant aneurysm Ipsilateral posterior cerebral artery aneurysm Ipsilateral distal PCA fusiform aneurysm |
| Cory et Al (6) | 1997 | 1 | CT MRI CCA |
Ipsilateral calcifications and hypodensities cingulate gyrus corpus callosum and frontoparietal parenchyma Ipsilateral focal infarctions corpus callosum Diffuse deep and subcortical white matter signal changes Mild cortical thickening Leptomeningeal enhancement Normal |
| Goldberg et Al (22) | 1997 | 1 | MRI | Ipsilateral increased signal surrounding anterior horn lateral ventricles and corpus callosum Diffuse leptomeningeal enhancement |
| Taylor et Al (23) | 1997 | 1 | MRI | Vascular malformation |
| Woolfenden et Al (9) | 1998 | 1 | MRI CCA |
Ipsilateral leptomeningeal enhancement, increased T2 signal in the white matter, loss of the normal cortical gyral pattern and enlargement of the ipsilateral ventricle Ipsilateral irregularities of arteries |
| Miedziak et Al (10) | 1998 | 1 | MRI | Ipsilateral white matter lesions on T2 Ipsilateral hypoplastic vertral and carotid artery |
| Chang et Al (24) | 1999 | 1 | MRI | Ipsilateral cerebral atrophy |
| Yano et Al (25) | 2000 | 1 | CT MRI |
Ipsilateral calcifications and hypodensities frontoparietal gyri Ipsilateral white matter lesions on T2 |
| Aynaci et Al (26) | 2001 | 1 | CT MRI |
Ipsilateral calcifications subcortical in the parietal lobe Ipsilateral white matter lesions on T2 |
| DeFelipe et Al (27) | 2001 | 1 | SPECT MRI |
Ipsilateral hypoperfusion Ipsilateral atrophy and blurring of sulci |
| Pichiecchio et Al (1) | 2002 | 1 | CT + MRI CCA |
No intracranial abnormalities Ipsilateral middle cerebral artery aneurysm |
| Moko et Al (5) | 2003 | 10 | MRI | Ipsilateral atrophy or white matter hyperintensity (N=4) Bilateral white matter hyperintensity (N=1) |
| Shah et Al (15) | 2003 | 1 | MRI FDG-PET |
Ipsilateral atrophy and hyperintensities on T2 Ipsilateral increased glucose uptake |
| Blaszczyk et Al (28) | 2003 | 19 | MRI SPECT |
White matter lesions, vascular malformations, cortex thickening, gyral effacement, cystic infarcts, intracranial calcifications. (N=11) Ipsiand contralateral hypoperfusion (N=13) |
| Sathornsumetee et Al (29) | 2005 | 1 | MRI | Ipsilateral atrophy |
| Aktekin et Al (30) | 2005 | 1 | MRI | Ipsilateral enlargement of the ventricle and poor demarcation of the white-gray matter |
| Okumura et Al (31) | 2006 | 1 | MR spectr MRI SPECT |
Normal Ipsilateral white matter hyperintensity Ipsilateral hyperperfusion |
| Aoki et Al (11) | 2006 | 1 | CT CCA |
Mass lesion with partial defect of the petrous bone Ipsilateral carotid artery giant fusiform aneurysm |
| Tollefson et Al (4) | 2007 | 54 | CT MRI |
Negative T2 abnormalities |
| Carreno et Al (32) | 2007 | 1 | MRI | Ipsilateral progressive hemispheric atrophy |
| Moon et Al (33) | 2008 | 1 | MRI DTI |
Ipsilateral atrophy Fiber derangement |
|
Legend: N= number of patients, CT= Computed Tomography, CCA= Conventional Catheter Angiography, MRI= Magnetic Resonance Imaging, FU= Follow-up, PET= Positron Emission Tomography, SPECT= Single Photon Emission Tomography, spectr= spectroscopy, DTI= Diffusion Tensor Imaging | ||||
On MRI white matter lesions on T2-weighted images were the most frequent finding, occurring in 31 out of 88 patients. Moko et Al published the largest case series on intracranial MRI appearances in patients with PRS, including ten patients. Three patients showed central nervous system (CNS) involvement clinically and on MRI. Two patients without clinical CNS involvement showed MR abnormalities. MRI abnormalities consisted either of cerebral atrophy or white matter hyperintensities. Five patients did not show CNS involvement, clinically or on MRI 5. Functional imaging was performed in sixteen of all reported PRS patients, giving rise to conflicting results. Some authors showed areas of hyperperfusion on single photon emission computed tomography (SPECT) and increased uptake in FDG-positron emission tomography (PET) while other authors depicted areas of hypoperfusion on SPECT in patients with PRS. Details on the different findings are shown in Table 1.
