Abstract
The effects of various nitric oxide compounds and their inhibitors on monkey ciliary muscle contraction in vitro were investigated in both the longitudinal and circular vectors. The responses to nitric oxide compounds in carbachol precontracted ciliary muscle consisted of an initial relaxation often followed by recovery to near carbachol precontracted levels while the compound was still present. Sodium nitroprusside produced the greatest relaxation responses (nearly 100% relaxation in both vectors at 10−3 M). The highest concentrations of isosorbide dinitrate (10−4 M) and L-arginine (10−3 M) produced relaxation responses of approximately 50% in both vectors. 8-Bromo cyclic GMP produced the smallest relaxation responses (25–35%). Nitric oxide synthase inhibition enhanced carbachol contraction up to 20% in the longitudinal but not the circular vector. Phosphodiesterase inhibition did not further enhance the relaxation response to L-arginine. Guanylate cyclase inhibition partially attenuated the relaxation response to sodium nitroprusside. Nitric oxide generating compounds were effective in relaxing precontracted monkey ciliary muscle in vitro. Endogenous production of nitric oxide is likely involved in the regulation of the contractile response in monkey ciliary muscle. Nitric oxide generating compounds may have potential value in therapeutic areas where modulation of ciliary muscle tension is desirable.
Keywords: ciliary muscle, nitric oxide, contraction, relaxation, carbachol, monkey
1. Introduction
Nitric oxide is synthesized from L-arginine by nitric oxide synthase. Nitric oxide activates soluble guanylate cyclase which results in an increase in intracellular levels of the second messenger cyclic GMP that in turn mediates relaxation responses. Nitric oxide synthase has been detected in ocular structures involved in aqueous humor drainage from the anterior portion of the eye (ciliary muscle and trabecular meshwork) as well as in layers of the retina and its circulation. Nitric oxide has the potential to be involved in both protective and damaging responses related to glaucoma. Inhibition or enhancement of its production in selected locations in the eye could be used to therapeutic advantage (review in (Becquet et al., 1997)).
In human glaucoma eyes there are dramatic reductions in staining indicative of nitric oxide synthase activity in the ciliary muscle and outflow pathways compared to control eyes (Chen et al., 1998, Nathanson and McKee, 1995a) that are unrelated to a general ocular decrease in nitric oxide synthase activity in glaucoma vs control eyes, the use of multiple glaucoma therapies, or the severity of the disease (Nathanson and McKee, 1995a). Concentrations of cGMP and nitrate are decreased in aqueous humor of subjects with primary open-angle glaucoma (POAG) (Doganay et al., 2002, Galassi et al., 2004). Arginine levels are significantly increased in vitreous humor of monkeys with experimental glaucoma (Carter-Dawson et al., 2002, Wamsley et al., 2002) and in aqueous humor of POAG patients (Hannappel et al., 1985).
Nitric oxide synthase activity, equated with NADPH-diaphorase (d) staining, localizes strongly to the longitudinal as compared to the circular regions of the ciliary muscle in normal human eyes (Chen et al., 1998, Nathanson and McKee, 1995a). Regionalized staining is less apparent in monkey ciliary muscle. Trabecular meshwork shows only minor staining. Ciliary processes, involved in aqueous humor production, show staining associated with the stroma and nonpigmented epithelial cells. The endothelial subtype of nitric oxide synthase is the predominant form found in both particulate and soluble fractions of the human ciliary muscle and outflow pathway tissue (Nathanson and McKee, 1995b).
Nitric oxide-mimicking nitrovasodilators can act at various sites in the anterior segment of the eye to potentially decrease IOP by increasing outflow facility, decreasing episcleral venous pressure, decreasing aqueous humor flow and increasing uveoscleral outflow. Nitrovasodilators relax bovine trabecular meshwork and ciliary muscle strips (Kamikawatoko et al., 1998, Wiederholt et al., 1994) and cat ciliary muscle strips (Goh et al., 1995) precontracted with carbachol (CARB) in vitro. IOP and aqueous humor formation are decreased in isolated pig eyes perfused with nitro-vasodilators, (Shahidullah et al., 2005) suggesting mechanisms independent of ocular vasculature. Conflicting results are reported for the effects of nitric oxide compounds on IOP in rabbits (Carreiro et al., 2009, Zamora and Keil, 2010).
