Summary
The Merci Retrieval System™ (Concentric Medical, Mountain View, CA, USA) was approved in 2004 for restoring blood flow in patients experiencing an acute stroke who are otherwise ineligible for intravenous tPA, or in whom intravenous tPA treatment has failed. However, this treatment results in successful recanalization in less than 60% of treatable vessels (Multi MERCI trial). We describe the use of a novel device, the Solitaire™ AB Neurovascular Remodeling Device (ev3 Inc., Irvine, CA, USA) that received the CE Mark for the treatment of neurovascular disease in 2007 and can recanalize arteries when MERCI has failed.
We describe a case of mechanical thrombectomy in which a new device, Solitaire™ AB, was used for acute cerebral ischemia in the setting of middle cerebral artery occlusion after unsuccessful recanalization with the MERCI® Retriever. Excellent angiographic and good clinical results were obtained without any complication.
The Solitaire™ AB device was able to retrieve and remove clots efficiently from the middle cerebral artery when the MERCI system failed, thereby offering a new alternative in the treatment of acute ischemic stroke.
Key words: thrombectomy, embolectomy, reperfusion, stroke, acute, interventional neuroradiology, MERCI, solitaire AB
Introduction
Several endovascular mechanical techniques for clot removal or lysis have been developed, and some are currently undergoing clinical trials. These include the use of lasers, angioplasty, ultrasonography, microsnares and MERCI which can physically grasp and remove a thrombus from the cerebral circulation1. This report describes the successful mechanical thrombectomy and recanalization of the middle cerebral artery (MCA) using the Solitaire™ AB device (ev3 Inc, Irvine, CA, USA)2 when the MERCI Retriever was unable to retrieve and remove clots in a patient with an acute ischemic stroke of the MCA territory.
Case Report
A 37-year-old Caucasian man was admitted to a primary stroke centre 1.5 hours after the onset of right hemiparesis and aphasia. Physical examination disclosed mute aphasia, homonymous hemianopia, sensory loss of the right face, arm, and leg, right facial paralysis and hemiplegia. The National Institutes of Health Stroke Scale (NIHSS) score was 23. A CT scan showed a hyperdensity of the left MCA without associated ischemic parenchymal changes. A total dose of 90 mg of tPA was administered intravenously without any change in neurological score at the end of the infusion, three hours after symptoms onset. Multimodal MRI performed after tPA infusion showed an infarct of 1/3 territory of the left MCA, perfusion-diffusion mismatch >20%, and occlusion of the left MCA.
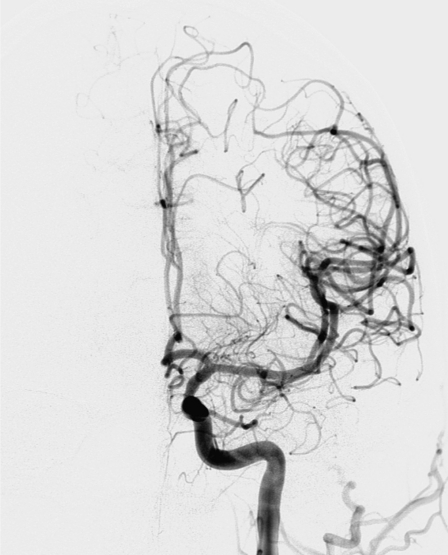
The patient was transferred to our tertiary stroke center for endovascular treatment. A diagnostic cerebral angiography performed five hours after stroke onset revealed a complete occlusion of the distal M1 of the left MCA (Figure 1). Mechanical thrombectomy was recommended for emergent neurovascular rescue. After obtaining informed consent, recanalization of the left MCA was initially attempted using the MERCI L5 Retriever (MC 18L microcatheter and L5 Retriever) with a 9F balloon guide catheter (Merci® Balloon Guide Catheter) (Concentric Medical, Mountain View, CA), according to the methodology described in the MERCI trial3. With the first attempt, the clot was moved proximally and blocked the intracranial ICA, anterior cerebral artery (ACA) and proximal MCA (Figure 3). The second attempt to recanalize with MERCI L5 Retriever was unsuccessful. Then, a Rebar® 18 microcatheter (ev3 Inc, Irvine, CA, USA) and a Synchro 14 microguidewire (Boston Scientific, Natick, MA, USA) were advanced into the intracranial circulation to catheterize the left MCA. With the tip of the microcatheter placed at the M1-M2 junction of the MCA, the guidewire was removed and the Solitaire™ AB device was advanced through the microcatheter and deployed. Once angiography demonstrated flow through the device, the balloon of the guiding catheter was inflated, and the device and the microcatheter were slowly retracted into the balloon catheter under constant aspiration. A thrombus was obtained with a single pass of the Solitaire™ AB (Figures 4, 6). Post-procedure angiography demonstrated successful recanalization of the entire left ICA, ACA and MCA, and reperfusion with normal antegrade flow into the distal MCA branches. There was no evidence of distal emboli (Figure 5). The patient was discharged to the primary stroke center 24 hours later. A control CT showed small areas of infarction within the deep MCA territory. NIHSS score at discharge ten days after stroke onset was 11 and modified Rankin scale score at 90 days was 3.
