Abstract
BACKGROUND:
Factors associated with asthma remission need to be determined, particularly when remission occurs in adulthood.
OBJECTIVE:
To evaluate airway responsiveness and inflammation in adult patients in asthma remission compared with adults with mild, persistent symptomatic asthma.
METHODS:
Adenosine monophosphate and methacholine responsiveness were evaluated in 26 patients in complete remission of asthma, 16 patients in symptomatic remission of asthma, 29 mild asthmatic patients and 15 healthy controls. Blood sampling and induced sputum were also obtained to measure inflammatory parameters.
RESULTS:
Perception of breathlessness at 20% fall in forced expiratory volume in 1 s was similar among groups. In subjects with symptomatic remission of asthma, responsiveness to adenosine monophosphate and methacholine was intermediate between mild asthma and complete asthma remission, with the latter group similar to controls. Asthma remission was associated with a shorter duration of disease. Blood immunoglobulin E levels were significantly increased in the asthma group, and blood eosinophils were significantly elevated in the complete asthma remission, symptomatic remission and asthma groups compared with controls. The suppressive function of regulatory T cells was lower in asthma and remission groups compared with controls.
CONCLUSION:
A continuum of asthma remission was observed, with patients in complete asthma remission presenting features similar to controls, while patients in symptomatic asthma remission appeared to be in an intermediate state between complete asthma remission and symptomatic asthma. Remission was associated with a shorter disease duration. Despite remission of asthma, a decreased suppressor function of regulatory T cells was observed, which may predispose patients to future recurrence of the disease.
Keywords: Airway inflammation, AMP responsiveness, Asthma, Asthma remission, Methacholine responsiveness, Regulatory T cells
Abstract
HISTORIQUE :
Il faut déterminer les facteurs associés à la rémission de l’asthme, notamment lorsque la rémission se produit à l’âge adulte.
OBJECTIF :
Évaluer la réactivité et l’inflammation des voies aériennes chez des patients adultes en rémission de l’asthme par rapport à celles d’adultes ayant un asthme symptomatique bénin et persistant.
MÉTHODOLOGIE :
Les chercheurs ont évalué la réactivité au monophosphate d’adénosine et à la méthacholine chez 26 patients en rémission complète de l’asthme, chez 16 patients en rémission symptomatique de l’asthme, chez 29 patients ayant un asthme bénin et chez 15 sujets témoins en santé. Ils ont également obtenu des échantillons de sang et des expectorations induites pour mesurer les paramètres inflammatoires.
RÉSULTATS :
La perception d’essoufflement après une diminution de 20 % du volume expiratoire maximal par seconde était similaire entre les groupes. Chez les sujets en rémission symptomatique de l’asthme, la réactivité au monophosphate d’adénosine et à la méthacholine se situait à michemin entre l’asthme bénin et la rémission complète de l’asthme, le dernier groupe ayant des résultats semblables à ceux des sujets témoins. La rémission de l’asthme s’associait à une maladie de plus courte durée. Les taux sanguins d’immunoglobuline E augmentaient considérablement dans le groupe atteint d’asthme, et les éosinophiles sanguins étaient très élevés dans les groupes de rémission complète de l’asthme, de rémission symptomatique et d’asthme par rapport aux sujets témoins. La fonction suppressive des lymphocytes T régulateurs était plus faible dans les groupes atteints d’asthme et en rémission que chez les sujets témoins.
CONCLUSION :
Les chercheurs ont observé un continuum de rémission d’asthme, les patients en rémission complète de l’asthme présentant des caractéristiques similaires à celles des sujets témoins, tandis que les patients en rémission symptomatique de l’asthme semblaient se trouver dans un état intermédiaire entre la rémission complète de l’asthme et l’asthme symptomatique. La rémission s’associait à une maladie de plus courte durée. Malgré la rémission de l’asthme, les chercheurs ont observé une diminution de la fonction suppressive des lymphocytes T régulateurs, qui pourrait prédisposer les patients à une future récurrence de la maladie.
Asthma is characterized by variable airway obstruction and hyper-responsiveness (AHR), which is attributed to airway inflammation and structural changes (1,2). We and others have provided evidence that airway inflammation and remodelling begin many years before the onset of asthma symptoms; however, once asthma has been diagnosed, it is considered to persist for life in the majority of patients (3–5).
Although relatively rare in adults, some patients may ‘grow out’ of asthma and, will therefore, be considered to be in asthma remission. There is evidence that even if symptoms have remitted in former mild-to-moderate asthmatic patients, residual airway inflammation on bronchoalveolar lavage or bronchial biopsies (6–8) can be observed in many subjects. Van den Toorn LM et al (7,8) and Warke et al (9) demonstrated persistant subepithelial fibrosis in subjects with symptomatic remission of asthma compared with healthy controls.
The mechanisms involved in symptomatic or complete remission of asthma, particularly in adulthood, are not well understood and need further investigation. The study of this phenomenon could help in determining how to possibly induce the remission of asthma. There is, therefore, a need to characterize these patients in so-called ‘asthma remission’ to determine the factors associated with remission and with recurrence of asthma in adulthood. In the present study, we compared pulmonary function, airway response to methacholine (MIT) and adenosine monophosphate (AMP), perception of respiratory symptoms, immunoglobulin (Ig) E levels, airway inflammation on induced sputum, and T cell function in patients in symptomatic or complete asthma remission and mild asthmatic patients.
