Abstract
Since initial reports over four decades ago, patients with angina-like chest pain whose coronary angiograms show no evidence of obstructive coronary artery disease and who have no structural heart disease continue to be a common occurrence for cardiologists. Many features of this patient population have remained constant with successive reports over time: a female predominance, onset of symptoms commonly between 40-50 years of age, pain that is severe and disabling, and inconsistent responses to conventional anti-ischemic therapy. Because patients may have had abnormal noninvasive testing that led to performance of coronary angiography, investigators have sought to demonstrate an association of this syndrome with myocardial ischemia. Abnormalities in coronary flow and metabolic responses to stress have been reported by several groups, findings consistent with a microvascular etiology for ischemia and symptoms, but others have questioned the presence of ischemia, even in patients selected for abnormal noninvasive testing. Despite considerable efforts by many groups over four decades, the syndrome remains controversial with regards to pathophysiology, diagnosis and management.
Keywords: Coronary, Microcirculation, Disease
As coronary angiography became more widely practiced in the 1960's, it was soon apparent that not all patients with clinical suspicion of coronary artery disease (CAD) had obstruction of epicardial coronary arteries. Several published series, including the National Heart, Lung, and Blood Institute-sponsored Coronary Artery Surgery Study and the Women's Ischemia Syndrome Evaluation (WISE) study, have reported that up to half of patients undergoing coronary angiography are found to have normal or non-obstructed epicardial coronary arteries (1-3). In 1967, Likoff et al. (4) reported 15 women ranging in age from 30 to 53 years with chest pain despite normal coronary angiograms (CPNCA), but with ECG abnormalities at rest (ST segment depression or T wave inversion) that were accentuated by exercise. Despite the ECG changes during exercise, the hemodynamic response--as assessed by pulmonary artery pressure, cardiac output, and oxygen consumption--were reported as normal in the 8 patients in whom these measurements were made. The authors of this article stated that “usual therapy of coronary artery disease was ineffective and unwarranted” in this setting. That same year, Kemp, Elliott and Gorlin (5) reported a series of 50 patients (62% women) with CPNCA, commenting that as a group, “these patients may frequently have the most severe pain syndromes, often proving refractory to conventional forms of therapy.” Of the 41 patients who underwent metabolic study during isoproterenol stress, 11 demonstrated myocardial lactate production supportive of myocardial ischemia. Four of these 11 patients had ischemic-appearing ECGs during exercise stress; however, 5 additional patients with ischemic-appearing ECGs during exercise stress did not demonstrate myocardial lactate production during isoproterenol infusion. In a 1973 editorial, Kemp noted the heterogeneity of patients included in studies of patients with CPNCA, making it difficult to derive clinical or mechanistic insights about this syndrome (6). The term “syndrome X” was used in this editorial (based on “group X” in the article under discussion) to denote the uncertainty of chest pain etiology in these patients, a term subsequently used by other investigators, but often with different criteria for its definition.
Twenty-five years ago, our group considered whether impaired coronary microcirculatory dilator responsiveness could limit blood flow response to stress, producing myocardial ischemia and angina, and published our initial findings during the inaugural year of the Journal (7). Stephen Epstein and I subsequently proposed “microvascular angina” as a suitable descriptor for this syndrome (8). Abnormalities in coronary flow and metabolic responses to stress were reported over the years by several groups, findings consistent with a microvascular etiology (by default, based on normal coronary angiograms) for ischemia and symptoms. Others, however, have questioned an ischemic cause for symptoms, even in patients selected for abnormal noninvasive testing such as ischemic appearance of stress ECG, designated by some groups as having “syndrome X.” In 1992, Paolo Camici, Stephen Epstein and I wrote a review article, entitled Pathophysiological Dilemma of Syndrome X (9). Despite considerable efforts by many groups since that time, the syndrome remains controversial with regards to pathophysiology, diagnosis and management.
Despite differences of opinion regarding cardiac versus noncardiac mechanisms of chest pain in this population, most groups have reported that patients with CPNCA and structurally normal hearts have a better prognosis with regards to serious cardiac events (myocardial infarction, cardiovascular deat h) compared with CAD patients (10-14). Although reassurance helps many patients, most continue to have chest pain resulting in emergency room evaluations, hospitalizations and repeat cardiac catheterizations, with adverse effects on quality of life, employment and health care costs (15-18).
Focus on the Coronary Microcirculation
In response to surrounding myocardial metabolic conditions, arterioles dilate or constrict in order to match flow appropriate to myocardial oxygen demands. Micropuncture measurements of pressure in small subepicardial arteries of the beating cat heart, however, showed that 40% to 50% of the total coronary resistance is imposed by pre-arteriolar arteries between 100 and 300 micron diameter (19, 20). The discovery of endothelium-derived relaxing factors (21)--nitric oxide (NO) in particular (22)--led to numerous studies of the role of the endothelium in coronary physiology, including levels of the circulation distal to epicardial arteries. In this regard, inhibition of NO synthesis with the L-arginine antagonist NG-monomethyl-L-arginine (L-NMMA) increased basal coronary vascular resistance and blunted the vasodilator response to the endothelium-dependent agonist acetylcholine in isolated perfused hearts (23). Small arteries (100- 300 micron diameter) and arterioles from cholesterol-fed animals contracted in response to doses of acetylcholine that produced relaxation in similar-sized small arteries from control animals, despite equivalent relaxation to a NO donor in the two groups (24-26). Microscopic examination of the vascular segments from these studies showed the endothelium to be structurally intact and free of atherosclerosis, although small lipid deposits or vacuoles were commonly seen within endothelial cells.
NO bioavailability may be diminished in experimental settings by a combination of reduced synthesis (which may be selective for specific signal transduction pathways) and accelerated degradation, resulting in impaired endothelium-dependent vascular relaxation and dilation. In this regard, accumulation of methylated arginines such as asymmetric dimethylarginine may compete with L-arginine for the substrate binding site on NO synthase (27). NO may also be degraded to biologically inactive nitrogen oxide compounds by the action of superoxide anions, which may be increased in hypercholesterolemia (28).
