Summary
Carotid-cavernous fistulas are abnormal arteriovenous communications either directly between the internal carotid artery and the cavernous sinus or between the dural branches of the internal and external carotid arteries. These fistulas predominantly present with ocular manifestations and they are treated mainly by endovascular techniques in most cases. A detailed review of the literature allowed us to make a complete analysis of the information available on the topic. We describe a case of a direct carotid-cavernous fistula occluded by endovascular implantation of a covered stent, showing the persistence of results after three years.
Key words: traumatic carotid cavernous fistula, covered stent, pseudoaneurysm, internal carotid artery, intracranial vasculature, interventional radiology
Introduction
A covered stent was successfully implanted to treat a direct carotid-cavernous fistula (CCF) in a 63-year-old woman. This procedure was undertaken following failure to obliterate the fistula by means of a detachable balloon due to carotid artery laceration. Immediate and six month control angiograms demonstrated complete occlusion of the fistula and normal lumen diameter of the cavernous carotid artery.
A carotid-cavernous fistula usually follows rupture of a cavernous sinus carotid aneurysm or may result from post-traumatic or iatrogenic injury to the artery3-16. Bruit, chemosis, exophthalmus, partial or complete ophthalmoplegia and headache are common clinical findings, which may manifest with an abrupt onset, reflecting severe venous hypertension3,6,27. Treatment options for high-flow CCF consist in endovascular obliteration by means of detachableballoons16 or coils10 and, less frequently, direct surgical repair27. Covered stents have been proposed recently as an alternative technique to obtain occlusion of CCF8,14,23.
Literature Review
Since 1981, CCF have been successfully treated with preservation of the internal carotid artery (ICA), usually achieved by placing detachable balloons in the venous compartment by arterial route through the vessel tear. However if the size of the fistula is too large the embolization balloon may retract to the parent ICA causing a partial or complete occlusion. Teng et Al26 suggested using a double balloon technique when the orifice of the fistula is too small for the passage of the deflated or slightly inflated balloon, or when the width of the cavernous sinus is smaller than the tear of the portant vessel. Moreover a delayed spontaneous deflation of the balloon over some weeks is expected. This results in a rate of pseudoaneurysm or pouch formation at the site of the healed CCF as high as 30%.
Since 1991 detachable coils have been proposed as an alternative procedure via transarterial or transvenous routes. The disadvantages of coils are an increased cost of the technique and possible coil occlusion of critical parenchymal veins afferent to the cavernous sinus. The mesh of coils within the CS should present, in case of large communications, an interface to the parent vessel, like an endovascular occlusion of intracranial aneurysms, with the risk of clot formation and acute thromboembolic complications that can be reduced with heparinization and antiplatelet therapy.
Halbach et Al1,4,5,7,11-13,17-22,25 described some failures of the platinum coil procedure in CCFs. In one case, finally treated with endovascular trapping of the ICA, the cast of platinum coils previously released into the CS projected through the fistula into the parent artery. In a second case closure of anterior drainage was achieved, but complicated by distal migration of platinum coils with aggravation of ocular symptoms. Endovascular cavernous sinus embolization with balloons or coils may be responsible for cranial nerve palsy. In 1999 Sencer et Al24 reported a case with mechanical compression of the lateral rectus muscle by balloons. These difficulties could be resolved by an intracranial covered stent. In 2001 Weber et Al28 described a case of direct carotid cavernous fistula cured by endovascular porous coronary stent. This kind of device is highly flexible and easily follows the curves of vessels.
In 2002 Kocer er Al14 reported a case of iatrogenic carotid cavernous fistula successfully treated with endovascular stent-graft (Jostent 12x4 mm). Covered stent or stent-graft provides waterproof sealing of the bridged vessel segment. Stent-graft has a cover of polytetrafluoroethylene (PTFE), used for many years as a graft material during vascular surgery. The stent-graft has a low incidence of complications, including acute stent thrombosis. Use of this device is the therapy of choice for acute coronary rupture. Jostent, provided by Jomed, barely follows the curves of intracranial vessels.
It can be used to reach only the first segment of intracranial ICA, the intracavernous segment. This poor navigability is closely connected to the length of the device.
In recent years, the use of covered stents has become a promising alternative to other endovascular techniques and it has been suggested that covered stents may be an efficient way to occlude a fistula preserving the parent artery.