METHODS
Subjects
Patients were recruited from advertisements and through the asthma clinic database. Twenty-six consecutive asthmatic subjects in complete remission (with a provocative concentration of methacholine inducing a 20% fall in forced expiratory volume in 1 s [PC20FEV1]) >16 mg/mL, having no asthma symptoms and not having used asthma medication for more than two years) (10), with a history of asthma confirmed by a physician diagnosis and follow-up, in addition to previous asthma symptoms and asthma medication use, were enrolled (Table 1). Six patients also had a positive methacholine test during their asthma period dating back to before the occurrence of remission. Furthermore, 16 asthmatic subjects in symptomatic remission for more than two years, but still presenting hyper-reactive airways (11,12), 29 subjects with mild asthma not using anti-inflammatory agents and 15 healthy controls with normal airway responsiveness (PC20 >16 mg/mL) were studied.
TABLE 1.
Characteristics of subjects in complete asthma remission during asthma period
| Subject | Asthma period |
Physician |
Asthma symptoms | Asthma medication | PC20 methacholine†, mg/mL | Emergency room visit for asthma | |
|---|---|---|---|---|---|---|---|
| Diagnosis | Follow–up* | ||||||
| 1 | 1987–2001 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | 7.0 | |
| 2 | 1988–1993 | Yes | Yes | B,CT,W,C | Bd | ||
| 3 | 1999–2002 | Yes | Yes | B | Bd | ||
| 4 | 1963–1970 | Yes | Yes | B, CT, W, C, Ph | Bd | ||
| 5 | 1988–2005 | Yes | Yes | B, CT, W, C | Bd, ICS | ||
| 6 | 1996–2000 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | ||
| 7 | 2003–2005 | Yes | Yes | B, W | Bd, ICS | 1.6 | |
| 8 | 1988–1995 | Yes | Yes | B | Bd | ||
| 9 | 2000–2006 | Yes | Yes | B, C | Bd, ICS | 1.0 | |
| 10 | 1999–2004 | Yes | Yes | B, C | Bd | 4.8 | |
| 11 | 2000–2005 | Yes | Yes | B, CT | Bd | 4.0 | |
| 12 | 1983–1987 | Yes | Yes | B, CT, W | Bd | Yes | |
| 13 | 1995–2006 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | ||
| 14 | 1972–1976 | Yes | Yes | B, C | Bd | ||
| 15 | 1996–2007 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | ||
| 16 | 1956–1960 | Yes | Yes | CT, C, Ph | ? | ||
| 17 | 1989–2004 | Yes | Yes | B, W, C | Bd, ICS | ||
| 18 | 1992–2001 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | ||
| 19 | 1996–2006 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | ||
| 20 | 1991–1994 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | ||
| 21 | 1981–1996 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | Yes | |
| 22 | 1966–1972 | Yes | Yes | B, CT, W | Bd | ||
| 23 | 1969–1971 | Yes | Yes | B | Bd | ||
| 24 | 2003–2006 | Yes | Yes | B, CT, W, C, Ph | Bd, ICS | 5.8 | |
| 25 | 1994–2005 | Yes | Yes | B, CT, C, Ph | Bd, ICS | ||
| 26 | 1999–2000 | Yes | Yes | B, CT, W | Bd | ||
Patients were seen regularly by physician during asthma period;
When available. B Breathlessness; Bd Inhaled bronchodilator; CT Chest tightness; C Cough; ICS Inhaled corticosteroids; PC20 Provocative concentration of methacholine causing a 20% fall in forced expiratory volume in 1 s; Ph Phlegm production; W Wheezing
All subjects provided informed written consent; the protocol was reviewed by the institutional ethics committee (Clinical Trial registration number: NCT 00526019).
The primary end point of the study was airway inflammation, assessed by airway responsiveness to AMP, and induced sputum eosinophil and neutrophil counts. Secondary end points were the following: airway response to methacholine (in symptomatic remission and current mild asthma compared with normal controls) and baseline FEV1, blood IgE levels and blood eosinophils, diurnal variation in peak expiratory flows (PEF) and the profile of peripheral blood regulatory T cells (Tregs).
Procedures
All patients visited the laboratory at study entry and one week later. They completed a respiratory questionnaire based on the European Community Respiratory Health Survey Questionnaire (13). Patients underwent physical examination, skin prick tests, baseline spirometry and a methacholine challenge according to American Thoracic Society criteria (14) using the method described by Juniper et al (15), with additional measures of modified Borg perception scores for breathlessness and chest tightness. Complete blood count and serum IgE levels were performed on blood samples. Sputum was induced using the method described by Pin et al (16) and modified by Pizzichini et al (17) by inhalation of hypertonic saline.
On the second visit, after baseline spirometry, an AMP inhalation test was performed using the 2 min tidal breathing method described by Cockcroft et al (18) for histamine and applied to AMP by Oosterhoff et al (19).
Induced sputum processing
Sputum was processed within 2 h of induction. Briefly, mucus plugs were selected from saliva and treated with four times their volume of dithiothreitol. An equal volume of 1×Dulbecco’s phosphate-buffered saline was then added and the suspension was filtered. Total cell count and viability were determined using the trypan blue exclusion method. Slides were prepared and stained with Diff-Quik (Dade Diagnostics Inc, USA) for differential cell count.
Regulatory T cell isolation
To study regulatory T cell function, eight healthy nonatopic controls, nine subjects with current mild asthma and nine asthmatic subjects in complete remission of asthma underwent peripheral blood sampling (150 mL) out of the allergy period. Peripheral blood mononuclear cells were isolated using lymphocyte separation media (Wisent, St-Bruno, Canada) density gradient centrifugation. Monocytes were separated from total lymphocytes by adhesion for 1 h at 37°C. CD4+CD25− and CD4+CD25+CD127− cells were isolated using a commercially available CD4+ T cell isolation Kit II and CD4+CD25+CD127dim/− Regulatory T cell Isolation Kit according to the manufacturer’s instructions (Miltenyi Biotec, USA). The purity of the CD4+CD25highFoxp3+CD127− cells was >90%.
