Abstract
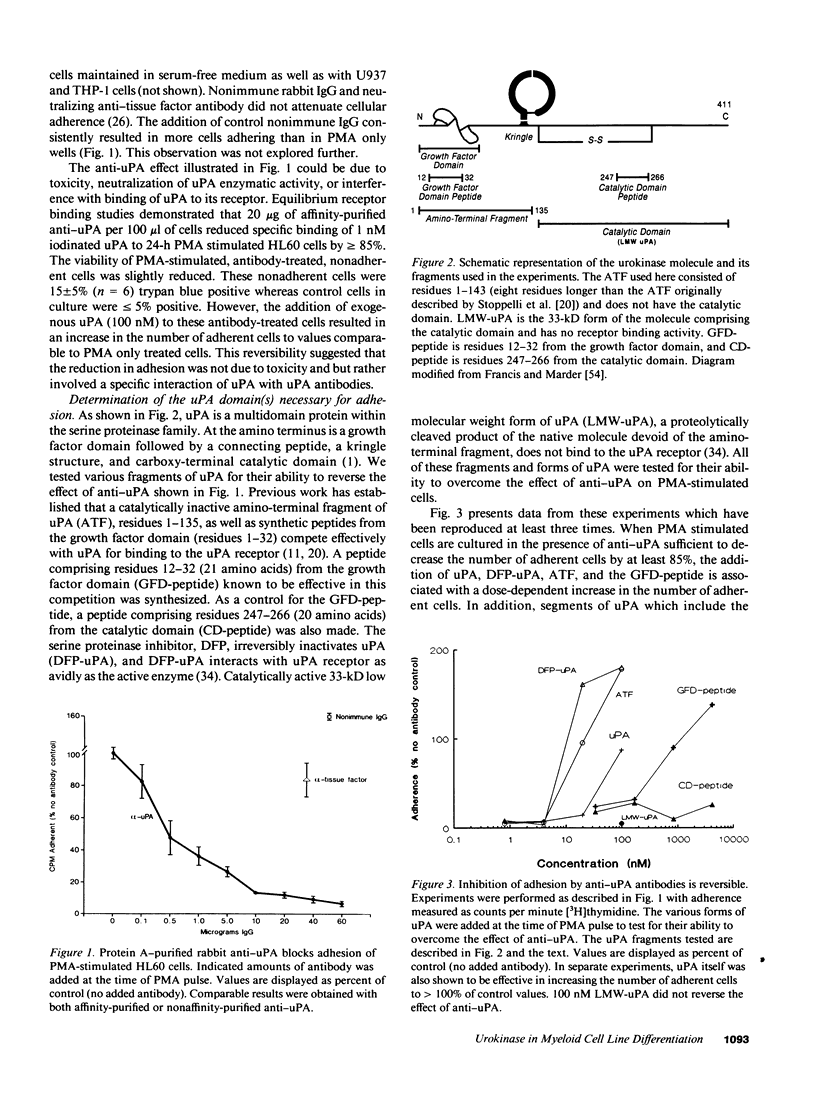
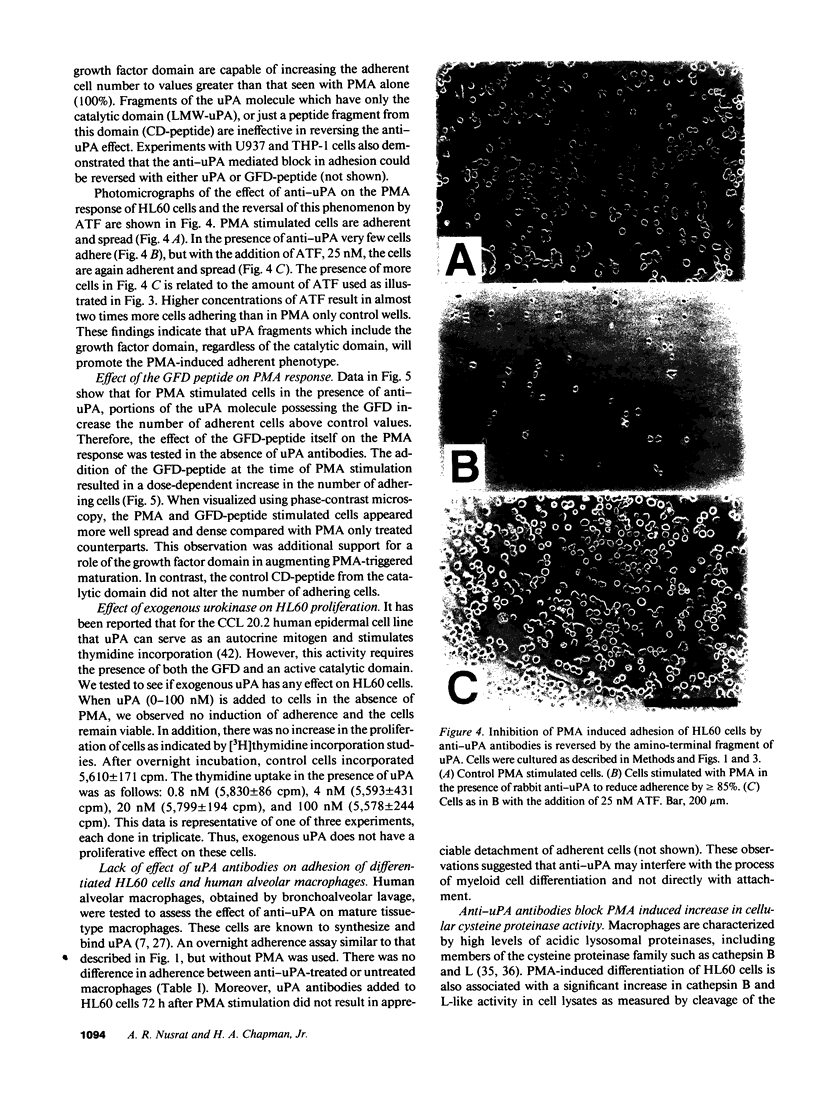
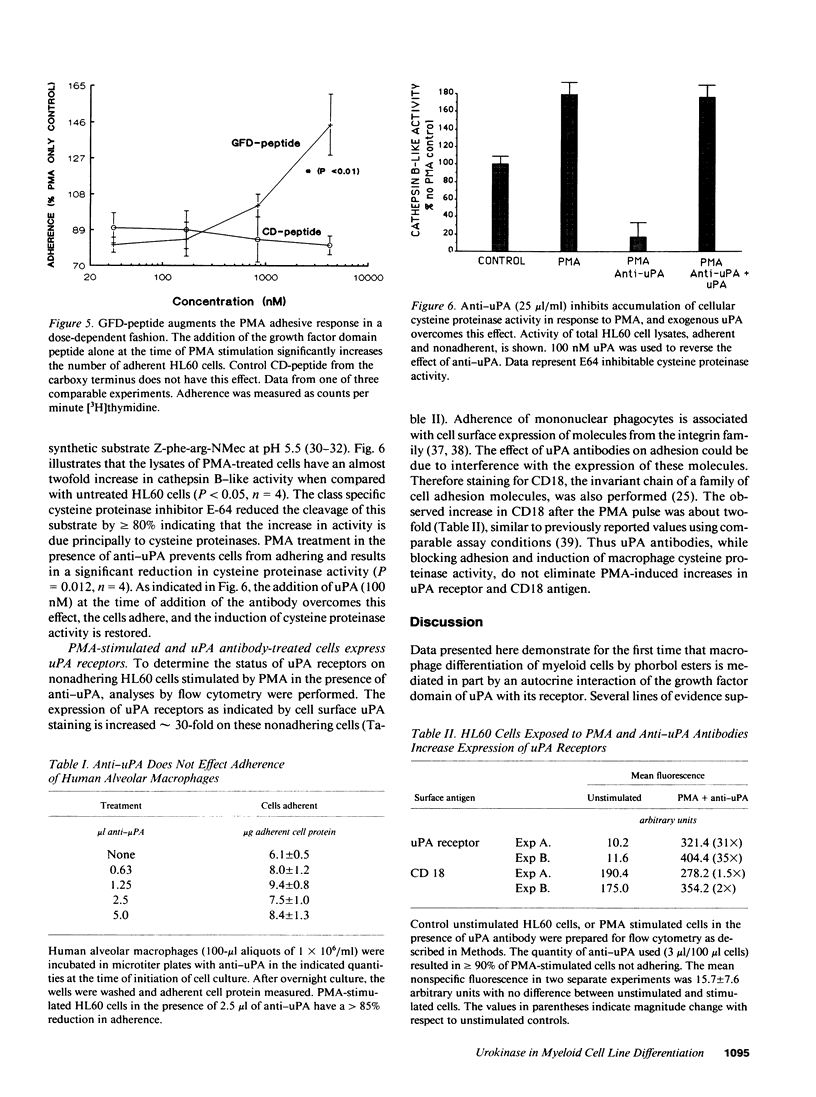
The human myeloid cell line HL60 secretes urokinase-type plasminogen activator (uPA) and expresses its receptor. When stimulated with phorbol myristate acetate (PMA), both secretion of uPA and the expression of its receptor are up-regulated, and these cells differentiate to an adherent phenotype. This adhesive response is markedly reduced in the presence of uPA antibodies. The PMA response is restored by the addition of native uPA, an amino-terminal fragment of uPA (residues 1-143) devoid of proteolytic activity, or a synthetic peptide (residues 12-32) from the uPA growth factor domain known to mediate receptor binding. In contrast, the addition of catalytically active low molecular weight uPA, which is missing the growth factor domain, or a peptide from the catalytic domain (residues 247-266) is ineffective. The influence of uPA antibodies on a second marker of macrophage differentiation, cysteine proteinase activity, was also examined. Cysteine proteinase activity of HL60 cells is increased in PMA-treated cells after 24 h but it fails to increase in the presence of anti-uPA. This increase in cathepsin B-like activity is also restored by exogenous uPA. These experiments indicate that an autocrine interaction of the growth factor domain of uPA with its receptor mediates an essential step in PMA-mediated myeloid cell differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving B. M., Krishnamurti C., Liu Y. P., Lucas D. L., Wright D. G. Stimulated production of urokinase and plasminogen activator inhibitor-2 by the human promyelocytic leukemia cell line HL-60. Thromb Res. 1988 Jul 15;51(2):175–185. doi: 10.1016/0049-3848(88)90061-8. [DOI] [PubMed] [Google Scholar]
- Amici C., Benedetto A., Saksela O., Salonen E. M., Vaheri A. Plasminogen activator and its enhancement in differentiating mouse Friend erythroleukemia cells. Int J Cancer. 1989 Jan 15;43(1):171–176. doi: 10.1002/ijc.2910430131. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Appella E., Robinson E. A., Ullrich S. J., Stoppelli M. P., Corti A., Cassani G., Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987 Apr 5;262(10):4437–4440. [PubMed] [Google Scholar]