In 2007 Gomez et Al9 presented their experience using balloon-expandable covered stents to treat CCF, focusing on arterial wall reconstruction. It was the first series with midterm follow-up between three months and 3.5 years. Seven post-traumatic direct CCF were treated using polytetrafluoroethylene (PTFE)-covered stents between April 2003 and September 2006. Five were treated with covered stents alone. One patient with transection of the internal carotid artery (ICA) first underwent bare stent placement to provide support for the covered stent. One patient had to be treated with coils and nBCA. Control angiograms obtained in the seven patients demonstrated occlusion of the fistula and preservation of the ICA in all cases. There was no mortality and no immediate postprocedural morbidity. The authors described PTFE-covered stents as a promising intracranial therapeutic alternative to treat CCF to preserve the parent artery by reconstructing the arterial wall.
In the same year, Archondakis et Al2 treated eight patients with post-traumatic CCF by positioning a covered stent in the intracranial internal carotid artery (ICA). They received periodic clinical and angiographic follow-up to evaluate the patency and the stability of clinical results. In all cases, the symptoms related to the CCF regressed after treatment and did not recur in the follow-up. Two patients presented residual filling of the CCF at the end of the procedure. The angiographic follow-up revealed a good patency of the ICA in six out of seven patients. One patient presented an ICA asymptomatic occlusion. One patient required transvenous coil occlusion of the cavernous sinus. The search strategy was principally based on article references read on the web or in scientific journals, after a complete analysis and review of all national and international literature on CCF treatment.
Analysis
Although the therapeutic goal is the occlusion of the fistula with preservation of the ICA, in case of failure of a preservative procedure or further difficulties during an endovascular treatment, trapping must be considered. Therefore previous balloon test occlusion must be always performed. The use of stents for management of this type of intracranial arterial disease has been limited by the interventionalist's inability to negotiate the relatively stiff covered stent with the balloon assembly through the tortuous intracranial vessels such as the carotid artery system without damaging the vessel in the process.
There are few reports in the literature on the long-term follow-up of treatment of CCF with a stent-graft. When a traditional endovascular procedure cannot be performed, the stent-graft could be used before trapping, always after balloon test occlusion. We expect a positive outcome from the initial experiences. Future technical developments of this device will overcome some of the current limitations, responsible for our failure 2,9,15.
Illustrative case report
In September 2004, a 63-year-old woman presented to an outside facility with a soft pulsatile mass in the right orbitozygomatic region and complaints of right double vision, progressively established a few days prior to admission. The patient had undergone surgical reduction of right zygomatic and maxillar fractures three weeks before, following craniofacial trauma due to a motor vehicle accident. Physical and neurological examination revealed right eye pulsatile proptosis associated with ipsilateral complete right ophthalmoplegia without mydriasis. Magnetic resonance angiography (MRA) disclosed a right carotid-cavernous fistula.
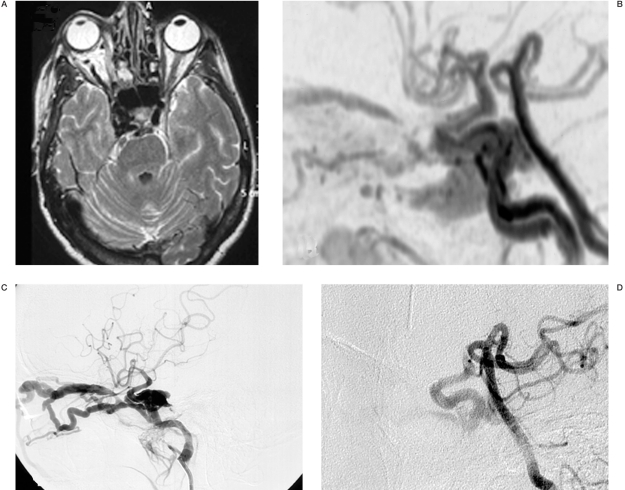
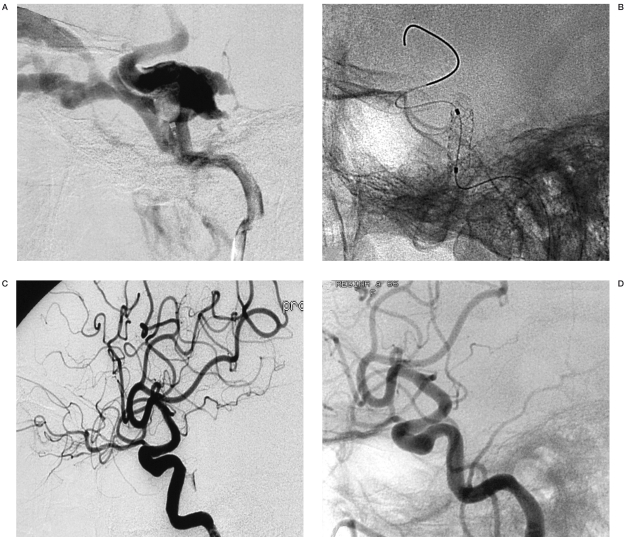