Suppression assays
Lyophilized CellTrace (Invitrogen, Canada) carboxyfluorescein diacetate succinimidyl ester (CFSE) was diluted in dimethyl sulfoxide immediately before use (5 mM working solution). Freshly isolated CD4+CD25− cells were resuspended in phosphate-buffered saline/0.1% bovine serum albumin at 1×106 cells/mL, and 1 μl/mL of CFSE stock solution was added yielding a final concentration of 5 μM. Labelling was performed according to the manufacturer’s protocol. For the proliferation assay, 96-well round bottom plates (Corning Life Sciences, USA) were coated with 10 μg/mL anti-CD3 antibody (eBio-sciences, USA). CFSE-labelled CD4+CD25− T cells were cultured at 2×104 cells/well with 4×104 irradiated autologous monocytes. Unlabelled CD4+CD25high CD127low regulatory T cells at different ratios (1:1 or 1:0.5) were added to CFSE-labelled CD4+CD25− T cells. After five days in culture, cells were harvested, washed and analyzed by flow cytometry for fluorescence signal on a Coulter EPICS XL-MCL flow cytometer using Expo32 software (Beckman Coulter, Canada).
Statistical analysis
Results were expressed as mean ± SD, geometric means for PC20 methacholine and AMP, or percentages according to the distribution of data. PC20 values for methacholine and AMP were log transformed for analysis. For statistical comparisons, a PC20 >16 mg/mL was considered to be equal to 16 mg/mL. The subjects’ demographic characteristics were analyzed using Fisher’s exact test or one-way ANOVA for categorical and qualitative data, respectively. Posteriori comparisons were performed using Tukey’s multiple comparison test. Relationships between pulmonary function parameters and airway inflammation (sputum eosinophils) were measured using Pearson’s or Spearman’s correlation coefficients. The univariate normality assumption was verified with the Shapiro-Wilk test. The Brown and Forsythe’s variation of Levene’s test statistic were used to verify the homogeneity of variances. P≤0.05 was considered to be statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute Inc, USA).
Results
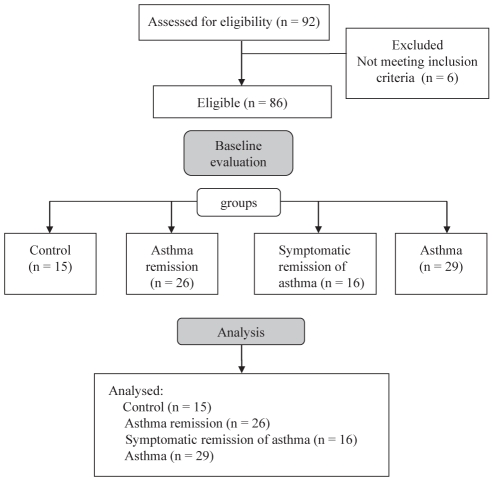
Of the 92 subjects assessed for eligibility, six did not meet the inclusion criteria. A study flow diagram is presented in Figure 1.
Figure 1).
Study flow diagram
Table 2 summarizes the demographic characteristics of the subjects at study enrollment. Atopy was more prevalent in patients with previous or current asthma diagnosis than in controls. Family history of asthma and/or allergy or their absence did not appear to be a predictor of asthma remission nor of symptomatic remission (P=0.31).
TABLE 2.
Demographic characteristics
| Control (n=15) |
Asthma group |
P* | |||
|---|---|---|---|---|---|
| Remission (n=26) | Symptomatic remission (n=16) | Asthma (n=29) | |||
| Age, years, mean ± SD | 31±12 | 33±11 | 37±17 | 32±11 | 0.46 |
| Sex, M/F | 5/10 | 11/15 | 6/10 | 11/18 | 0.72/0.97 |
| Atopy | 6 | 21 | 11 | 27 | 0.0002 |
| Family history | 0.31 | ||||
| Asthma and allergy | 2 | 5 | 2 | 6 | |
| Asthma only | 1 | 0 | 2 | 2 | |
| Allergy only | 6 | 17 | 11 | 19 | |
| No asthma, no allergy | 6 | 4 | 1 | 2 | |
| Smoking habit, NS/ex/S | 12/3/0 | 24/1/1 | 12/4/0 | 25/5/1 | 0.37 |
| Asthma medication | – | – | – | Bd (prn) | |
Data presented as n or n/n, unless otherwise indicated.
χ2 or Fisher’s exact test categorical data, ANOVA for quantitative data. BD Bronchodilator; ex Exsmokers; NS Never smokers; prn As needed; S Smokers
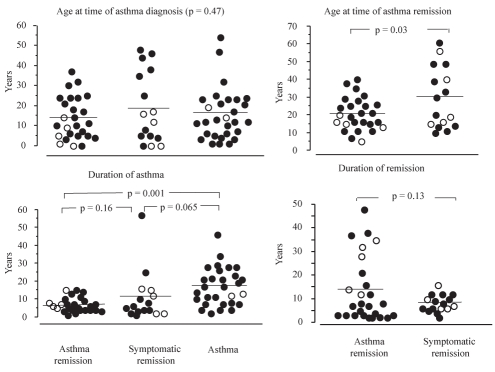
The age at time of asthma diagnosis was not significantly different among the groups (P=0.47 [Figure 2]). Patients in the complete remission group were generally younger than those of the symptomatic remission group at time of asthma remission (P=0.03). The duration of asthma was not significantly different between asthma remission and symptomatic remission groups.
Figure 2).