- Bach R., Nemerson Y., Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981 Aug 25;256(16):8324–8331. [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Burnett D., Crocker J., Vaughan A. T. Synthesis of cathepsin B by cells derived from the HL60 promyelocytic leukaemia cell line. J Cell Physiol. 1983 Jun;115(3):249–254. doi: 10.1002/jcp.1041150306. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Lesser M., Yoo O. H., Orlowski M. Increased cathepsin B-like activity in alveolar macrophages and bronchoalveolar lavage fluid from smokers. Am Rev Respir Dis. 1986 Sep;134(3):538–541. doi: 10.1164/arrd.1986.134.3.538. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Cubellis M. V., Andreasen P., Ragno P., Mayer M., Danø K., Blasi F. Accessibility of receptor-bound urokinase to type-1 plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4828–4832. doi: 10.1073/pnas.86.13.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso M., Pucci M., Fibbi G., Dini G. Interaction of urokinase with specific receptors abolishes the time of commitment to terminal differentiation of murine erythroleukaemia (Friend) cells. Br J Haematol. 1987 Jul;66(3):289–294. doi: 10.1111/j.1365-2141.1987.tb06912.x. [DOI] [PubMed] [Google Scholar]
- Eierman D. F., Johnson C. E., Haskill J. S. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989 Mar 15;142(6):1970–1976. [PubMed] [Google Scholar]
- Ellis V., Wun T. C., Behrendt N., Rønne E., Danø K. Inhibition of receptor-bound urokinase by plasminogen-activator inhibitors. J Biol Chem. 1990 Jun 15;265(17):9904–9908. [PubMed] [Google Scholar]
- Fibbi G., Ziche M., Morbidelli L., Magnelli L., Del Rosso M. Interaction of urokinase with specific receptors stimulates mobilization of bovine adrenal capillary endothelial cells. Exp Cell Res. 1988 Dec;179(2):385–395. doi: 10.1016/0014-4827(88)90277-7. [DOI] [PubMed] [Google Scholar]
- Francis C. W., Marder V. J. Physiologic regulation and pathologic disorders of fibrinolysis. Hum Pathol. 1987 Mar;18(3):263–274. doi: 10.1016/s0046-8177(87)80009-6. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Chaplin D. D., Kiely J. M., Unanue E. R. Regulation of interleukin 1 gene expression by adherence and lipopolysaccharide. J Immunol. 1987 Jun 1;138(11):3799–3802. [PubMed] [Google Scholar]