The Coronary Microcirculation in Humans
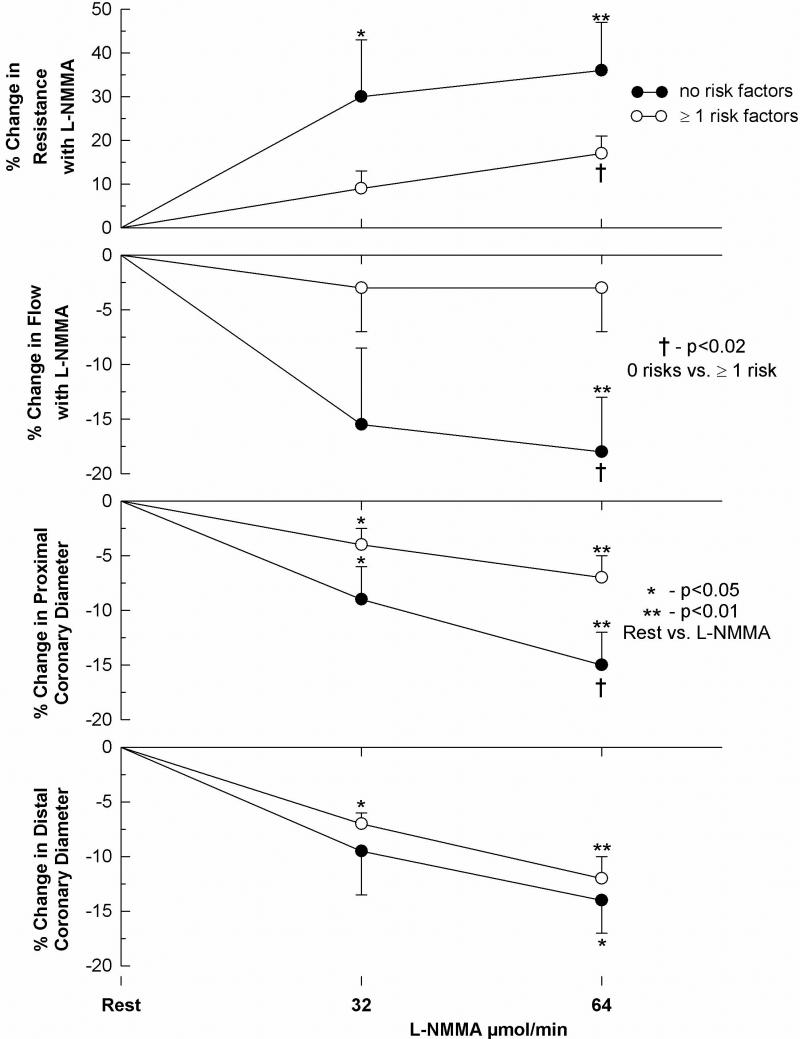
Several groups have reported evidence for dysfunction of the coronary circulation at a level too small to be assessed visually by measurement of coronary flow responses to endothelium-dependent and endothelium-independent vasodilators in patients undergoing cardiac catheterization, generally for chest pain evaluation (29-31). Quyyumi et al. (31) investigated the contribution of NO to coronary circulatory dynamics by infusing L-NMMA into coronary arteries of 31 patients (17 women) with CPNCA, although most had one or more risk factors for atherosclerosis. Inhibition of NO synthesis caused a 10-15% constriction of epicardial arteries and 15-20% reduction in coronary blood flow in patients who had no risk factors for atherosclerosis (Figure 1). In contrast, patients with risk factors had less of a constrictor effect on the coronary circulation, consistent with reduced NO bioactivity. Further, co-infusion of L-NMMA with acetylcholine caused greater attenuation of the dilator effects of this agonist in patients without risk factors than those with risk factors, indicating that the effect of acetylcholine on normal arteries, regardless of size, is largely mediated through enhanced NO release (Figure 2). Coronary microvascular dysfunction in some cases, however, may be independent of the endothelium. Thus, Reis et al. (32) of the WISE study group reported that of 159 women undergoing invasive study, 74 (47%) had what they defined as subnormal coronary flow responses (<2.5 ratio increase from baseline) to intracoronary adenosine. Age and the number of years post-menopause correlated inversely with reduced coronary flow reserve, but not lipid and hormone levels, blood pressure or left ventricular ejection fraction. A subsequent report from the WISE study group with 210 women undergoing this testing indicated that conventional atherosclerosis risk factors accounted for less than 20 percent of the observed variability in the coronary flow response to adenosine, suggesting the role of other, yet unidentified factors responsible for microvascular dysfunction (33).
1. Contribution of Nitric Oxide to Epicardial and Microvascular Coronary Tone.
The effect of risk factors for coronary atherosclerosis on the change in resting coronary vascular tone with L-NMMA, an antagonist of NO synthesis. The percent change in the coronary vascular resistance, flow, and proximal and distal epicardial diameters with L-NMMA are compared in patients with ≥ 1 risk factor (open circles) and in patients without risk factors (solid circles). Results expressed as mean ± SEM. † P<0.001, and denotes differences in the response to L-NMMA by repeat-measures analysis of variance between patients with and patients without risk factors. *P<0.05, and **P,0.01 denote differences between rest and post-LNMMA measurements in each group. (From reference 31, with permission)
2. Atherosclerosis Risk Factors and Coronary Tone During Endothelial Stimulation.

The impact of risk factors for coronary atherosclerosis on the vascular effects of acetylcholine before and after inhibition of nitric oxide synthesis with L-NMMA.. The percent change in the coronary vascular resistance, flow, and proximal and distal epicardial diameters with acetylcholine are compared in patients with ≥ 1 risk factor (open circles) and in patients without risk factors (solid circles) for atherosclerosis. Responses to the peak dose of acetylcholine are compared before and after L-NMMA in the two groups. Results expressed as mean ± SEM. †P<0.03, and denotes differences in the response to acetylcholine between patients with and patients without risk factors. *P<0.05, and **P,0.01 denote differences between control and post-LNMMA measurements in each group. (From reference 31, with permission)
The authors of these studies concluded that microvascular dysfunction may exist in patients with CPNCA, and limit coronary flow during stress. The clinical implications of these findings, however, are uncertain: Similar abnormalities of coronary microvascular function may exist in asymptomatic subjects who have no indication for cardiac catheterization and invasive study of coronary dynamics.