Follow-up observation of imaging findings is limited. Only Cory et Al monitored a child with PRS for nearly two years and demonstrated that cerebral calcifications were relatively unchanged but that the underlying white matter lesions had progressed together with progression of facial atrophy 6. We specially searched the literature for possible associations between PRS and neurovascular abnormalities. The results are depicted in Table 2. We found one patient with an AVM 7, one patient with a dural fistula 8, two cases with vascular congenital malformations 9,10 and three patients with intracranial aneurysm 1,3,11. Vascular malformations consisted of one patient with reversible arterial caliber changes and one patient with an ipsilateral hypoplastic verbral and carotid artery 9,10.
Table 2.
Overview of literature on intracranial vascular abnormalities in patients with PRS between 1988 and 2008.
| Authors | Date | Sex/Age | Symptoms | Vascular Abnormalities |
|---|---|---|---|---|
| Hirata et Al (7) | 1988 | M/39 | Epilepsy | Ipsilateral AVM |
| Schievink et Al (8) | 1995 | F/45 | Pulsatile tinnitus Retroorbital pain |
Ipsilateral carotid-jugular fistula and carotid dissection |
| Schievink et Al (3) | 1996 | M/5 | Diplopia Left-sided ptosis Left-sided headaches Progressive vision loss left eye |
Ipsilateral giant aneurysm Ipsilateral cavernous giant aneurysm Contralateral cavernous giant aneurysm Ipsilateral posterior cerebral artery aneurysm Ipsilateral distal PCA fusiform aneurysm |
| Woolfenden et Al (9) | 1998 | M/35 | Hemiplegic migraine | Ipsilateral reversible arterial caliber changes |
| Miedziak et Al (10) | 1998 | F/23 | Transient Numbness contralateral upper and lower extremities |
Ipsilateral hypoplastic vertebral and carotid artery |
| Pichiecchio et Al (1) | 2002 | F/32 | Migraine | Ipsilateral middle cerebral artery aneurysm |
| Aoki et Al (11) | 2006 | F/56 | Facial pain and left-sided headaches |
Ipsilateral carotid artery giant fusiform aneurysm |
| Legend: M=Male, F=Female | ||||
Schievink et Al described a child with PRS and multiple intracranial aneurysms. This child was treated for a giant aneurysm of the left cavernous carotid artery at the age of five years. Treatment consisted of carotid ligation in the neck and a superficial temporal artery-middle cerebral artery bypass. At the age of 12 the patient was similarly treated for a giant aneurysm of the cavernous carotid artery on the opposite site, which had progressed from a previously noted small dilatation. At age 21 the patient underwent endovascular treatment for a de novo saccular aneurysm of the left posterior cerebral artery at the P1-P2 junction and a fusiform aneurysm of the distal left posterior cerebral artery3. Pichiecchio et Al published the case of 32-year-old woman with PRS who had migraine and an intracranial aneurysm 1. More recently Aoki et Al described the case of a 56-year-old women with a giant fusiform aneurysm extending from the petrous segment of the ACI to the cavernous segment11.