The patient was then referred to our department for treatment (Figure 1). The patient underwent a four-vessel cerebral angiography and right vertebral artery injection during compression of the ipsilateral ICA. The angiograms demonstrated a carotid-cavernous fistula with retrograde flow in the superior and inferior ophthalmic veins. Following a bolus of 5000 I.U. of heparin and 1000 mg of acetylsalicylate, a detachable balloon device (Goldbal 1, Balt) was placed within the fistula by the transarterial route. However, angiographic control following balloon inflation showed persistence of the fistula due to carotid artery laceration. A covered stent (Jostent Graftmaster 4 mmx19 mm, Jomed) was then advanced into the right internal carotid artery and inflated in the cavernous carotid siphon, covering the dissecting arterial segment and across the fistular orifice. Immediate control angiogram showed occlusion of the fistula (Figure 2). At the end of the procedure a load of 300 mg of clopidogrel was administered. The immediate post-procedure period was uneventful and the patient experienced regression of proptosis, while pulsation was absent. The right ophthalmoplegia persisted after the procedure. The patient was kept in a protocol of aspirin 300 mg and clopidogrel 75 mg daily for one month and aspirin 300 mg alone thereafter. Six months after the procedure, neurological examination revealed only a mild right sixth cranial nerve palsy. The first control angiogram (six months later) showed complete occlusion of the fistula and normal lumen diameter of the internal carotid artery. The last angiogram (October 2007), three years after the endovascular procedure, shows persistence of the result achieved.
Figure 1.
Figure 2.
Discussion
Optimal management of high-flow CCF consists in occlusion of the fistula with preservation of the internal carotid artery patency16. For this purpose, endovascular techniques have been used during recent decades for embolization of the fistula by means of balloons16 or coils10. To date, endovascular placement of a detachable balloon device within the fistula by a transarterial route has been established as the gold standard procedure3,6,16,27. Occasionally, transarterial obliteration of the fistula is achievable only by occlusion of the internal carotid artery, by means of multiple balloon devices. Alternatively, a transvenous balloon placement may be performed, usually by the inferior petrous sinus or superior ophthalmic vein route, particularly when the internal carotid artery appears too tortuous or severely stenotic11,16. Failure of the detachable balloon technique may follow prolapse, migration, deflation or rupture of the device, resulting in carotid artery occlusion or distal thromboembolic episodes 16. In such cases, direct surgical repair of the fistula may be attempted27. Late adverse events of endovascular treatment of CCF include formation of false aneurysm or fistula re-formation 17.
Covered stents have been successfully used in the management of aortic, coronary and peripheral vessel pathologies such as arteriovenous fistulas, aneurysms and ruptures 7,19. They originally had a sandwich-like design to fix a thin polytetrafluoroethylene (PTFE) membrane between two stainless stents. The PTFE membrane is a compatible polymer that can be manufactured in the form of distensible microporous membranes; it is the best available covering material for the stent grafts in terms of patency rates20,22. Later, new self-expanding covered stents (made of Nitinol) arose and their design was refined to extend the membrane up to the stent edges because restenosis was noted to develop at the proximal and distal ends, which were not covered with the membrane 5. In the neurovascular field, covered stents have been employed in the treatment of cervical8,18 and skull base1,21 arterial dissections, pseudoaneurysms and fistulas, as an alternative to the combined use of uncovered devices and trans-stent coil embolization. Application of covered stents for intracranial neurovascular disease is a recently explored treatment option. Thus far, a few cases of intracranial aneurysms 8,4,13,22,25 and fistulas 8,14,23 treated by means of covered stent have been published in the literature. Overall, a successful direct obliteration of a CCF with implantation of a covered stent in the internal carotid artery has been reported in only five cases 8,14.