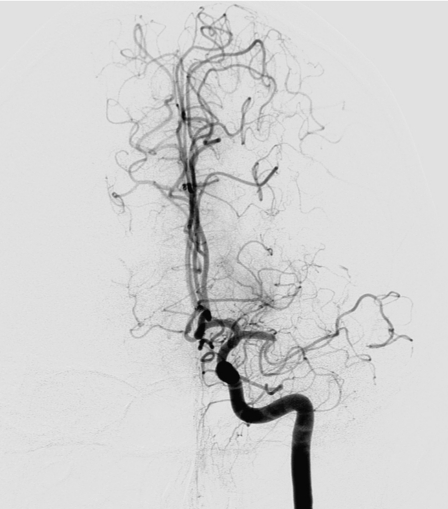
Figure 1.
Diagnostic cerebral angiography revealed a complete occlusion of the left MCA (distal part of M1 and proximal part of M2).
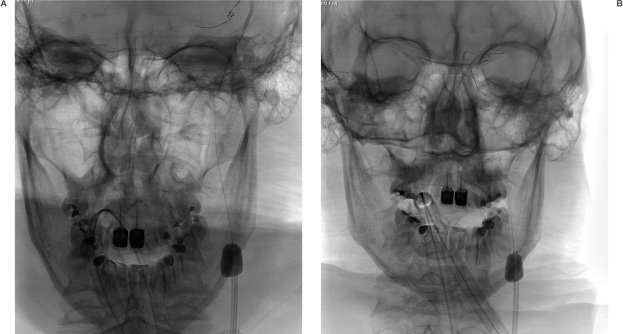
Figure 2.
A,B) First attempt to recanalize the artery with L5 MERCI device (MC 18L microcatheter and L5 Retriever).
Figure 3.
During the first attempt to recanalize the artery, the clot was moved proximally and blocked the anterior cerebral artery (ACA) and the MCA.
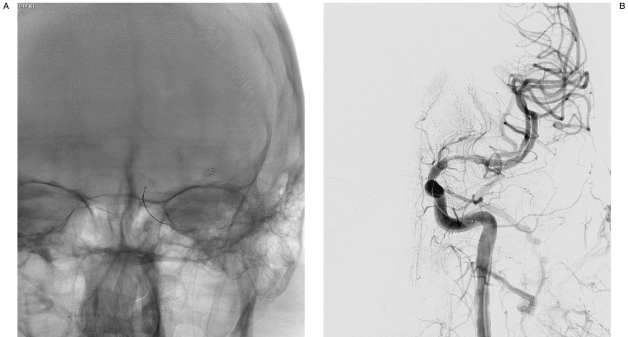
Figure 4.
A,B) The Solitaire™ AB device was deployed (A), and angiography demonstrated flow through the device (B).