In the living cynomolgus monkey eye, nitrovasodilators decrease IOP and, at certain concentrations, decrease the resistance to fluid outflow (Schuman et al., 1994). At low concentrations, it is possible the nitrovasodilators relax the ciliary muscle and increase outflow through the uveoscleral route (Bill, 1967; Inomata et al., 1972, Poyer et al., 1995). At higher concentrations, the nitro-vasodilators may act directly at the trabecular meshwork to cause relaxation and enhance conventional outflow (Rao et al., 2001, Sabanay et al., 2000, Sabanay et al., 2006). Small increases in outflow are produced by modulation of the nitric oxide system in organ-cultured human anterior segments (Schneemann et al., 2002). Intravenous administration of L-arginine into human volunteers lowers IOP and increases nitrite levels in the aqueous humor compared to controls, presumably as a result of conversion to nitric oxide by nitric oxide synthase (Chuman et al., 2000).
Nitric oxide also mediates the accommodative and dis-accommodative fine control of focus by intrinsic nerve cells within the ciliary muscle (Tamm et al., 1995).
The present study investigates the effects of various nitro-vasodilators on contractile properties of the monkey ciliary muscle in vitro to determine whether or not the responses are similar to those found in studies conducted in other species. Responses in both the longitudinal (LONG) (outflow facility and uveoscleral outflow relevant) and circular (CIRC) (accommodation relevant) vectors of the monkey ciliary muscle were measured and compared (Poyer et al., 1993).
2. Materials and methods
2.1. Ciliary muscle specimens and measurement of mechanical responses
Eyes from cynomolgus (Macaca fascicularis, n = 7) and rhesus (Macaca mulatta, n = 56) monkeys of either sex, ranging in age from 2.5 to 26 years, that were euthanized for other nonocular studies at the Wisconsin National Primate Research Center or by other investigators at the University of Wisconsin, were obtained fresh at the time of euthanasia. Ciliary muscle strips (approximately 5 mm in the CIRC vector × 5 mm in the LONG vector), were prepared and mounted in a 4 ml perfusion chamber that was maintained at 34 °C and was perfused continuously with warmed oxygenated (95% 02/5% CO2) Krebs solution (ionic composition (mM): Na+ 143.3, K+ 5.9, Ca+2 2.6, Mg+2 1.2, Cl− 128.3, 2.2, 24.9, 1.2, glucose 11.1, pH 7.4) or Krebs solution containing the experimental compounds, at a flow rate of 8 ml/min, via tubing attached to a peristaltic pump. Selection of the perfusand was made by transferring the end of the tubing to a different solution bottle in the water bath while the pump ran continuously. A small air bubble was formed when the solutions were switched that could be followed through the clear tubing, allowing precise determination of the time the new solution entered the muscle chamber. All solutions had an initial pH of 7.4. Muscle strips mounted in the chamber were allowed to equilibrate (up to 90 min) until a resting tension of 40–215 mg was reached. The strip was then exposed to 10−6 M carbachol (CARB, Aldrich Chemical Co., Inc, Milwaukee, WI), a near-maximal concentration, (Poyer et al., 1993) to determine the responsiveness of the tissue. The effect of sequentially higher concentrations of nitric oxide agonist and antagonist solutions on CARB-precontracted ciliary muscle was then determined for 15–20 min exposure intervals. After removal of the nitric oxide solutions, a final exposure to CARB was done to determine whether the contractile response of the ciliary muscle to CARB alone had been altered with time or treatment.
Contractile force was measured in both the LONG and CIRC vectors by two force transducers (Aurora 400A force transducer system (amplifier contained within), 50 mN ~ 5 g, Aurora Scientific, Inc., Aurora, Ontario, Canada). Output from the transducer system was recorded on a two-channel flatbed recorder.