Discussion
Compared to the healthy population central nervous system involvement seems to occur more often in patients with PRS, most commonly consisting of migraine-type headache, focal epilepsy and trigeminal neuralgia. A recent global internet survey of 205 patients with PRS estimated the central nervous system involvement to be over fifty percent 2. The presenting symptom in our patient was a slowly progressive daily headache. For more than two years this never led to a diagnostic work-up. Eventually the subacute aggravation of his headache led to the diagnosis. This aggravation was probably due to increased mass effect of the giant aneurysm caused by acute intramural hemorrhage. Another diagnostic problem we faced was the explanation for his secondary deterioration with decreased consciousness and development of left-sided hemiparesis. Occurrence of vasospasm was unlikely since no subarachnoid hemorrhage was present. The hydrocephalus only partially explained his neurological deficit and we believed the left-sided hemiparesis to have a thromboembolic origin. However, because this patient probably suffers from an underlying condition affecting the entire cerebral vasculature, other causes for this event could not be excluded. How a thrombus of a giant aneurysm of the left middle cerebral artery could give rise to embolisms in the right hemisphere with sparing of the ipsilateral circulation remains unanswered.
Confronted with two rare disorders in the same child we searched the literature for an association between children with PRS and giant aneurysms, hoping that this would facilitate our decision-making. We found one other child with the same association 3. Because other abnormalities, apart from the giant aneurysm, were seen on CT, we reviewed the literature published in the last 20 years (1988-2008) reporting on intracranial abnormalities of any sort in patients with PRS. As shown in Table 1, ipsilateral calcifications (both cortical and subcortical) and atrophy on CT, and hyperintense white matter lesions on T2-weighted MRI are the most prominent intracranial features of PRS. Functional imaging yields inconsistent results. Although there are no exact data on the prevalence of PRS in the general population, we believe that based on this review intracranial abnormalities are not uncommon in PRS.
In contrast, intracranial vascular abnormalities are rarely associated with PRS. Our patient is the second child reported with intracranial aneurysmal disease with an associated Parry-Romberg syndrome. In the adult population we found two cases of PRS with an intracranial aneurysm1,11. Other reported neurovascular abnormalities include one AVM 7, one dural fistula 8 and two cases with vascular congenital malformations 9,10.
In recent literature, aneurysms seen in the pediatric population account for around 7% of all aneurysms 12. Their features differ significantly from the aneurysms in adults especially in their sexual prevalence, location, morphology and etiology. Giant aneurysms are known to be of increased incidence in this group. Recently the Toronto group and the Bicêtre group published two large series on this subject reporting an incidence of giant aneurysms of 29.7%12,13.
When children present with one or more intracranial aneurysm, a generalized connective tissue disorder is suspected. However, quite often the underlying disease remains unknown despite extensive investigations 14. In our patient the possible underlying disorder may be PRS, since both entities are extremely rare and a coincidental occurrence seems highly unlikely. Speculating on a common etiology of both disorders is beyond our expertise because both diseases are associated with a wide variety of conditions, such as trauma, infections, connective tissue disorders, familial occurrence, inflammatory diseases, etc.12,15,16.
Interestingly an Australian group recently described a chronic sympathetic hyperactivity in the areas of distribution of the trigeminal nerve of the affected side of PRS patients17. In earlier reports several authors also proposed that cranial vasculitis in PRS is the cause of trigeminal dysfunction5. In view of these findings, an inflammatory process as a common etiology for PRS and giant seems logical and reasonable, although the primary trigger remains obscure.
Although very rare, we consider the described association between PRS and giant aneurysm important for clinical practice. When confronted with a patient with PRS presenting with neurological symptoms, we recommend including intracranial vascular diseases in the differential diagnosis. At the moment, we lack the knowledge on precise prevalence data to recommend routine screening for intracranial abnormalities in patients with PRS.