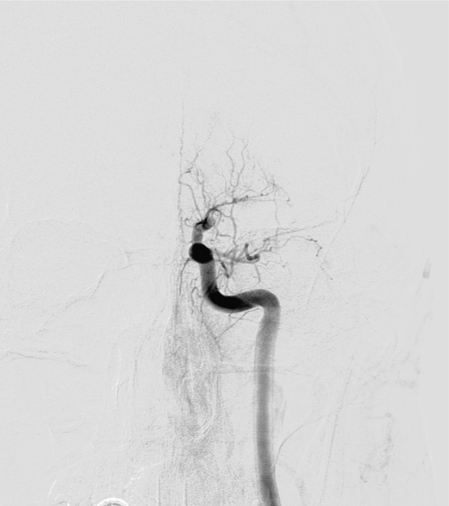
Figure 5.
Postprocedure angiography demonstrated successful recanalization of the entire left ICA, ACA and MCA, and reperfusion with normal antegrade flow into the distal MCA branches, after Solitaire™ AB was used.
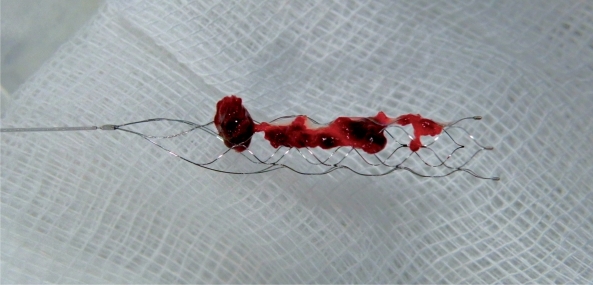
Figure 6.
A thrombus was retrieved with a single pass of the Solitaire™ AB device.
Discussion
We describe the use of the novel Solitaire™ AB Neurovascular Remodeling Device that can recanalize the main cerebral arteries when MERCI has failed. The Solitaire™ AB device unique design is laser-cut from a nitinol plate into a honeycomb pattern that results in a self-expanding, overlap platform that allows for versatility in multiple applications. The delivery system is a stainless steel pushwire and electrolytically detachable using the NDS-2 Solitaire™ Detachment System (ev3 Inc., Irvine, CA, USA). The Solitaire™ AB offers the unique capability of being able to be fully deployed and then completely retrieved if it has not been detached. This system has proven reliable and safe, with no reported failures of detachment or premature detachment2,4. The Solitaire™ AB is available in a variety of diameters and lengths. The Solitaire™ AB Neurovascular Remodeling Device is primarily used for intracranial aneurysm treatment, but its relatively low radial force, high flexibility, thin wall, and ability to be retrieved and repositioned may permit thrombus or foreign body retrieval2.
Like other endovascular techniques such as intra-arterial thrombolysis5 or mechanical thrombectomy6,7,8, Solitaire™ AB might increase the rate of successful recanalization in patients with acute ischemic stroke compared to intravenous tissue plasminogen activator which achieves early recanalization in less than 30% of patients with large vessel occlusions (middle cerebral, basilar artery, and carotid terminus)3,9,10. In fact, the combination of a high NIHSS score and hyperdensity MCA sign observed in our patient implies a large clot burden, usually with a poor response to intravenous tissue plasminogen activator (tPA) 9.
The hypothetical or potential complications of performing mechanical thrombectomy with the Solitaire™ AB include the complications experienced with other devices including dissection or rupture of the artery during the catheterization with the guidewire and the microcatheter. Other potential complications with the Solitaire™ AB include the dissection or perforation of the artery or an atherosclerotic plaque during the deployment and recovery process as well as possible clot fragmentation resulting in distal emboli. To minimize the risk for potential complications, we have these recommendations for the use of Solitaire™ AB for clot retrieval: (1) Conduct the catheterization with the tip of the guidewire bent, (2) begin the deployment of the Solitaire™ AB in a straight segment of the artery and avoid starting the deployment on a curve of the artery, and (3) when recovering the device, the withdrawal should be soft, firm, and continuous.