2.2. Compounds
The following nitric oxide generating compounds and precursors were studied at concentrations of 10−7 to 10−3 M: sodium nitroprusside (SNP), a nonnitrate vasodilator; isosorbide dinitrate (ISDN), an organic nitrate vasodilator; L-arginine (L-arg), an endogenous nitric oxide synthase substrate; 8-bromo cyclic 3′,5′ guanosine monophosphoric acid (8-Br cGMP), a cell permeable form of the presumed endogenous mediator of the relaxation response. Inhibitors included Nw-Nitro- L-arginine methyl ester hydrochloride (L-NAME), a nonselective nitric oxide synthase inhibitor; 3-isobutyl-L-methylxanthine (IBMX), a nonselective inhibitor of phosphodiesterases; methylene blue, a nonselective inhibitor of soluble guanylate cyclase; 1H-(1,2,3) oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ), a potent and selective inhibitor of soluble guanylate cyclase. Solutions containing the various agents were kept in subdued light until needed, were used within 2 h of compounding and were monitored closely for changes in pH. IBMX and 8-Br cGMP were obtained from Tocris Bioscience, Ellisville, MO. All other compounds were from Sigma Chemical Co., St. Louis, MO.
For inhibitor studies, in the cases of IBMX and ODQ (with pretreatment), the ciliary muscle was incubated with the inhibitor for 40 min before the addition of CARB plus the inhibitor for another 20 min followed by the addition of L-arg (in the presence of IBMX) or SNP (in the presence of ODQ). In the case of ODQ (no pretreatment) and methylene blue, CARB contraction was carried out for 20 min in the presence of these inhibitors before the addition of SNP.
2.3. Data analysis
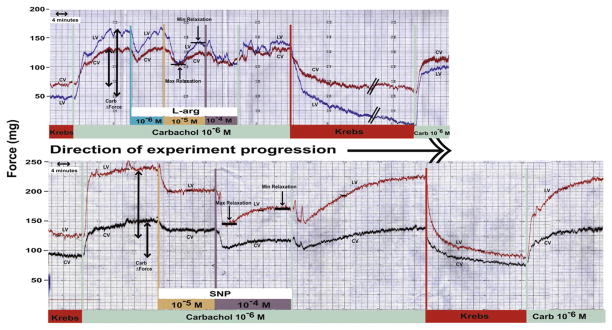
Additions of test compounds to CARB-contracted ciliary muscle often resulted in biphasic responses (Fig. 1) in which there was an initial relaxation of the contraction response in the presence of both CARB plus the test compound followed by a partial recovery or enhancement of the CARB contraction in the presence of the test compound. Therefore data for both relaxation and recovery responses to a given compound are presented. Results are expressed as the mean ± S.E.M. % change in CARB contraction force from Krebs baseline and are compared to 0.0 by the two-tailed paired t-test for the response of a given compound or by the two-tailed unpaired t-test when the responses to two compounds are compared. For results obtained in the presence of inhibitors, the % change in the CARB contraction force in the presence of the inhibitor was used for the analysis.
Fig. 1.
Response of carbachol precontracted monkey ciliary muscle to L-arginine (top) and SNP (bottom) illustrating the biphasic nature of the relaxation response. Longitudinal vector (LV), circular vector (CV). Examples of what is referred to as maximum relaxation and minimum relaxation in the presence of the test agent and carbachol are noted. Also, the carbachol change in contraction force is indicated which was used for calculating the % change in responses.
3. Results
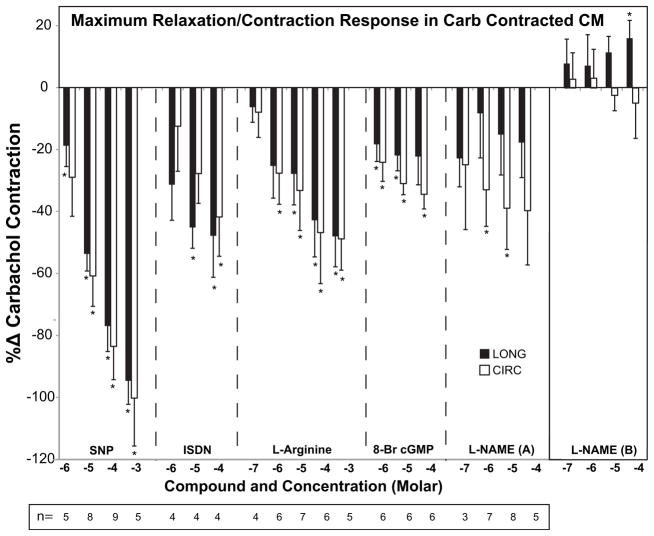
SNP, ISDN, L-arg, and 8-Br cGMP produced concentration-dependent relaxation of CARB-contracted ciliary muscle (Fig. 2, Table 2). Nearly complete relaxation was achieved in both CIRC and LONG vectors with the 10−3 M concentration of SNP. This relaxation was only slightly reversed as the incubation continued in the presence of both CARB and SNP. The effect of SNP was not completely reversed upon continued CARB-only infusion but was completely reversed after washout with Krebs solution followed by CARB infusion (not shown). The relaxation response to ISDN, was of lesser magnitude (approx 40–50% reduction in contraction in both vectors) and was not maintained during continued incubation. L-arg, a substrate of nitric oxide synthase, maximally relaxed CARB-contracted ciliary muscle by 50% in both vectors and partially maintained the relaxation as the incubation continued. 8-Br cGMP produced a differential relaxation response of 35% in CIRC and 22% in LONG vectors (not significant by the paired t-test) that was partially maintained with continued incubation.