Characteristics of asthma and remission. Age at time of diagnosis, duration of asthma, age at time of asthma remission and duration of remission are compared among groups. Closed circles represent atopic patients, open circles represent nonatopic patients
When questionned about the circumstances surrounding asthma remission and the possible reasons why they became asymptomatic, patients (complete remission, n/symptomatic remission, n) mentioned the following:
Environmental improvement by better control of environment and/ or reduced exposure to allergens (5/6);
Increased time allocation for sports and/or training (5/5);
Less intensive sports or less sport in arenas (2/1);
Smoking cessation (1/0);
Puberty (1/0);
Important weight loss following bariatric surgery (1/0);
Desensitizing vaccines (1/0); and
Stress reduction after retirement (1/0).
Finally, 11 patients in complete remission and eight in symptomatic remission could not identify any possible reasons for the origin of their remission.
MIT and airway response to AMP
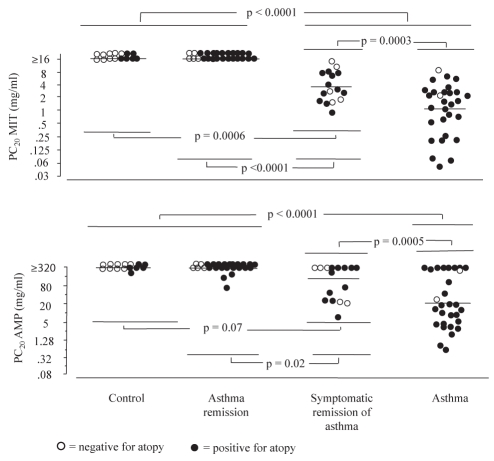
The mean MIT was similar in control and asthma remission groups (both PC20 MIT >16 mg/mL [inclusion criterion]). Patients in the asthma group demonstrated significant hyper-responsiveness (geometric mean 1.1mg/mL; P<0.0001) compared with control and asthma remission groups (Figure 3). The symptomatic remission of asthma group (3.5 mg/mL) was significantly different from the control (P=0.0006) and the complete remission groups (P<0.0001), and from the asthma group (P=0.0003).
Figure 3).
Airway responsiveness to methacholine (MIT) and adenosine monophosphate (AMP). Airway response to challenges was not significantly different within groups. Patients in symptomatic asthma remission were significantly less responsive to MIT and AMP than asthma patients, and significantly more responsive to both challenges than patients in complete asthma remission, whose responsiveness was similar to that of controls. Bar represents mean. PC20 Provocative response to methacholine causing a 20% fall in forced expiratory volume in 1 s
Mean airway responsiveness to AMP, expressed as the PC20 AMP (the provocative dose inducing a 20% fall in FEV1), was similar in control (312 mg/mL) and asthma remission groups (287 mg/mL); patients in the asthma group demonstrated airway hyper-responsiveness, with a mean PC20 AMP of 25 mg/mL (P<0.0001 compared with remission and control groups). The symptomatic remission of asthma group had a mean PC20 of 106 mg/mL, significantly different from the asthma (P=0.0005) and the complete remission groups (P=0.02), but not statistically different from the control group (P=0.07) (Figure 3).
Borg scores for the perception of respiratory symptoms for patients who had a 20% fall of FEV1 at PC20 AMP were not significantly different in the groups with symptomatic remission of asthma and asthma for breathlesness (median [95% CI]: symptomatic remission: 0 [0 to 1.8]) and asthma: 0.5 (0.5 to 1.8); P=0.28) and chest tightness (respectively, 1.0 [0 to 2.7] and 1.0 [0.6 to 2.6]; P=0.81).
Correlation of airway response to AMP and MIT
MIT was correlated to AMP response in patients with asthma (r=0.6; P=0.001) and symptomatic remission (r=0.67; P<0.0001). Correlations for patients in complete remission and for controls could not be calculated due to the inclusion criteria of a PC20 methacholine >16 mg/mL.
FEV1 and FVC
Baseline FEV1 and FVC, and FEV1/FVC ratio before methacholine inhalation tests varied slightly but not significantly among the groups (Table 3). Borg scores for perception of respiratory symptoms at 20% fall of FEV1 on methacholine inhalation test were not significantly different in the groups in symptomatic remission of asthma and asthma for breathlesness (median [95% CI]: symptomatic remission: 0 (0 to 1.6) and asthma: 0.5 (0.5 to 1.7; P=0.27) and chest tightness (0.5 [0 to 2.0] and 1.2 [0.8 to 2.1], respectively; P=0.65).
TABLE 3.
Expiratory flow and volume data
| Controls |
Asthma group |
|||
|---|---|---|---|---|
| Asthma remission | Symptomatic remission | Asthma | ||
| FEV1, % predicted* | 103±15 | 104±13 | 95±13 | 95±13 |
| FVC, % predicted* | 108±13 | 112±14 | 109±13 | 106±11 |
| FEV1/FVC* ratio | 0.80±0.10 | 0.78±0.05 | 0.74±0.09 | 0.75±0.08 |
| AM PEF, % predicted† | 93 (84–103) | 94 (85–103) | 85 (77–94) | 86 (78–94) |
| Δ AM PEF, %† | 5 (3–9) | 7 (5–8) | 7 (2–12) | 11 (3–19) |
| Δ PM PEF, %† | 6 (2–9) | 6 (5–8) | 7 (3–12) | 11 (3–20) |
Data presented as mean ± SD;
Data presented as mean (95% CI). Δ Daily variations in morning (AM) or afternoon/evening (PM) peak expiratory flow (PEF) over a period of one week; FEV1 Forced expiratory volume in 1 s; FVC Forced vital capacity
Diurnal variation in PEF
Daily measurements of morning (AM) and afternooon (PM) PEF over one week are summarized in Table 3. The magnitude of variations of AM and PM PEF was not significantly different between groups (P=0.54 and P=0.48, respectively).