The Case For Myocardial Ischemia
Several conditions have been proposed to account for coronary microvascular dysfunction, including altered autonomic tone (34,35), insulin resistance (36-38), enhanced ion transport across cell membranes (39), increased endothelin-1 release (40,41), estrogen deficiency (42), and endothelial dysfunction (43-45). In many cases, patients were selected for invasive study based on an abnormal test, such as treadmill stress ECG or nuclear perfusion imaging, consistent with inducible ischemia. Such tests, however, could be “false positive” not only for epicardial coronary artery disease (by selection), but also false positive for ischemia, whatever the etiology. Accordingly, metabolic evidence for ischemia might support claims of coronary microvascular dysfunction sufficient to limit appropriate blood to myocardium during stress. Thus, Buffon et al. (46) investigated metabolic evidence of ischemia during stress by measuring lipid hydroperoxides and conjugated dienes--molecules generated upon reoxygenation of ischemic tissue--as metabolic markers of ischemia in arterial and great cardiac vein blood. Samples were drawn before and after rapid atrial pacing in 9 patients (4 women) with CPNCA, 7 of whom had ischemic-appearing ST segment depression during exercise stress and 5 of whom had reversible nuclear perfusion defects. These measurements were compared with those of 5 patients with mitral valve disease who underwent this study and served as controls. Curiously, levels of these molecules were higher in great cardiac vein blood than arterial blood prior to pacing in patients, whereas the reverse was true in controls. In patients, but not controls, great cardiac venous levels of these molecules increased after pacing (160 bpm or heart rate at development of ST segment depression for 3 minutes) that induced ST segment depression and chest pain in all but 1 patient.
Buchthal et al. (47) from the WISE study group reported that 7 of 35 women with CPNCA who underwent nuclear magnetic resonance (NMR) spectroscopy had findings compatible with myocardial ischemia dur ing repetitive hand-grip exercise. This conclusion was based on reduction in spectral signals from the phosphate of phosphocreatine relative to the phosphates of adenosine triphosphate that was similar to the decline in the ratio of these high energy phosphate spectra recorded in patients with CAD. The frequency of exercise-induced nuclear perfusion defects and abnormal brachial artery endothelial testing, however, was similar for the 7 women with reduction in the ratio of high energy phosphate spectra and the 28 women with lesser reduction (or actual increases) in these spectra. No coronary flow dynamic data were reported to ascertain relevance to invasive measures of microvascular function.
Magnetic Resonance Imaging and Subendocardial Ischemia
Invasive measures of coronary microvascular dynamics and performance of nuclear magnetic resonance spectroscopy are unavailable to most cardiologists. Further, widely accepted values for “normal” versus “abnormal” responses to these tests are lacking. Cardiac magnetic resonance imaging (MRI) is being used with increasing frequency to demonstrate ischemia in patients with suspected CAD, including the emergency department setting for patients presenting with chest pain, with sufficient resolution to demonstrate subendocardial ischemia or infarction (48). Panting et al. (49) performed cardiac MRI in 20 patients (16 women) with CPNCA and ischemic-appearing ECG responses to exercise stress and in 10 age and sex-matched control subjects. Fourteen of the patients had undergone nuclear perfusion imaging studies: None showed reversible perfusion abnormalities following stress to suggest inducible myocardial ischemia. Images were obtained at baseline and during adenosine infusion to dilate the coronary microcirculation and maximally increase coronary blood flow. In controls, adenosine increased perfusion in the endocardium and the epicardium by analysis of short axis slices of the left ventricle. In contrast, patients showed less increase in endocardial perfusion, but preserved increase in epicardial perfusion in response to adenosine. During the adenosine infusion, 19 of 20 patients experienced chest pain--often intense in severity--whereas 4 of 10 controls experienced chest pain, generally mild in severity. The investigators concluded that coronary microvascular dysfunction may limit appropriate increases in endocardial blood flow. Left unexplained is why ischemic chest pain should have occurred when endocardial perfusion was not actually reduced by adenosine in this study.
On the other hand, Vermeltfoort et al. (50) performed adenosine-stress cardiac MRI in 20 patients (15 women) with CPNCA--all of whom had ischemic-appearing ECGs and/or reversible nuclear perfusion defects during exercise stress--and reported comparable increases in subendocardial and subepicardial signal intensity following adenosine infusion. As highlighted by Camici in an accompanying editorial (51), transient reductions in subendocardial signal intensity, commonly noted in this study, are likely an artifact of the first pass sequence, unlike the sustained signal loss in the subendocardium seen in patients with CAD which is generally believed to represent subendocardial ischemia.
The Case Against Myocardial Ischemia
Although many of the studies cited abov e support the paradigm that coronary microvascular dysfunction in the absence of structural heart disease may precipitate myocardial ischemia, other studies--beginning with the report from the Montreal Heart Institute (52) that prompted Kemp's 1973 editorial--have questioned the existence of myocardial ischemia in patients with CPNCA who have structurally normal hearts, including those with ischemic-appearing stress ECGs or other non-invasive testing suggestive of inducible ischemia. Such studies have included analysis of coronary sinus metabolites of carbohydrates and fatty acids (53) and pH monitoring of coronary sinus blood before and during rapid atrial pacing (54), concluding that most patients with CPNCA do not show metabolic evidence of myocardial ischemia despite chest pain with stress.