The use of a covered stent for CCF management, compared with other endovascular materials, potentially carries several advantages, thus appearing an attractive alternative. Unlike balloons, there is no risk of rupture or deflation of the device, while the possibility of pseudoaneurysm formation is virtually eliminated 14,25.
A covered stent provides ready fistula obliteration with preservation of the parent arterial lumen 8,14. Moreover, a lower risk of distal thromboembolic episodes is likely, at least in the early stages, compared to that associated with internal carotid artery sacrifice by means of balloon devices 14. Ultimately, we feel that, from a theoretical standpoint, a covered stent offers a potentially permanent exclusion from the circulation of an arteriovenous fistula, preventing re-formation by a definitive reconstruction of the parent vessel walls. However, the mechanical properties of the currently available covered stents, in terms of longitudinal flexibility and section diameter, imply technical difficulties in their application. Advancement and navigation of the rigid covered stent, originally designed for coronary pathology, into the tortuous intracranial arterial tree may be inherently related to several risks as long as technically demanding maneuvers are required. Moreover, in cases of wide internal carotid arteries, there may be need of further stent expansion by means of angioplasty balloon 8. Local vasospasm following irritation and direct vessel injury are the main concerns in that respect 8,14,22 Nevertheless, as in our case, two recent series of 11 and 24 patients respectively, treated with covered stents for intracranial CCFs and aneurysms, found no evidence of vasospasm or vessel injury8,22. Late stent thrombosis following intimal hyperplastic reaction is also a concern regarding the use of covered stents. Felber et Al8 reported no late occlusions of the devices in nine patients treated by means of covered stents for extracranial and intracranial aneurysms and arteriovenous fistulas, with follow-up ranging from three months to five years. Similarly, in a series of 24 patients with intracranial aneurysms treated with endovascular deployment of covered stent, evidence of minimal intimal hyperplasia was observed in only one patient during a follow-up period of six to 24 months22. Yet, several measures may circumvent these limitations, such as injection of papaverine into the ICA during, and standard antiplatelet drug medication after procedure. Furthermore, case-to-case patient selection based on careful scrutiny of angiographic Pattern and appropriately designed devices suitable for intracranial use will be essential to rationalize indications and the optimize results of this promising endovascular technique. Although the worldwide experience of covered stent employment for the management of CCF is so far very limited, it appears to be an effective alternative, particularly in cases in which other traditional procedures have failed. Stent patency on a long-term basis and associated risk of late ischemic attacks following covered stent placement within the intracranial internal carotid artery are the issues to be determined in the future.
References
- 1.Alexander MJ, Smith TP, Tucci DL. Treatment of an iatrogenic petrous carotid artery pseudoaneurysm with a Symbiot covered stent: Technical case report. Neurosurgery. 2002;50:658–662. doi: 10.1097/00006123-200203000-00047. [DOI] [PubMed] [Google Scholar]
- 2.Archondakis E, Pero G, et al. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. Am J Neuroradiol. 2007;28:342–347. [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow DL, Spector RH, et al. Classification and treatment of spontaneous carotid-cavernous fistulas. J Neurosurg. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 4.Chiaradio JC, Guzman L, et al. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery. Neurosurgery. 2002;50:213–216. doi: 10.1097/00006123-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JE, Savvas G, Gomori JM. Petrous carotid artery pseudoaneurysm in bilateral carotid fibromuscular dysplasia: treatment by means of self-expanding covered stent. Surgical Neurology. 2007;68(2):220. doi: 10.1016/j.surneu.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 6.Debrun GM, Viñuela F, et al. Indications for treatment and classification of 132 carotid cavernous fistulas. Neurosurgery. 1988;22:285–289. doi: 10.1227/00006123-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Elsner M, Auch-Schwelk W, et al. Coronary stent grafts covered by a polytetrafluoroethylene membrane. Am J Cardiol. 1999;84:335–338. doi: 10.1016/s0002-9149(99)00289-1. [DOI] [PubMed] [Google Scholar]