References
- 1.Pichiecchio A, Uggetti C, et al. Parry-Romberg syndrome with migraine and intracranial aneurysm. Neurology. 2002;59:606–608. doi: 10.1212/wnl.59.4.606. [DOI] [PubMed] [Google Scholar]
- 2.Stone J. Parry-Romberg syndrome: a global survey of 205 patients using the Internet. Neurology. 2003;61:674–676. doi: 10.1212/wnl.61.5.674. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI, Mellinger JF, Atkinson JL. Progressive intracranial aneurysmal disease in a child with progressive hemifacial atrophy (Parry-Romberg disease): case report. Neurosurgery. 1996;38:1237–1241. doi: 10.1097/00006123-199606000-00038. [DOI] [PubMed] [Google Scholar]
- 4.Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56:257–263. doi: 10.1016/j.jaad.2006.10.959. [DOI] [PubMed] [Google Scholar]
- 5.Moko SB, Mistry Y, et al. Parry-Romberg syndrome: intracranial MRI appearances. J Craniomaxillofac Surg. 2003;31:321–324. doi: 10.1016/s1010-5182(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 6.Cory RC, Clayman DA, et al. Clinical and radiologic findings in progressive facial hemiatrophy (Parry-Romberg syndrome) Am J Neuroradiol. 1997;18:751–757. [PMC free article] [PubMed] [Google Scholar]
- 7.Hirata K, Katayama S, et al. Arteriovenous malformation with crossed total hemiatrophy: a case report. J Neurol. 1988;235:165–167. doi: 10.1007/BF00314309. [DOI] [PubMed] [Google Scholar]
- 8.Schievink WI, Piepgras DG, Nichols DA. Spontaneous carotid-jugular fistula and carotid dissection in a patient with multiple intracranial arachnoid cysts and hemifacial atrophy: a generalized connective tissue disorder? Case report. J Neurosurg. 1995;83:546–549. doi: 10.3171/jns.1995.83.3.0546. [DOI] [PubMed] [Google Scholar]
- 9.Woolfenden AR, Tong DC, et al. Progressive facial hemiatrophy: abnormality of intracranial vasculature. Neurology. 1998;50:1915–1917. doi: 10.1212/wnl.50.6.1915. [DOI] [PubMed] [Google Scholar]
- 10.Miedziak AI, Stefanyszyn M, et al. Parry-Romberg syndrome associated with intracranial vascular malformations. Arch Ophthalmol. 1998;116:1235–1237. doi: 10.1001/archopht.116.9.1235. [DOI] [PubMed] [Google Scholar]
- 11.Aoki T, Tashiro Y, et al. Parry-Romberg syndrome with a giant internal carotid artery aneurysm. Surg Neurol. 2006;65:170–173. doi: 10.1016/j.surneu.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Lasjaunias P, Wuppalapati S, et al. Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst. 2005;21:437–450. doi: 10.1007/s00381-004-1125-x. [DOI] [PubMed] [Google Scholar]
- 13.Agid R, Souza MP, et al. The role of endovascular treatment for pediatric aneurysms. Childs Nerv Syst. 2005;21:1030–1036. doi: 10.1007/s00381-005-1152-2. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, McGirt MJ, et al. Intracranial aneurysms in the pediatric population: case series and literature review. Surg Neurol. 2005;63:424–432. doi: 10.1016/j.surneu.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Shah JR, Juhasz C, et al. Rasmussen encephalitis associated with Parry-Romberg syndrome. Neurology. 2003;61:395–397. doi: 10.1212/wnl.61.3.395. [DOI] [PubMed] [Google Scholar]
- 16.Lewkonia RM, Lowry RB. Progressive hemifacial atrophy (Parry-Romberg syndrome) report with review of genetics and nosology. Am J Med Genet. 1983;14:385–390. doi: 10.1002/ajmg.1320140220. [DOI] [PubMed] [Google Scholar]