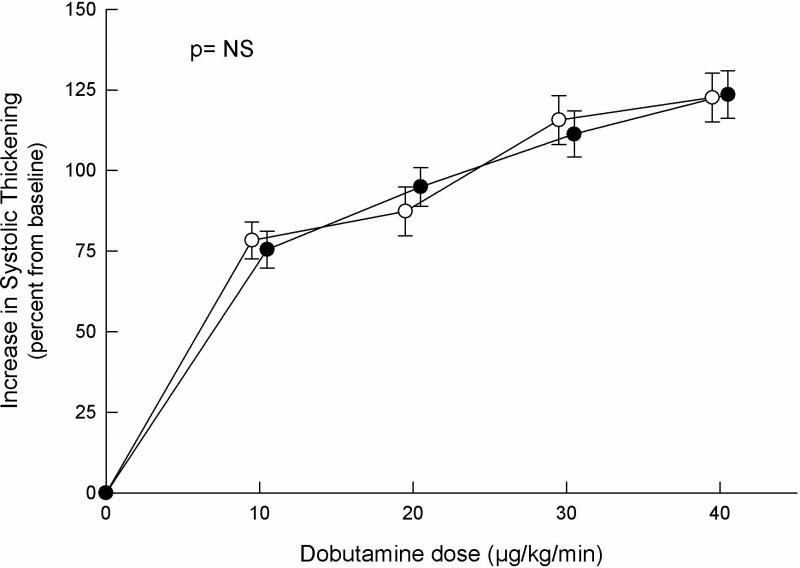
Other groups have evaluated cardiac function during stress to determine whether ischemic appearance of the ECG during stress is associated with diminished regional or global wall motion consistent with inducible myocardial ischemia, as recognized in CAD. Thus, Nihoyannopoulos et al. (55) reported normal left ventricular systolic function by echocardiography immediately following exercise and during rapid atrial pacing in 18 patients with CPNCA despite experiencing chest pain with ischemic-appearing ST segment depression during these stresses. We evaluated 70 consecutive patients (44 women) with CPNCA, of whom 22 had ischemic-appearing ECG responses during treadmill exercise and 13 had reversible perfusion defects, albeit without correlation between these two non-invasive tests (56). The results of exercise testing and dobutamine stress echocardiography from these 70 patients were compared to those of 26 healthy subjects. The transesophageal route for imaging was used in order to maximize the number of ventricular segments visualized and the quality of images for assessment of contractility. Dobutamine infused in step-wise increments to 40 mcg/kg/min induced chest pain in 59 patients, but in none of the control subjects. Ischemic-appearing ST segment depression developed in 22 patients and in 2 control subjects. Wall motion abnormalities occurred in none of the patients or the control subjects, and no differences were observed in transmural contractile response to dobutamine between patients and control subjects (Figure 3). Indeed, of the 70 patients, the quantitative myocardial contractile response to dobutamine was virtually identical in the 22 patients with ST segment depression and the 48 patients without this ECG response during infusion.
3. Left Ventricular Functional Responses to Stress.
Comparison of the quantitative myocardial response to the infusion of incremental doses of dobutamine in healthy control subjects (open circles) and in patients with chest pain and normal coronary angiograms (solid circles). Results expressed as mean ±S EM. P value corresponds to comparison of the different responses by repeat-measures analysis of variance. (From reference 56, with permission)
Thus, despite the frequent provocation of characteristic chest pain, including those with ischemic-appearing ST segment depression, patients with CPNCA do not demonstrate concomitant regional wall motion abnormalities, but instead show a quantitatively normal myocardial contractile response to stress that argue against inducible ischemia. In rebuttal to this conclusion, however, Maseri et al. (57) proposed that patchy microvascular constriction (or absence of appropriate vasodilation) may produce myocardial ischemia during stress that does not affect myocardial contractility because of compensatory vasodilation of adjacent arterioles.
Prognostic Implications of Coronary Endothelial Dysfunction
Although reports from large cohorts of patients with CPNCA suggest a benign prognosis (10-18), at least for life-threatening cardiac events, studies incorporating assessment of endothelial function indicate that subsets of patients may be at higher risk of serious cardiovascular events (58-61). These studies support the prognostic significance of coronary endothelial dysfunction at the epicardial or microvascular level. The relevance to patients with CPNCA, however, is unclear. Similar demonstration of endothelial dysfunction in a population of comparable age and risk factor profile, but free of chest pain symptoms, might have identified similar cardiovascular risk.
Investigators from the WISE study group reported the prognostic implications of NMR spectroscopy testing in women with CPNCA, extending findings from their previous publication (47). This series included 74 women with no obstructive plaques on coronary angiograms, of whom 60 had no reduction in the ratio of phosphocreatine-to-adenosine triphosphate signals during hand-grip stress and 14 showed reduction in this ratio (62). At 3 years, 87% of the group with a normal ratio of high energy phosphate signals were free of cardiovascular events, versus 57% in the abnormal spectral signal ratio group. The difference in events, however, was primarily due to increased frequency of repeat hospitalizations for chest pain and repeat coronary angiography, and not myocardial infarction or cardiovascular death.
Abnormal Cardiac Pain Perception: The Sensitive Heart
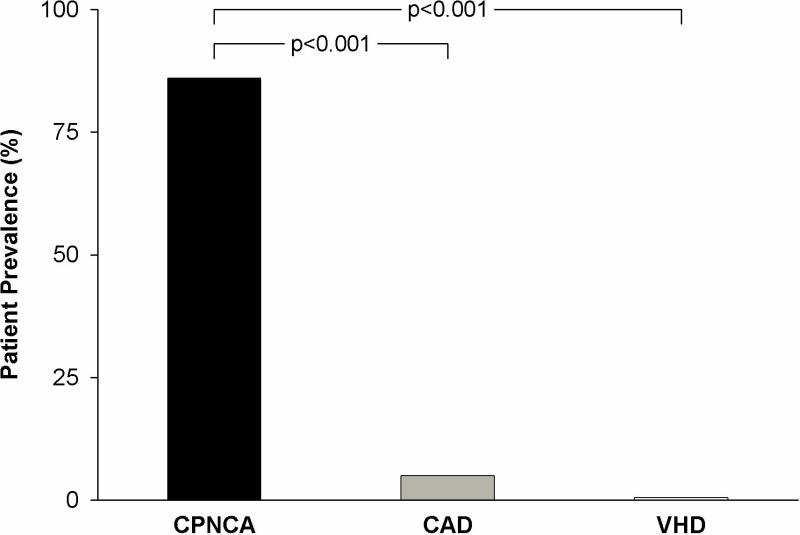
The observation that patients with CPNCA commonly experience their characteristic chest pain during the performance of diagnostic cardiac catheterization has led several groups to consider abnormal cardiac pain perception as a fundamental abnormality in this patient population, initia lly demonstrated by simple catheter movement or saline injections within the heart (63-67). We found that in 36 patients (21 women) with CPNCA, characteristic chest pain could be provoked in 86% by electrical stimulation (right ventricular pacing) at a heart rate 5 beats faster than their resting heart rate, with the pain worsened by increasing the stimulus intensity (64). In over half of these patients, pain could also be provoked by simply injecting contrast media into the left coronary artery (Figure 4). In contrast, pain responses to these maneuvers were rarely encountered in patients with CAD or valvular heart disease. Other potent stimuli for pain provocation in CPNCA patients are dipyridamole and adenosine infusion (64,65). Exaggerated pain sensitivity has also been demonstrated within the esophagus of patients with CPNCA (68), lending speculation that the mechanism of exaggerated visceral pain sensitivity may be neurophysiologically linked to whatever is responsible for anxiety and panic disorders commonly noted with patients with CPNCA (67, 69-71).