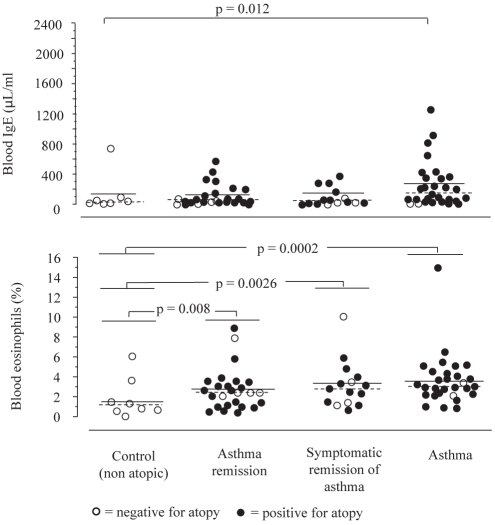
Blood IgE and eosinophils
Blood IgE levels were significantly different between control (median: 19.0 μL/mL) and asthma (166.5 μL/mL) groups (P=0.012 [Figure 4]). Blood IgE levels in the asthma remission (55.0 μL/mL) and symptomatic remission (45.0 μL/mL) groups were not significantly different from control or asthma groups. Besides, nonatopic controls had significantly less blood eosinophils (median 1.0%) than subjects of the remission (median 2.4%; P=0.008), symptomatic remission (median 2.8%; P=0.0026) and asthma (median 3.0%; P=0.0002) groups (Figure 4).
Figure 4).
Blood immunoglobulin E (IgE) and blood eosinophils. There were significantly higher levels of blood IgE in the asthma group than in the control group; no significant differences were found among the other groups. Nonatopic controls had fewer blood eosinophils than patients of the remission, symptomatic remission or asthma groups. Bar represents mean, dashed bar represents median
Inflammatory cells in induced sputum
Evaluation of inflammation was performed using induced sputum. Total cell number was not significantly different among the groups (ie, P>0.05, [Table 4]). There were no significant differences in neutrophil or eosinophil percentages among the groups.
TABLE 4.
Inflammatory cells in sputum
| Controls (nonatopic) |
Asthma group |
|||
|---|---|---|---|---|
| Remission | Symptomatic remission | Asthma | ||
| Total cell count, ×106/g* | 2.1 (1.7–11.5) | 3.2 (0.5–5.2) | 2.9 (1.4–6.5) | 2.4 (0.6–4.9) |
| Neutrophils, %* | 8.0 (4–13) | 23.5 (1–55) | 27.6 (2–31) | 23.8 (4–57) |
| Eosinophils, %* | 0 (0–9) | 0.5 (0–8) | 1.1 (0–4) | 2.5 (0–14) |
Data presented as median (minimum-maximum).
P>0.05
Correlation with airway response to AMP
The patients in the symptomatic remission and current mild asthma groups who had negative AMP challenges had lower blood IgE levels and lower blood and sputum eosinophils; PC20 AMP correlated with blood IgE levels (r=−0.347; P<0.0001), blood eosinophils (r=−0.27; P=0.002) and sputum eosinophils (r=−0.58; P<0.0001).
Treg function
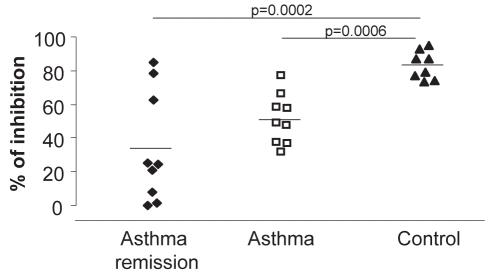
There were no significant differences in the percentage of CD4+CD25highFoxp3+CD127− cells among controls, asthma and complete asthma remission groups (9.1±1.8%, 7.8±0.6% and 5.2±0.6%, respectively). The suppressive function of Tregs was analysed by co-culture of these Tregs with CD4+CD25− effector cells. The capacity of Tregs to suppress proliferation of effector T cells was higher in healthy controls (83.1±3.0% of inhibition) than in the asthma group (51.3±4.9%; P=0.0006) or the complete remission of asthma group (34.0±10.9%; P=0.0002). There were no differences in the suppressive function of Tregs between the complete asthma remission and asthma group (Figure 5).
Figure 5).
The suppressive function of regulatory T cells was higher in healthy controls than in asthmatic subjects (P=0.0006) and in complete remission subjects (P=0.002). There was no difference between the suppressive function of regulatory T cells in the asthma and complete asthma remission groups
DISCUSSION
In the present study, patients in asthma remission presented features similar to controls with normal airway hyper-responsiveness. Remission was associated with shorter disease duration. Patients in symptomatic asthma remission appeared to be in an intermediate state between complete remission of asthma and symptomatic asthma.
Asthma remission may be observed in adolescence, but occurs less frequently in adulthood. There appears to be a continuum of remission, with patients having no symptoms but still evidence of airway hyper-responsiveness or inflammation. In this regard, persistent airway obstruction, increased PEF variability and AHR to methacholine or cold air have been observed during symptomatic asthma remission (20–24).