Due to our limited experience, we do not have a proven technique that maximizes efficacy. We recommend deploying Solitaire™ AB within the thrombus with the distal end of the device placed distal to the thrombus. We recommend leaving the device deployed for a few minutes, to permit rapid oxygenation of the brain and facilitate the thrombus "gripping" in the mesh of the device, and letting the natural factors of thrombolysis circulate across the affected artery and branches. Upon deployment of the Solitaire™ AB we recommend obtaining an angiogram to verify the reperfusion and detect any distal embolus. During recovery of the device, we recommend temporary occlusion of the carotid artery with a balloon guide catheter, and then under continuous aspiration through the guiding catheter, gently withdraw the device and the microcatheter simultaneously, without resheathing the device. Some clinicians attempt to resheath the device to better trap the thrombus. We recommend caution when the Solitaire AB enters the balloon guiding catheter to minimize the possibility of "squeezing" the thrombus off the device causing emboli.
An advantage that Solitaire™ AB has over other devices is the ability to be used as a stent when an artery cannot be opened due to adherent thrombus or an atherosclerotic lesion to recanalize the artery. It can be useful in some patients with good collateral circulation that may preserve the viability of brain parenchyma distal to an intracranial arterial occlusion for hours or days after the presenting event11.
If the Solitaire™ AB is used as a clot retriever, we do not believe it is necessary to administer anti-platelet or anti-thrombotic agents during and after the procedure. If the Solitaire™ AB is left as an implant, it will be necessary to administer an anti-platelet regime. At the Hospital Universitari Germans Trias i Pujol , we use a dose of clopidogrel 3x75 mg by nasogastric probe, and 900 mg of intravenous lysine acetyl salicylate (inyesprin®) during the procedure, and continue post-procedure with aspirin 325 mg/day and clopidogrel 75 mg/day for three months and continue with aspirin thereafter.
The introduction of a new device, Solitaire™ AB, that is able to remove emboli from the intracranial circulation when the MERCI retriever has failed, provides a novel tool for rapid revascularization of ischemic brain tissue with the potential for excellent clinical outcomes. In our case, complete thrombus removal was achieved in only a few minutes with a single pass of the Solitaire™ AB used as a clot retriever. Plainly our report must be confirmed by larger series and controlled studies.
Acknowledgment
The authors acknowledge Ms. Rosa Garcia, interventional neuroradiology nurse, for her excellent work and cooperation in our daily practice.
References
- 1.Martinez H, Zoarski GH, et al. Mechanical thrombectomy of the internal carotid artery and middle cerebral arteries for acute stroke by using the retriever device. Am J Neuroradiol. 2004;25:1812–1815. [PMC free article] [PubMed] [Google Scholar]
- 2.Henkes H, Flesser A, et al. A novel microcatheter-delivered, highly-flexible and fully-retrievable stent, specifically designed for intracranial use. Interventional Neuroradiology. 2003;9:391–393. doi: 10.1177/159101990300900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith WS for the Multi MERCI Investigators. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, Part I. Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 4.Henkes H, Drepper P. Technical note on VDS. A system for the endovascular electrolytical detachment of platinum coils at variable length. Interventional Neuroradiology. 2002;8:197–200. doi: 10.1177/159101990200800212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 6.Gobin YP, Starkman S, et al. MERCI I: a phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke. 2004;35:2848–2854. doi: 10.1161/01.STR.0000147718.12954.60. [DOI] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, et al. for the MERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1440. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 8.Smith WS, Sung G, et al. Mechanical thrombectomy for acute ischemic stroke final results of the multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 9.Del Zoppo GJ, Poeck K, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 10.The National Institute of Neurological Disorders and Stroke rtPA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Eng J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 11.Kelly ME, Turner RD, et al. Revascularization of symptomatic subacute cerebrovascular occlusions with a self-expanding intracranial stent system. Neurosurgery. 2009;64(1):72–78. doi: 10.1227/01.NEU.0000334049.12472.B7. [DOI] [PubMed] [Google Scholar]
- 12.IMS trial investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the interventional management of stroke study. Stroke. 2004;35:904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]