Fig. 2.
Effects of nitric oxide compounds on carbachol (CARB)-precontracted monkey ciliary muscle (CM) strips. Data are expressed as the %Δ in the initial CARB contraction force for each vector (longitudinal = LONG, black bars; circular = CIRC, white bars) versus the concentration of each compound. N is the number of different monkey CMs represented. A given CM may have received more than one concentration of a given agent. Maximal relaxation responses of CM contraction response in the presence of CARB + test agent are shown (for L-NAME this is shown in part (A)). Continued incubation of the CM with the given agent in the presence of CARB resulted in partial attenuation of the relaxation response (not shown) or enhancement of contraction response, as was the case for L-NAME (B). Significantly different from 0.0: *p < 0.05, minimum. See data in Table 2.
Table 2.
Effects of nitric oxide compounds on carbachol (CARB)-precontracted monkey ciliary muscle (CM) strips.
| Agent, conc (M) | Maximum relaxation response % change in CARB ΔForce
|
Minimum relaxation or enhanced contraction response (agent still present) % change in CARB ΔForce
|
n | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LONG
|
CIRC
|
LONG
|
CIRC
|
||||||
| mean | sem | mean | sem | mean | sem | mean | sem | ||
| SNP | |||||||||
| 10−6 | −18.78* | 6.73 | −28.95 | 12.59 | −9.83 | 7.63 | −21.77 | 13.58 | 5 |
| 10−5 | −53.68* | 5.54 | −60.79* | 9.82 | −33.88* | 9.19 | −40.94* | 13.17 | 8 |
| 10−4 | −76.95* | 8.22 | −83.53* | 10.75 | −67.29* | 7.55 | −68.67* | 10.79 | 9 |
| 10−3 | −94.64* | 7.59 | −100.21* | 15.47 | −85.79* | 11.63 | −84.75* | 19.74 | 5 |
| ISDN | |||||||||
| 10−6 | −31.31 | 11.49 | −12.44 | 14.53 | −4.36 | 4.17 | 11.15 | 12.55 | 4 |
| 10−5 | −45.13* | 6.70 | −27.72 | 9.61 | −7.67 | 7.62 | −3.21 | 4.17 | 4 |
| 10−4 | −47.80* | 13.39 | −41.73* | 12.68 | −13.26 | 8.65 | 2.02 | 3.95 | 4 |
| L-Arginine | |||||||||
| 10−7 | −6.34 | 4.85 | −7.95 | 8.12 | 24.87* | 5.66 | 25.18* | 4.72 | 4 |
| 10−6 | −25.30 | 10.40 | −27.63* | 10.04 | −1.21 | 12.24 | −0.31 | 13.49 | 6 |
| 10−5 | −27.86* | 10.00 | −33.22* | 12.91 | −0.96 | 10.48 | 9.24 | 10.51 | 7 |
| 10−4 | −42.84* | 11.81 | −46.82* | 16.48 | −12.37 | 7.18 | −14.41* | 5.59 | 6 |
| 10−3 | −48.04* | 9.80 | −48.84* | 10.08 | −19.00 | 11.91 | −11.62 | 8.83 | 5 |
| 8-Br cGMP | |||||||||
| 10−6 | −18.33* | 5.53 | −24.12* | 6.16 | −1.04 | 6.17 | −4.54 | 4.63 | 6 |
| 10−5 | −21.95* | 4.92 | −30.98* | 3.60 | −8.24 | 6.01 | −15.57 | 5.11 | 6 |
| 10−4 | −22.27 | 9.13 | −34.45* | 4.75 | −10.44 | 6.31 | −22.60* | 3.07 | 6 |
| L-NAME | |||||||||
| 10−7 | −22.86 | 9.18 | −24.90 | 20.93 | 10.48 | 10.48 | 3.74 | 11.35 | 3 |