- Genton C., Kruithof E. K., Schleuning W. D. Phorbol ester induces the biosynthesis of glycosylated and nonglycosylated plasminogen activator inhibitor 2 in high excess over urokinase-type plasminogen activator in human U-937 lymphoma cells. J Cell Biol. 1987 Mar;104(3):705–712. doi: 10.1083/jcb.104.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Radnik R. A., Garoni J. A., Kreisberg J. I. Urokinase-dependent adhesion loss and shape change after cyclic adenosine monophosphate elevation in cultured rat mesangial cells. J Clin Invest. 1988 Dec;82(6):1992–2000. doi: 10.1172/JCI113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudewicz P. W., Gilboa N. Human urokinase-type plasminogen activator stimulates chemotaxis of human neutrophils. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1176–1181. doi: 10.1016/s0006-291x(87)80193-6. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Haskill S., Johnson C., Eierman D., Becker S., Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988 Mar 1;140(5):1690–1694. [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheimer J. C., Nong Y. H., Remold H. G. IFN-gamma, tumor necrosis factor-alpha, and urokinase regulate the expression of urokinase receptors on human monocytes. J Immunol. 1988 Dec 15;141(12):4229–4234. [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Functional characteristics of receptor-bound urokinase on human monocytes: catalytic efficiency and susceptibility to inactivation by plasminogen activator inhibitors. Blood. 1989 Sep;74(4):1396–1402. [PubMed] [Google Scholar]
- Kirchheimer J. C., Wojta J., Christ G., Binder B. R. Proliferation of a human epidermal tumor cell line stimulated by urokinase. FASEB J. 1987 Aug;1(2):125–128. doi: 10.1096/fasebj.1.2.3038646. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen L. S., Kellerman G. M., Behrendt N., Picone R., Danø K., Blasi F. A 55,000-60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J Biol Chem. 1988 Feb 15;263(5):2358–2363. [PubMed] [Google Scholar]
- Ossowski L. In vivo invasion of modified chorioallantoic membrane by tumor cells: the role of cell surface-bound urokinase. J Cell Biol. 1988 Dec;107(6 Pt 1):2437–2445. doi: 10.1083/jcb.107.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone R., Kajtaniak E. L., Nielsen L. S., Behrendt N., Mastronicola M. R., Cubellis M. V., Stoppelli M. P., Pedersen S., Danø K., Blasi F. Regulation of urokinase receptors in monocytelike U937 cells by phorbol ester phorbol myristate acetate. J Cell Biol. 1989 Feb;108(2):693–702. doi: 10.1083/jcb.108.