- 17.Drummond PD, Hassard S, Finch PM. Trigeminal neuralgia, migraine and sympathetic hyperactivity in a patient with Parry-Romberg syndrome. Cephalalgia. 2006;26:1146–1149. doi: 10.1111/j.1468-2982.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 18.Fry JA, Alvarellos A, et al. Intracranial findings in progressive facial hemiatrophy. J Rheumatol. 1992;19:956–958. [PubMed] [Google Scholar]
- 19.Leao M, da Silva ML. Progressive hemifacial atrophy with agenesis of the head of the caudate nucleus. J Med Genet. 1994;31:969–971. doi: 10.1136/jmg.31.12.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terstegge K, Kunath B, et al. MR of brain involvement in progressive facial hemiatrophy (Romberg disease): reconsideration of a syndrome. Am J Neuroradiol. 1994;15:145–150. [PMC free article] [PubMed] [Google Scholar]
- 21.Derex L, Isnard H, Revol M. Progressive facial hemiatrophy with multiple benign tumors and hamartomas. Neuropediatrics. 1995;26:306–309. doi: 10.1055/s-2007-979779. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg-Stern H, deGrauw T, et al. Parry-Romberg syndrome: follow-up imaging during suppressive therapy. Neuroradiology. 1997;39:873–876. doi: 10.1007/s002340050525. [DOI] [PubMed] [Google Scholar]
- 23.Taylor HM, Robinson R, Cox T. Progressive facial hemiatrophy: MRI appearances. Dev Med Child Neurol. 1997;39:484–486. doi: 10.1111/j.1469-8749.1997.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang SE, Huh J, et al. Parry-Romberg syndrome with ipsilateral cerebral atrophy of neonatal onset. Pediatr Dermatol. 1999;16:487–488. doi: 10.1046/j.1525-1470.1999.0le16.x. [DOI] [PubMed] [Google Scholar]
- 25.Yano T, Sawaishi Y, et al. Progressive facial hemiatrophy after epileptic seizures. Pediatr Neurol. 2000;23:164–166. doi: 10.1016/s0887-8994(00)00168-5. [DOI] [PubMed] [Google Scholar]
- 26.Aynaci FM, Sen Y, et al. Parry-Romberg syndrome associated with Adie's pupil and radiologic findings. Pediatr Neurol. 2001;25:416–418. doi: 10.1016/s0887-8994(01)00333-2. [DOI] [PubMed] [Google Scholar]
- 27.DeFelipe J, Segura T, et al. Neuropathological findings in a patient with epilepsy and the Parry-Romberg syndrome. Epilepsia. 2001;42:1198–1203. doi: 10.1046/j.1528-1157.2001.45800.x. [DOI] [PubMed] [Google Scholar]
- 28.Blaszczyk M, Krolicki L, et al. Progressive facial hemiatrophy: central nervous system involvement and relationship with scleroderma en coup de sabre. J Rheumatol. 2003;30:1997–2004. [PubMed] [Google Scholar]
- 29.Sathornsumetee S, Schanberg L, et al. Parry-Romberg syndrome with fatal brain stem involvement. J Pediatr. 2005;146:429–431. doi: 10.1016/j.jpeds.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Aktekin B, Oguz Y, et al. Cortical silent period in a patient with focal epilepsy and Parry-Romberg syndrome. Epilepsy Behav. 2005;6:270–273. doi: 10.1016/j.yebeh.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Okumura A, Ikuta T, et al. Parry-Romberg syndrome with a clinically silent white matter lesion. Am J Neuroradiol. 2006;27:1729–1731. [PMC free article] [PubMed] [Google Scholar]
- 32.Carreno M, Donaire A, et al. Parry Romberg syndrome and linear scleroderma in coup de sabre mimicking Rasmussen encephalitis. Neurology. 2007;68:1308–1310. doi: 10.1212/01.wnl.0000259523.09001.7a. [DOI] [PubMed] [Google Scholar]
- 33.Moon WJ, Kim HJ, et al. Diffusion tensor imaging and fiber tractography in Parry-Romberg syndrome. Am J Neuroradiol. 2008;29:714–715. doi: 10.3174/ajnr.A0967. [DOI] [PMC free article] [PubMed] [Google Scholar]