4. Chest Pain During Cardiac Catheterization: The Sensitive Heart.
Prevalence of typical chest pain experienced during cardiac catheterization in 36 patients with chest pain and normal coronary angiograms (CPNCA), 44 patients with coronary artery disease (CAD) and 10 patients with valvular heart disease (VHD) provoked by pacing from apex of right ventricle at 5 beats over resting heart rate and/or injection of contrast media into the left coronary artery. (Adapted from reference 64, with permission)
Rosen et al. (72) measured regional cerebral blood flow by positron emission tomography as an index of neuronal activity at rest and during dobutamine stress in 8 patients (6 women) with CPNCA and ischemic-appearing ECG responses to exercise stress, and 8 control subjects. Dobutamine precipitated severe chest pain and ST segment depression in all patients, although echocardiography showed increased left ventricular contractility. Patients and controls showed similar increases in blood flow in the hypothalamus, thalami, right orbito-frontal cortex and anterior temporal lobes. In patients, but not controls, increased blood flow was also noted in the right anterior insula/frontal operculum junction. In a previous study of identical design but with CAD patients, dobutamine infusion provoked chest pain and echocardiographic evidence of myocardial ischemia: No increased blood flow in the right insula was noted in these patients (73). Thus, patients with this syndrome may have an altered pattern of cortical activation by visceral afferent signals, which could contribute to abnormal pain perception during cardiac stress even in the absence of ischemia.
Treatment Trials and Management Approaches
Perhaps the most frustrating aspect of the syndrome of CPNCA is the management of chest pain symptoms, as was recognized in the earliest reports in the literature. Numerous therapies have been reported to be successful in clinical trials generally including small numbers of patients, including nitrates (74), beta blockers (75,76), calcium channel blockers (77), angiotensin-converting enzyme inhibitors (78), tricyclic antidepressants (79), aminophylline (80), estrogen replacement therapy (81) and L-arginine (82). The WISE study group is currently conducting randomized clinical trials with quinapril (NCT00150826) and ranolazine (NCT00570089). The clinical experience, however, has generally been that pain relief with medical therapy is not sustained over time, and patients are commonly prescribed a large number of drugs. Another management consideration is gastroespophageal testing, that may reveal acid reflux disease or esophageal motility disorders (68). Other groups have proposed non-pharmacologic approaches to pain symptoms in this patient population, including exercise training (83), transcendental meditation (84), cognitive behavioral therapy (85), and transcutaneous electrical nerve stimulation or spinal cord stimulation (86-89).
Absence of widely accepted understanding of pathophysiology and approach to diagnosis and treatment should not preclude a sympathetic appreciation of symptoms, which can be debilitating for some patients. My management approach is to perform vasodilator stress cardiac MRI: If convincing evidence of subendocardial ischemia is present, then anti-ischemic therapy seems appropriate. If no ischemia is evident, I use a combination of beta blocker and imipramine, and encourage enrollment in an aerobic exercise program. This approach is supported by several of the randomized clinical trials mentioned previously (75,76,79,83), but the combination has not been validated by proper clinical trial design.
Future Research Directions
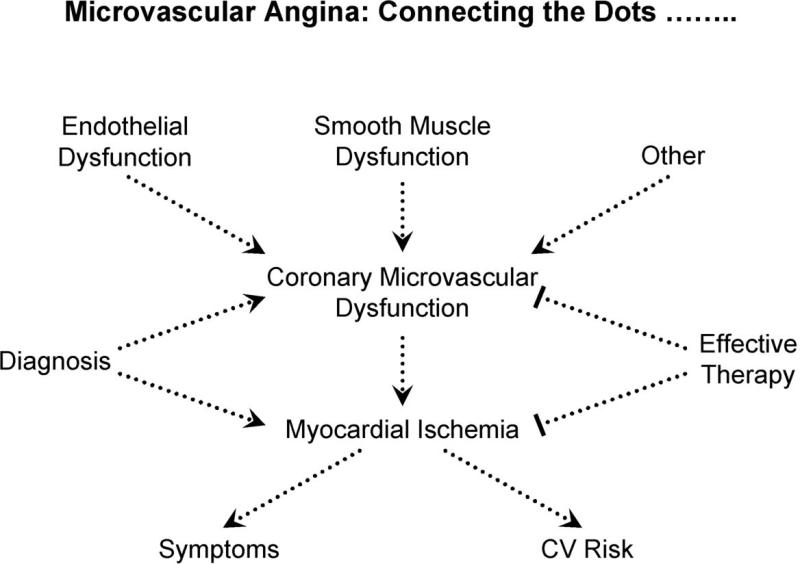
The studies cited in this review have generally focused on selected aspects of the syndrome of CPNCA in the absence of structural heart disease. Necessary to resolve the continuing debate and legitimize a specific diagnosis (e.g., microvascular angina) is to “connect the dots”, by defining a coherent pathophysiology that links coronary microvascular dysfunction by some mechanism—and localized to that level of the circulation by exclusion of epicardial disease, perhaps using computerized tomographic coronary angiography--with evidence of myocardial ischemia (Figure 5). For the diagnosis to be clinically relevant, testing that separates those patients whose symptoms are due to myocardial ischemia from those whose pain is non-ischemic should be validated by multiple groups, and strategies for effective management must be supported by randomized clinical trials. This last point is critical because patients suffer from pain that is susceptible to placebo effect, without lasting benefit. If conventional noninvasive stress testing cannot reliably identify patients with inducible ischemia as a cause of chest pain, then testing that is not widely available (such as NMR spectroscopy) must be validated by several centers to support referral of patients for specialized testing. Greater experience with cardiac MRI, which is more widely available, may support use of this testing, but validation of findings from multiple centers is necessary. To be clinically useful, results of such testing must translate into effective treatment and improved quality of life for patients.