- 8.Felber S, Henkes H, et al. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery. 2004;55:631–639. doi: 10.1227/01.neu.0000134455.02947.1f. [DOI] [PubMed] [Google Scholar]
- 9.Gomez F, Escobar W, et al. Treatment of Carotid Cavernous Fistulas Using Covered Stents: Midterm Results in Seven Patients. Am J Neuroradiol. 2007;28:1762. doi: 10.3174/ajnr.A0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guglielmi G, Viñuela F, et al. Carotid-cavernous fistula caused by a ruptured intracavernous aneurysm: Endovascular treatment by electrothrombosis with detachable coils. Neurosurgery. 1992;31:591–596. doi: 10.1227/00006123-199209000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Halbach VV, Higashida RT, et al. Transvenous embolization of direct carotid cavernous fistulas. Am J Neuroradiol. 1988;9:741–749. [PMC free article] [PubMed] [Google Scholar]
- 12.Halbach VV, Higashida RT, et al. Transarterial platinum coil embolization of carotid cavernous fistula. Am J Neuroradiol. 1991;12:429–433. [PMC free article] [PubMed] [Google Scholar]
- 13.Islak C, Kocer N, et al. Bare stent graft technique: a new method of endoluminal vascular reconstruction for the treatment of giant and fusiform aneurysms. Am J Neuroradiol. 2002;23:1589–1595. [PMC free article] [PubMed] [Google Scholar]
- 14.Kocer N, Kizilkilik O, et al. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. Am J Neuroradiol. 2002;23:442–446. [PMC free article] [PubMed] [Google Scholar]
- 15.La Tessa G, Pasqualetto L, et al. Traumatic carotid cavernous fistula: failure of endovascular treatment with two stent-grafts. Interventional Neuroradiology. 2005;11:369–375. doi: 10.1177/159101990501100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis AI, Tomsick TA, Tew JM., Jr Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36:239–245. doi: 10.1227/00006123-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Lewis AI, Tomsick TA, et al. Long-term results in direct carotid-cavernous fistulas after treatment with detachable balloons. J Neurosurg. 1996;84:400–404. doi: 10.3171/jns.1996.84.3.0400. [DOI] [PubMed] [Google Scholar]
- 18.Marotta TR, Buller C, et al. Autologous veincovered stent repair of a cervical internal carotid artery pseudoaneurysm: Technical case report. Neurosurgery. 1998;42:408–412. doi: 10.1097/00006123-199802000-00138. [DOI] [PubMed] [Google Scholar]
- 19.May J, White G, et al. Transluminal placement of a prosthetic graft-stent device for treatment of subclavian artery aneurysm. J Vasc Surg. 1993;18:1056–1059. [PubMed] [Google Scholar]
- 20.Ming-Hua Li, Bu-Lang Gao, et al. Management of pseudoaneurysm in the intracranial segment of the ICA with covered stents specially designed for use in the intracranial vasculature: technical notes. Neuroradiology. 2006;48:841–846. doi: 10.1007/s00234-006-0127-7. [DOI] [PubMed] [Google Scholar]
- 21.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412–419. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- 22.Saatci I, Cekirge HS, et al. Treatment of internal carotid artery aneurysms with covered stent: experience in 24 patients with mid-term follow-up results. Am J Neuroradiol. 2004;25:1742–1749. [PMC free article] [PubMed] [Google Scholar]
- 23.Sellar R. The use of intra-cranial covered stents to treat a dural fistula. Neurointerventionalist. 2002;3:74–76. [Google Scholar]
- 24.Sencer S, Minareci M, Poyanli A. Treatment of direct carotid cavernous fistula. Am J Neuroradiol. 1999;20:1465–1466. [PMC free article] [PubMed] [Google Scholar]
- 25.Singer RJ, Dake MD, et al. Covered stent placement for neurovascular disease. Am J Neuroradiol. 1996;18:507–509. [PMC free article] [PubMed] [Google Scholar]
- 26.Teng MH, Chang CY, et al. Cavernous fistulas. Am J Neuroradiol. 2000;21:1753–1756. [PMC free article] [PubMed] [Google Scholar]
- 27.Tu Y-K, Liu H-M, Hu S-C. Direct surgery of carotid cavernous fistulae and dural arteriovenous malformations of the cavernous sinus. Neurosurgery. 1997;41:798–806. doi: 10.1097/00006123-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Weber W, Henkes H, et al. Cure of a direct carotid cavernous fistula by endovasculat stent deployment. Cerebrovascular disease. 2001;12:272–275. doi: 10.1159/000047715. [DOI] [PubMed] [Google Scholar]