Sears et al (25) investigated factors predicting persistence or relapse of wheezing in a New Zealand birth cohort, and showed that sensitization to house dust mites, airway hyper-responsiveness, female sex, smoking, and early age at onset were factors involved in the persistence of wheezing or relapse. Furthermore, Taylor et al (26) reported data from the latter cohort, showing that one of three asthmatic children was considered to be in remission at 18 years of age, although relapses were not easily predicted. Other studies showed that some children may outgrow their asthma at puberty (27,28), but 30% to 80% redevelop symptomatic asthma in adulthood (28–32). Regarding the factors associated with a recurrence of asthma symptoms, development of chronic airflow limitation in childhood and persistent airway inflammation have been proposed (6–8,33,34). In the present study, airway inflammation evaluated by induced sputum showed no significant difference between asthma and symptomatic or complete asthma remission. This is consistent with the study by Van den Toorn et al (35), who found that residual inflammation exists in asthma remission. There is evidence that even if symptoms have remitted in former mild-to-moderate asthmatic patients, residual airway inflammation often persists. Pumputiene et al (6) reported increased airway T cell and eosinophil activity (assessed by serum interleukin-2 and eosinophil cationic protein levels) in atopic and nonatopic children despite the absence of asthma symptoms. Warke et al (9) showed that eosinophil numbers were raised in the bronchoalveolar lavage fluid of atopic children who had apparently outgrown their asthma compared with healthy controls. However, there was no relationship between duration of remission and degree of airway eosinophilia, but the authors suggested that the latter could be a risk factor for future relapses (9). Although there were a slightly higher number of inflammatory cells in asthmatic subjects in our study, globally, the number of these cells was not different among the groups. This was also shown in a recent study by Broekema et al (36), in which asthma remission and asthma without an accelerated decline in lung function demonstrated a similar sputum inflammatory profile.
Tregs contribute to the maintenance of immunological tolerance in the airways, evidenced by impaired function of these cells in asthma and allergy (37). Thus, peripheral blood CD4+CD25+ T cells derived from atopic donors were shown to be suppressive in allergen-stimulated cultures. This suppressive activity was similar to healthy controls. Ling et al (38) showed that FOXP3 Treg cells were defective in atopic individuals. This defect was particularly marked during seasonal pollen exposure. Verhagen et al (39) reported an absence of CD4+CD25+FOXP3+ cells in allergic dermatitis, suggesting a dysregulated control of inflammation. We evaluated the function of these cells in a subgroup of subjects in complete asthma remission and compared them with asthmatic patients and controls. Although the number of Tregs was similar in all groups, we found that subjects in complete remission still had decreased suppressive function of Tregs. In asthmatic subjects, this is a feature that may indicate an increased risk of future recurrence of the disease.
To characterize subjects in asthma remission, Vonk et al (40) reported outcomes in a cohort of 119 allergic asthmatic children initially evaluated at five to 14 years of age and followed for a mean of 30 years. In this group, complete remission of asthma was present only in a small subset of asthmatic patients, while one-half of the subjects showed symptomatic remission. A higher FEV1 in childhood and improvement in FEV1 over the years were associated with both complete and symptomatic asthma remission when older (11,40).
Panhuysen et al (41) also investigated the outcome of asthma in 181 patients 13 to 44 years of age. When retested 25 years later, 40% reported no respiratory symptoms, 25% showed an FEV1 >90% predicted while 21% showed no AHR. Complete remission of asthma after 25 years was associated with younger age and less severe airway obstruction when compared with the initial evaluation. Absence of AHR was associated with younger age, a higher FEV1, a shorter untreated period between onset of asthma symptoms and treatment from the first visit, and a lower total serum IgE level. Neither sex nor atopy were significant determinants of the outcome for both asthma and AHR. Results of the present study support the hypothesis that milder disease and earlier intervention are associated with a beneficial outcome (41). Finally, in a study by De Marco et al (42) involving 18,156 subjects zero to 44 years of age who attended the clinical stage of the European Community Respiratory Health Survey, family history of asthma or allergy was associated with a reduced possibility of remission throughout life, while early contact with older children was associated with an increased possibility.
Indirect challenges may more closely reflect acute changes in airway inflammation (43–46). Agents such as adenosine 5′-monophosphate (AMP) act through the activation of bronchial mast cells; thus, 5′AMP could better reflect airway inflammation than methacholine response (42). In the present study, airway responsiveness to AMP decreased progressively from asthma to asymptomatic AHR, and even further in the complete remission of asthma group, with the latter group exhibiting an AMP response similar to controls.
We do not know whether patients in symptomatic or complete asthma remission who participated in the present study will experience a recurrence of the disease. They had many features similar to controls: baseline FEV1, and FVC and FEV1/FVC ratio, low PEF variations, and similar levels of blood IgE and blood eosinophils. Family history of asthma and/or allergy was similar in all groups. However, although there were few indications of airway inflammation, atopy, which is suspected to be a factor predisposing to asthma recurrence (47), was more prevalent in the remission groups than in the controls. Moreover, evaluation of Treg function in the complete asthma remission group showed that suppressive function of these cells was reduced and was comparable with the symptomatic asthma group, suggesting that the mechanism of immune regulation in these patients is still abnormal and may predispose to disease recurrence.
Poor perception of symptoms has been implicated in asymptomatic subjects with AHR, although this is an unlikely explanation (48). However, perception during symptomatic asthma remission appears to be different. Van den Toorn et al (8) investigated perception scores of dyspnea after a 20% fall in FEV1 during methacholine and AMP-induced bronchoconstriction challenges, and concluded that they were not different between subjects in symptomatic remission and subjects with symptomatic asthma (as in our study), thereby making blunted dyspnea perception an unlikely explanation for the stated discrepancy.
We previously observed that subjects with asymptomatic AHR had a lesser degree of airway inflammation and remodelling on bronchial biopsies compared with symptomatic asthma subjects – features that became similar to patients with asymptomatic asthma when they subsequently developed symptomatic asthma (3). These data suggest that combined increases in airway inflammation and remodelling may reach a threshold supporting the development of respiratory symptoms. Consequently, the opposite may be true in asthma remission, with asthma symptoms decreasing with reduced airway inflammation and remodelling.