5. Connecting the Dots.....
Future research should establish a coherent pathophysiology that links coronary microvascular dysfunction with myocardial ischemia. For the diagnosis to be clinically relevant, testing that separates those patients whose symptoms are due to myocardial ischemia from those whose pain is non-ischemic should be validated by multiple groups, and strategies for effective management must be supported by randomized clinical trials.
Acknowledgments
The author is supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health, and previously served on the Data Safety and Monitoring Board of the Women's Ischemia Syndrome Evaluation (WISE) study. There are no conflicts of interest regarding testing, drugs or other treatments included in this manuscript.
Abbreviations
- CAD
coronary artery disease
- WISE
Women's Ischemia Syndrome Evaluation
- ECG
electrocardiogram
- CPNCA
chest pain despite normal coronary angiograms
- NO
nitric oxide
- L-NMMA
NG-monomethyl-L-arginine
- NMR
nuclear magnetic resonance
- MRI
magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content of this article represent the opini ons of the author and not necessarily those of the National Heart, Lung, and Blood Institute
References
- 1.Proudfit WL, Shirey EK, Sones FM. Selective cine coronary arteriography: Correlation with clinical findings in 1,000 patients. Circulation. 1966;33:901–10. doi: 10.1161/01.cir.33.6.901. [DOI] [PubMed] [Google Scholar]
- 2.Kemp HG, Kronmal RA, Vliestra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms. A CASS registry study. J Am Coll Cardiol. 1986;7:479–83. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 3.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol. 2001;87:93741. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 4.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276:1063–6. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 5.Kemp HG, Elliott WC, Gorlin R. The anginal syndrome with normal coronary arteriography. Trans Assoc Am Physicians. 1967;80:59–70. [PubMed] [Google Scholar]
- 6.Kemp HG. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32:375–6. doi: 10.1016/s0002-9149(73)80150-x. [DOI] [PubMed] [Google Scholar]
- 7.Cannon RO, Watson RM, Rosing DR, Epstein SE. Angina caused by reduced vasodilator reserve of the small coronary arteries. J Am Coll Cardiol. 1983;1:1359–73. doi: 10.1016/s0735-1097(83)80037-0. [DOI] [PubMed] [Google Scholar]
- 8.Cannon RO, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61:1338–43. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 9.Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation. 1992;85:883–92. doi: 10.1161/01.cir.85.3.883. [DOI] [PubMed] [Google Scholar]
- 10.Kemp HG, Vokonas PS, Cohn PF, Gorlin R. The anginal syndrome associated with normal coronary angiograms: Report of a six y ear experience. Am J Med. 1973;54:735–2. doi: 10.1016/0002-9343(73)90060-0. [DOI] [PubMed] [Google Scholar]
- 11.Pasternak RC, Thibault GE, DeSanctis RW, Hutter AM. Chest pain with angiographically insignificant coronary arterial obstruction: Clinical presentation and long-term follow-up. Am J Med. 1980;68:813–7. doi: 10.1016/0002-9343(80)90199-0. [DOI] [PubMed] [Google Scholar]
- 12.Dart AM, Davies HA, Dalal J, Ruttley M, Henderson AH. “Angina” and normal coronary arteriograms: A follow-up study. Eur Heart J. 1980;1:97–100. doi: 10.1093/oxfordjournals.eurheartj.a061112. [DOI] [PubMed] [Google Scholar]
- 13.Wielgosz AT, Fletcher RH, McCants CB, McKinnis RA, Haney TL, Williams RB. Unimproved chest pain in patients with minimal or no coronary disease: A behavioral phenomenon. Am Heart J. 1984;108:67–72. doi: 10.1016/0002-8703(84)90546-5. [DOI] [PubMed] [Google Scholar]
- 14.Papaniculaou MN, Califf RM, Hlatky MA, et al. Prognostic implications of angiographically normal and insignificantly narrowed coronary arteries. Am J Cardiol. 1986;58:1181–7. doi: 10.1016/0002-9149(86)90378-4. [DOI] [PubMed] [Google Scholar]
- 15.Day LJ, Sowton E. Clinical features and follow-up of patients with angina and normal coronary arteries. Lancet. 1976;2:334–7. doi: 10.1016/s0140-6736(76)92591-5. [DOI] [PubMed] [Google Scholar]
- 16.Ockene IS, Shay MJ, Alpert JS, Weiner BH, Dalen JE. Unexplained chest pain in patients with normal coronary arteriograms: A follow-up study of functional status. N Engl J Med. 1980;303:1249–52. doi: 10.1056/NEJM198011273032201. [DOI] [PubMed] [Google Scholar]
- 17.Isner JM, Salem DN, Banas JS, Levine HJ. Long-term clinical course of patients with normal coronary arteriography: Follow-up study of 121 patients with normal or nearly normal coronary arteriograms. Am Heart J. 1981;102:645–53. doi: 10.1016/0002-8703(81)90088-0. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: Results from the National Institutes of Health- National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 19.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251:H779–88. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- 20.Chilian WM, Layne SM, Klausner EC, Eastham CL, Marcus ML. Redistribution of coronary microvascular resistance produced by dipyridamole. Am J Physiol. 1989;256:H383–90. doi: 10.1152/ajpheart.1989.256.2.H383. [DOI] [PubMed] [Google Scholar]
- 21.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 22.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxant factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 23.Amezcua JL, Palmer RMJ, de Souza BM, Moncado S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989;97:1119–24. doi: 10.1111/j.1476-5381.1989.tb12569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborne JA, Siegman MJ, Sedar AW, Mooers SV, Lefer AM. Lack of endothelium-dependent relaxation in coronary resistance arteries of cholesterol-fed rabbits. Am J Physiol. 1989;256:C591–7. doi: 10.1152/ajpcell.1989.256.3.C591. [DOI] [PubMed] [Google Scholar]
- 25.Selke FW, Armstrong ML, Harrison DG. Endothelium-dependent vascular relaxation is abnormal in the coronary microcirculation of atherosclerotic primates. Circulation. 1990;81:1586–93. doi: 10.1161/01.cir.81.5.1586. [DOI] [PubMed] [Google Scholar]