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. J., Jr, Mason R. W., Chen P., Joseph L. J., Sukhatme V. P., Yee R., Chapman H. A., Jr Synthesis and processing of cathepsin L, an elastase, by human alveolar macrophages. Biochem J. 1989 Jan 15;257(2):493–498. doi: 10.1042/bj2570493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmarin A. G., Weil S. C., Rosner G. L., Griffin J. D., Arnaout M. A., Tenen D. G. Differential expression of CD11b/CD18 (Mo1) and myeloperoxidase genes during myeloid differentiation. Blood. 1989 Jan;73(1):131–136. [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O. Plasminogen activation and regulation of pericellular proteolysis. Biochim Biophys Acta. 1985 Nov 12;823(1):35–65. doi: 10.1016/0304-419x(85)90014-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Corti A., Soffientini A., Cassani G., Blasi F., Assoian R. K. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4939–4943. doi: 10.1073/pnas.82.15.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppelli M. P., Tacchetti C., Cubellis M. V., Corti A., Hearing V. J., Cassani G., Appella E., Blasi F. Autocrine saturation of pro-urokinase receptors on human A431 cells. Cell. 1986 Jun 6;45(5):675–684. doi: 10.1016/0092-8674(86)90782-8. [DOI] [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Kobayashi Y., Goto Y., Okumura H., Nakae S., Konno T., Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982 Apr;42(4):1530–1536. [PubMed] [Google Scholar]
- Valinsky J. E., Reich E., Le Douarin N. M. Plasminogen activator in the bursa of Fabricius: correlations with morphogenetic remodeling and cell migrations. Cell. 1981 Aug;25(2):471–476. doi: 10.1016/0092-8674(81)90065-9. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. L., Francis G. E. Differentiation-linked secretion of urokinase and tissue plasminogen activator by normal human hemopoietic cells. J Exp Med. 1987 Jun 1;165(6):1609–1623. doi: 10.1084/jem.165.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. L., Jacobs P., Dowdle E. B. The secretion of plasminogen activators by human myeloid leukemic cells in vitro. Blood. 1983 Mar;61(3):568–574. [PubMed] [Google Scholar]