CONCLUSION
We showed that asthma remission presents as a continuum, some previously asthmatic subjects being in asymptomatic remission but showing a persistence of both AHR and airway inflammation while others (so-called ‘complete asthma remission’) have neither. However, even in complete remission of asthma, Treg function appears to remain abnormal compared with normal controls and may predispose to disease recurrence.
Acknowledgments
This study was supported by GlaxoSmithKline Canada (CRIF-FRIC) and by a local funds program.
REFERENCES
- 1.Busse WW. Inflammation in asthma: The cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102:S17–S22. doi: 10.1016/s0091-6749(98)70002-8. [DOI] [PubMed] [Google Scholar]
- 2.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987;136:225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Laprise C, Laviolette M, Boutet M, Boulet LP. Asymptomatic airway hyperresponsiveness: Relationships with airway inflammation and remodelling. Eur Respir J. 1999;14:63–73. doi: 10.1034/j.1399-3003.1999.14a12.x. [DOI] [PubMed] [Google Scholar]
- 4.Chakir J, Laviolette M, Boutet M, Laliberte R, Dube J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996;75:735–44. [PubMed] [Google Scholar]
- 5.Ulrik CS. Outcome of asthma: Longitudinal changes in lung function. Eur Respir J. 1999;13:904–18. doi: 10.1034/j.1399-3003.1999.13d35.x. [DOI] [PubMed] [Google Scholar]
- 6.Pumputiene I, Emuzyte R, Dubakiene R, Firantiene R, Tamosiunas V. T cell and eosinophil activation in mild and moderate atopic and nonatopic children’s asthma in remission. Allergy. 2006;61:43–8. doi: 10.1111/j.1398-9995.2006.00986.x. [DOI] [PubMed] [Google Scholar]
- 7.Van den Toorn LM, Overbeek SE, De Jongste JC, et al. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001;164:2107–13. doi: 10.1164/ajrccm.164.11.2006165. [DOI] [PubMed] [Google Scholar]
- 8.Van den Toorn LM, Overbeek SE, Prins JB, Hoogsteden HC, De Jongste JC. Dyspnoea perception during clinical remission of atopic asthma. Eur Respir J. 2002;19:1047–50. doi: 10.1183/09031936.02.01712001. [DOI] [PubMed] [Google Scholar]
- 9.Warke TJ, Fitch PS, Brown V, et al. Outgrown asthma does not mean no airways inflammation. Eur Respir J. 2002;19:284–7. doi: 10.1183/09031936.02.00882002. [DOI] [PubMed] [Google Scholar]
- 10.Vonk JM, Postma DS, Boezen HM, et al. Childhood factors associated with asthma remission after 30 year follow-up. Thorax. 2004;59:925–9. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rönmark E, Lindberg A, Watson L, Lundback B. Outcome and severity of adult onset asthma. Report of the obstructive lung disease in northern Sweden studies (OLIN) Respir Med. 2007;101:2370–7. doi: 10.1016/j.rmed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Holm M, Omenaas E, Gislason T, et al. Remission of asthma: A prospective longitudinal study from northen Europe (RHINE study) Eur Respir J. 2007;30:62–5. doi: 10.1183/09031936.00121705. [DOI] [PubMed] [Google Scholar]
- 13.European Community Respiratory Health Survey Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS) 1996;9:687–95. doi: 10.1183/09031936.96.09040687. [DOI] [PubMed] [Google Scholar]
- 14.ATS statement Standardization of spirometry – 1987 update. Am Rev Respir Dis. 1987;136:1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 15.Juniper E, Cockcroft DW, Hargreave FE. Canadian Thoracic Society. Lund: AB Draco; 1992. Histamine and methacholine inhalation tests: Tidal breathing method Laboratory procedure and standardization. [Google Scholar]
- 16.Pin I, Gibson PG, Kolendowicz R, et al. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. 1992;47:25–9. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzichini E, Hargreave FE, Pizzichini M. Assessment of airway inflammation. In: Barnes P, Grunstein M, Leff A, Woolcock A, editors. Asthma. Vol. 2. Philadelphia: Lippincott-Raven; 1997. pp. 1433–50. [Google Scholar]
- 18.Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: A method and clinical survey. Clin Allergy. 1977;7:235–43. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 19.Oosterhoff Y, de Jong JW, Jansen MA, Koeter GH, Postma DS. Airway responsiveness to adenosine 5′-monophosphate in chronic obstructive pulmonary disease is determined by smoking. Am Rev Respir Dis. 1993;147:553–8. doi: 10.1164/ajrccm/147.3.553. [DOI] [PubMed] [Google Scholar]
- 20.Kerrebijn KF, Fioole AC, van Bentveld RD. Lung function in asthmatic children after year or more without symptoms or treatment. BMJ. 1978;1:886–8. doi: 10.1136/bmj.1.6117.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber W, Eber E, Steinbrugger B, Modl M, Weinhandl E, Zach MS. Atopy, lung function and bronchial responsiveness in symptom-free paediatric asthma patients. Eur Respir J. 1997;10:1041–5. doi: 10.1183/09031936.97.10051041. [DOI] [PubMed] [Google Scholar]
- 22.Boulet LP, Turcotte H, Brochu A. Persistence of airway obstruction and hyperresponsiveness in subjects with asthma remission. Chest. 1994;105:1024–31. doi: 10.1378/chest.105.4.1024. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu Y, Fujimoto K, Yasuo M, et al. Airway hyper-responsiveness in young adults with asthma that remitted either during or before adolescence. Respirology. 2009;14:217–23. doi: 10.1111/j.1440-1843.2008.01413.x. [DOI] [PubMed] [Google Scholar]