- 26.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res. 1992;70:465–76. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- 27.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthase in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 28.Minor RL, Myers PR, Guerra R, Bates JN, Harrison DG. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990;86:2109–16. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 30.Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29–37. doi: 10.1172/JCI116183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quyyumi AA, Dakak N, Andrews NP, et al. Nitric oxide in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995;95:1747–55. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis SE, Hollubkov R, Smith Conrad, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 33.Wessel TR, Arant CB, McGorray SP, et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: results from the NHLBI Women's Ischemia Syndrome Evaluation (WISE). Clin Cardiol. 2007;30:69–74. doi: 10.1002/clc.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosano GM, Ponikowski P, Adamopoulos s, et al. Abnormal autonomic control of the cardiovascular system in syndrome X. Am J Cardiol. 1994;73:1174–9. doi: 10.1016/0002-9149(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 35.Frobert O, Molgaard H, Botker HE, Bagger JP. Autonomic balance in patients with angina and a normal coronary angiogram. Eur Heart J. 1995;16:1356–60. doi: 10.1093/oxfordjournals.eurheartj.a060742. [DOI] [PubMed] [Google Scholar]
- 36.Chauhan A, Foote J, Petch MC, Schofield PM. Hyperinsulinemia, coronary artery disease and syndrome X.. J Am Coll Cardiol. 1994;23:364–8. doi: 10.1016/0735-1097(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 37.Swan JW, Walton C, Godsland IF, Crook D, Oliver MF, Stevenson JC. Insulin resistance syndrome as a feature of cardiological syndrome X in non-obese men. Br Heart J. 1994;71:41–4. doi: 10.1136/hrt.71.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botker HE, Moller N, Schmitz O, Bagger JP, Nielsen TT. Myocardial insulin resistance in patients with syndrome X. J Clin Invest. 1997;100:1919–27. doi: 10.1172/JCI119722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaspardone A, Ferri C, Crea F, et al. Enhanced activity of sodium-lithium countertransport in patients with cardiac syndrome X. A potential link between cardiac and metabolic syndrome X.. J Am Coll Cardiol. 1998;32:2031–4. doi: 10.1016/s0735-1097(98)00470-7. [DOI] [PubMed] [Google Scholar]
- 40.Kaski JC, Elliott PM, Salomone O, et al. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br Heart J. 1995;74:620–4. doi: 10.1136/hrt.74.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanza GA, Luscher TF, Pasceri V, et al. Effects of atrial pacing on arterial and coronary sinus endothelin-1 levels in syndrome X. Am J Cardiol. 1999;84:1187–91. doi: 10.1016/s0002-9149(99)00532-9. [DOI] [PubMed] [Google Scholar]
- 42.Rosano GM, Collins P, Kaski JC, Lindsay DC, Sarrel PM, Poole-Wilson PA. Syndrome X in women is associated with oestrogen deficiency. Eur Heart J. 1995;16:610–4. doi: 10.1093/oxfordjournals.eurheartj.a060963. [DOI] [PubMed] [Google Scholar]
- 43.Egashira K, Inou T, Hirooka Y, Yamada A, Urabe Y, Takeshita A. Evidence of impaired endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary angiograms. N Engl J Med. 1993;328:1659–64. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- 44.Zeiher AM, Krause T, Shachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–52. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 45.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 46.Buffon A, Rigattieri S, Santini SA, et al. Myocardial ischemia-reperfusion damage after pacing-induced tachycardia in patients with cardiac syndrome X. Am J Physiol Heart Circ Physiol. 2000;279:H2627–33. doi: 10.1152/ajpheart.2000.279.6.H2627. [DOI] [PubMed] [Google Scholar]
- 47.Buchthal SD, den Hollander JA, Bairey-Merz CN, et al. Abnormal myocardial phosphorous-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 48.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–7. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 49.Panting JR, Gatehouse PD, Yang G-Z, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–53. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 50.Vermeltfoort IAC, Bondarenko O, Raijmakers WRP, et al. Is subendocardial ischemia present in patients with chest pain and normal coronary angiograms? A cardiovascular MR study. Eur Heart J. 2007;28:1554–8. doi: 10.1093/eurheartj/ehm088. [DOI] [PubMed] [Google Scholar]
- 51.Camici PG. Is the chest pain in cardiac syndrome X due to subendocardial ischemia? Eur Heart J. 2007;28:1539–40. doi: 10.1093/eurheartj/ehm167. [DOI] [PubMed] [Google Scholar]
- 52.Arbogast R, Bourassa MG. Myocardial function during atrial pacing in patients with angina pectoris and normal coronary arteriograms: comparison with patients having significant coronary artery disease. Am J Cardiol. 1973;32:257–63. doi: 10.1016/s0002-9149(73)80130-4. [DOI] [PubMed] [Google Scholar]
- 53.Camici PG, Marraccini P, Lorenzoni R, Buzzigoli G, Pecori N. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: response to pacing stress. J Am Coll Cardiol. 1991;17:1461–70. doi: 10.1016/0735-1097(91)90632-j. [DOI] [PubMed] [Google Scholar]
- 54.Rosano GM, Kaski JC, Arie S, et al. Failure to demonstrate myocardial ischaemia in patients with angina and normal coronary arteries. Evaluation by continuous coronary sinus pH monitoring. Eur Heart J. 1997;17:1175–80. doi: 10.1093/oxfordjournals.eurheartj.a015034. [DOI] [PubMed] [Google Scholar]
- 55.Nihoyannopoulos P, Kaski JC, Crake T, Maseri A. Absence of myocardial. dysfunction during stress in patients with syndrome X. J Am Coll Cardiol. 1991;18:1463–70. doi: 10.1016/0735-1097(91)90676-z. [DOI] [PubMed] [Google Scholar]
- 56.Panza JA, Laurienzo JM, Curiel RV, et al. Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29:293–301. doi: 10.1016/s0735-1097(96)00481-0. [DOI] [PubMed] [Google Scholar]
- 57.Maseri A, Crea F, Kaski JC, Crake T. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol. 1991;17:499–506. doi: 10.1016/s0735-1097(10)80122-6. [DOI] [PubMed] [Google Scholar]
- 58.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 59.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 60.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 61.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women. Results from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:722–5. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 62.Johnson BD, Shaw LJ, Buchtal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease. Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–9. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro LM, Crake T, Poole-Wilson PA. Is altered cardiac sensation responsible for chest pain in patients with normal coronary arteries? Clinical observation during cardiac catheterization. BMJ. 1988;296:170–1. doi: 10.1136/bmj.296.6616.170-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cannon RO, III, Quyyumi AA, Schenke WH, et al. Abnormal cardiac pain sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol. 1990;16:1359–6. doi: 10.1016/0735-1097(90)90377-2. [DOI] [PubMed] [Google Scholar]
- 65.Lagerqvist B, Sylven C, Waldenstrom A. Lower threshold for adenosine-induced chest pain in patients with angina and normal coronary angiograms. Br Heart J. 1992;68:282–5. doi: 10.1136/hrt.68.9.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosen SD, Uren NG, Kaski JC, Tousoulis D, Davies GJ, Camici PG. Coronary vasodilator reserve, pain perception, and sex in patients with syndrome X. Circulation. 1994;90:50–60. doi: 10.1161/01.cir.90.1.50. [DOI] [PubMed] [Google Scholar]
- 67.Pasceri V, Lanza GA, Buffon A, Montenero AS, Crea F, Maseri A. Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X. J Am Coll Cardiol. 1998;31:62–6. doi: 10.1016/s0735-1097(97)00421-x. [DOI] [PubMed] [Google Scholar]
- 68.Cannon RO, Cattau EL, Yakshe PN, et al. Coronary flow reserve, esophageal motility, and chest pain in patients with angiographically normal coronary arteries. Am J Med. 1990;88:217–22. doi: 10.1016/0002-9343(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 69.Bass C, Wade C, Hand D, Jackson G. Patients with angina and normal and near normal coronary arteries: clinical and psychosocial state 12 months after angiography. Br Med J. 1983;287:1505–8. doi: 10.1136/bmj.287.6404.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beitman BD, Mukerji V, Lamberti JW, et al. Panic disorder in patients with chest pain and angiographically normal coronary arteries. Am J Cardiol. 1989;63:1399–1403. doi: 10.1016/0002-9149(89)91056-4. [DOI] [PubMed] [Google Scholar]
- 71.Rutledge T, Reis SE, Olson M, et al. History of anxiety disorder is associated with a decreased likelihood of angiographic coronary artery disease in women with chest pain: The WISE study. J Am Coll Cardiol. 2001;37:780–5. doi: 10.1016/s0735-1097(00)01163-3. [DOI] [PubMed] [Google Scholar]
- 72.Rosen SD, Paulesu E, Wise RJ, Camici PG. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart. 2002;87:513–9. doi: 10.1136/heart.87.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosen SD, Paulesu E, Frith CD, et al. Central neural correlates of angina pectoris as a model of visceral pain. Lancet. 1994;344:147–150. doi: 10.1016/s0140-6736(94)92755-3. [DOI] [PubMed] [Google Scholar]
- 74.Lanza GA, Manzoli A, Bia E, Crea F, Maseri A. Acute effects of nitrates on exercise testing in patients with syndrome X: clinical and pathophysiological implications. Circulation. 1994;90:2695–2700. doi: 10.1161/01.cir.90.6.2695. [DOI] [PubMed] [Google Scholar]
- 75.Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–6. doi: 10.1016/s0002-9149(99)00450-6. [DOI] [PubMed] [Google Scholar]
- 76.Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propanolol therapy in syndrome X. Am J Cardiol. 1989;63:286–90. doi: 10.1016/0002-9149(89)90332-9. [DOI] [PubMed] [Google Scholar]
- 77.Cannon RO, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56:242–6. doi: 10.1016/0002-9149(85)90842-2. [DOI] [PubMed] [Google Scholar]
- 78.Kaski JC, Rosano GM, Gavrielides S, Chen L. Effects of angiotensin-converting enzyme inhibition on exercise-induced angina and ST segment depression in patients with microvascular angina. J Am Coll Cardiol. 1994;23:652–7. doi: 10.1016/0735-1097(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 79.Cannon RO, III, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–7. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 80.Elliott PM, Krzyzowska-Dickinson K, Calvino R, Hann C, Kaski JC. Effect of oral aminophylline in patients with angina and normal coronary arteriograms (cardiac syndrome X). Heart. 1997;77:523–6. doi: 10.1136/hrt.77.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosano GMC, Peters NS, Lefroy D, et al. 17-beta-Estradiol therapy lessens angina in postmenopausal women with syndrome X. J Amer Coll Cardiol. 1996;27:1500–5. doi: 10.1016/s0735-1097(96)00348-8. [DOI] [PubMed] [Google Scholar]
- 82.Lerman A, Burnett JC, Jr, Higano ST, McKinley LJ, Holmes DJ., Jr Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–8. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 83.Eriksson BE, Tyni-Lenne R, Svedenhag J, et al. Physical training in syndrome X: Physical training counteracts deconditioning and pain in syndrome X. J Am Coll Cardiol. 2000;36:1619–25. doi: 10.1016/s0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]
- 84.Cunningham C, Brown S, Kaski JC. Effects of transcendental meditation on symptoms and electrocardiographic changes in patients with cardiac syndrome X. Am J Cardiol. 2000;85:653–5. doi: 10.1016/s0002-9149(99)00828-0. [DOI] [PubMed] [Google Scholar]
- 85.Mayou RA, Bryant BM, Sanders C, Bass C, Klimes I, Forfar C. A controlled trial of cognitive behavioural therapy for non-cardiac chest pain. Psychological Medicine. 1997;27:1021–31. doi: 10.1017/s0033291797005254. [DOI] [PubMed] [Google Scholar]
- 86.Sanderson JE, Woo KS, Chung HK, Chan WW, Tse LK, White HD. The effect of transcutaneous electrical nerve stimulation on coronary and systemic hemodynamics in syndrome X. Coron Artery Dis. 1996;7:547–52. doi: 10.1097/00019501-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 87.Anderson C, Hole P, Oxhoj H. Spinal cord stimulation as a pain treatment for angina pectoris. Pain Clin. 1995;8:333–9. [Google Scholar]
- 88.Eliasson T, Albertsson P, Hardhammar P, Emanuelsson H, Augustinsson LE, Mannheimer C. Spinal cord stimulation in angina pectoris with normal coronary arteriograms. Coron Artery Dis. 1993;4:819–27. doi: 10.1097/00019501-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Lanza GA, Sestito A, Sandric S, et al. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J. 2001;2:25–30. [PubMed] [Google Scholar]