- 24.Koh YY, Kang H, Yoo Y, Yu J, Nah KM, Kim CK. Peak expiratory flow variability and exercise responsiveness in methacholine-hyperresponsive adolescents with asthma remission. J Asthma. 2005;42:17–23. doi: 10.1081/jas-200028014. [DOI] [PubMed] [Google Scholar]
- 25.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DR, Cowan JO, Greene JM, Willan AR, Sears MR. Asthma in remission: Can relapse in early adulthood be predicted at 18 years of age? Chest. 2005;127:845–50. doi: 10.1378/chest.127.3.845. [DOI] [PubMed] [Google Scholar]
- 27.Barbee RA, Murphy S. The natural history of asthma. J Allergy Clin Immunol. 1998;102:S65–72. doi: 10.1016/s0091-6749(98)70006-5. [DOI] [PubMed] [Google Scholar]
- 28.Martin AJ, McLennan LA, Landau LI, Phelan PD. The natural history of childhood asthma to adult life. Br Med J. 1980;280:1397–400. doi: 10.1136/bmj.280.6229.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van den Toorn LM, Overbeek SE, Prins JB, Hoogsteden HC, de Jongste JC. Asthma remission: Does it exist? Curr Opin Pulm Med. 2003;9:15–20. doi: 10.1097/00063198-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Phelan PD, Robertson CF, Olinsky A. The Melbourne asthma study: 1964–1999. J Allergy Clin Immunol. 2002;109:189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 31.Bronnimann S, Burrows B. A prospective study of the natural history of asthma. Chest. 1986;90:480–4. doi: 10.1378/chest.90.4.480. [DOI] [PubMed] [Google Scholar]
- 32.Roorda RJ, Gerritsen J, van Aalderen WMC, et al. Risk factors for the persistence of respiratory symptoms in childhood asthma. Am Rev Respir Dis. 1993;148:1490–5. doi: 10.1164/ajrccm/148.6_Pt_1.1490. [DOI] [PubMed] [Google Scholar]
- 33.Martinez FD. Links between pediatric and adult asthma. J Allergy Clin Immunol. 2001;107(Suppl 5):449S–55S. doi: 10.1067/mai.2001.114993. [DOI] [PubMed] [Google Scholar]
- 34.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 35.Van den Toorn LM, Prins JB, de Jongste JC, et al. Benefit from anti-inflammatory treatment during clinical remission of atopic asthma. Respir Med. 2005;99:779–87. doi: 10.1016/j.rmed.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Broekema M, Volbeda F, Timens W, et al. Airway eosinophilia in remission and progression of asthma: Accumulation with a fast decline of FEV(1) Respir Med. 2010;104:1254–62. doi: 10.1016/j.rmed.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Bellinghausen I, Klostermann B, Knop J, Saloga J. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J Allergy Clin Immunol. 2003;111:862–8. doi: 10.1067/mai.2003.1412. [DOI] [PubMed] [Google Scholar]
- 38.Ling EM, Smith T, Nguyen XD, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 39.Verhagen J, Akdis M, Traidl-Hoffmann C, et al. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol. 2006;117:176–83. doi: 10.1016/j.jaci.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Vonk JM, Postma DS, Boezen HM, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004;59:925–9. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panhuysen CI, Vonk JM, Koëter GH, et al. Adult patients may outgrow their asthma. A 25-year follow-up study. Am J Respir Crit Care Med. 1997;155:1267–72. doi: 10.1164/ajrccm.155.4.9105065. [DOI] [PubMed] [Google Scholar]
- 42.De Marco R, Pattaro C, Locatelli F, Svanes C, ECRHS Study Group Influence of early life exposures on incidence and remission of asthma throughout life. J Allergy Clin Immunol. 2004;113:845–52. doi: 10.1016/j.jaci.2004.01.780. [DOI] [PubMed] [Google Scholar]
- 43.Joos GF, O’Connor B, Anderson SD, et al. ERS Task Force Indirect airway challenges. Eur Respir J. 2003;21:1050–68. doi: 10.1183/09031936.03.00008403. [DOI] [PubMed] [Google Scholar]
- 44.Van Den Berge M, Meijer RJ, Kerstjens HM, et al. PC20 adenosine 5′ monophosphate is more closely associated with airway inflammation in asthma than PC20 methacholine. Am J Respir Crit Care Med. 163:1546–50. doi: 10.1164/ajrccm.163.7.2010145. 3001; [DOI] [PubMed] [Google Scholar]
- 45.Van den Berge M, Kerstjens HA, Postma DS. Provocation with adenosine 5′ monophosphate as a marker of inflammation in asthma, allergic rhinitis and chronic obstructive pulmonary disease. Clin Exp Allergy. 2002;32:824–30. doi: 10.1046/j.1365-2222.2002.01385.x. [DOI] [PubMed] [Google Scholar]
- 46.De Meer G, Heederik D, Postma DS. Bronchial responsiveness to adenosine 5′-monophosphate (AMP) and methacholine differ in their relation with airway allergy and baseline FEV1. Am J Respir Crit Care Med. 2002;165:327–331. doi: 10.1164/ajrccm.165.3.2104066. [DOI] [PubMed] [Google Scholar]
- 47.De Marco R, Locatelli F, Cerveri I, Bugiani M, Marinini A, Giammanco G. Italian study on asthma in young adults study group. Incidence and remission of asthma: A retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110:228–35. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 48.Boulet LP, Prince P, Turcotte H, et al. Clinical features and airway inflammation in mild asthma versus asymptomatic airway hyperresponsiveness. Respir Med. 2006;100:292–9. doi: 10.1016/j.rmed.2005.04.026. [DOI] [PubMed] [Google Scholar]