| 10−6 | −8.31 | 14.41 | −32.99* | 11.79 | 9.59 | 13.26 | 4.17 | 12.41 | 7 |
| 10−5 | −15.21 | 13.01 | −38.93* | 13.32 | 15.27 | 6.87 | −3.18 | 6.61 | 8 |
| 10−4 | −17.76 | 11.30 | −39.72 | 17.52 | 21.36* | 7.62 | −6.50 | 15.16 | 5 |
Data are expressed as the %Δ in the initial CARB contraction force for each vector (longitudinal = LONG; circular = CIRC) versus the concentration of each compound. N is the number of different monkey CMs represented. A given CM may have received more than one concentration of a given agent. Bold values indicate values that are significant. Significantly different from 0.0:
p < 0.05, minimum. Initial CARB contraction forces (mean ± S.E.M.) were as follows: SNP, 80.0 ± 9.2 mg LONG, 43.8 ± 6.4 mg CIRC; ISDN, 51.9 ± 20.6 mg LONG, 26.4 ± 9.4 mg CIRC; L-arg, 66.6 ± 9.8 mg LONG, 40.2 ± 6.7 mg CIRC; 8-Br cGMP, 99.8 ± 17.1 mg LONG, 54.4 ± 7.5 mg CIRC; L-NAME, 54.7 ± 10.3 mg LONG, 21.4 ± 2.5 mg CIRC.
Nonspecific inhibition of nitric oxide synthase with L-NAME produced a variable initial relaxation (A) followed by an enhancement of contraction (B, +20%) in the LONG but not the CIRC vectors.
Final CARB contraction force in the absence of each agent or after Krebs washout at the conclusion of the testing with all agents was unchanged compared to the initial CARB contraction force.
Table 1 shows the responses to the various agents in monkey ciliary muscle compared to those reported for other species. Overall, responses in monkey ciliary muscle to all agents were greater than in bovine ciliary muscle. Relaxation responses to SNP were similar in cat and monkey ciliary muscle. Likewise, monkey and bovine ciliary muscle showed similar relaxation responses to 8-Br cGMP.
Table 1.
Effect of nitric oxide compounds (10−4 M) on carbachol-induced ciliary muscle contraction in different species.
| Monkey | Bovine (Wiederholt et al., 1994) | Bovine (Kamikawatoko et al., 1998) | Cat (Goh et al., 1995) | |
|---|---|---|---|---|
| SNP (10−4 M) | −77 ± 8§(LONG, n = 9), −84 ± 11§(CIRC) | −45.5§ (n = 8) | −48.6 ± 6.3 (n = 6) | −80 (n = 4) |
| ISDN (10−4 M) | −48 ± 13*(LONG, n = 4), −42 ± 13*(CIRC) | −12 (n = 10) | ||
| L-arg (10−4 M) | −43 ± 12*(LONG, n = 6), −47 ± 16*(CIRC) | |||
| 8-Br cGMP (10−4 M) | −22 ± 9(LONG, n = 6), −34 ± 5§(CIRC) | −13.3§ (n = 8) | −36 ± 3.5 (n = 5) | |
| L-NAME (10−4 M) | +21 ± 8*(LONG, n = 5), −6 ± 15(CIRC) | +8.5 ± 1§ (L-NAG@, 10−4 M, n = 7) |
Data are % change (mean ± S.E.M. (if available)) in carbachol-induced contraction by 10−6 M (monkey, bovine (Wiederholt)); 3 × 10−6 M (cat); 10−5 M (bovine (Kamikawatoko)) carbachol.
p < 0.05;
p < 0.001.
L-NAG (L-nitroarginine) is another inhibitor of nitric oxide synthase. LONG = longitudinal vector; CIRC = circular vector.
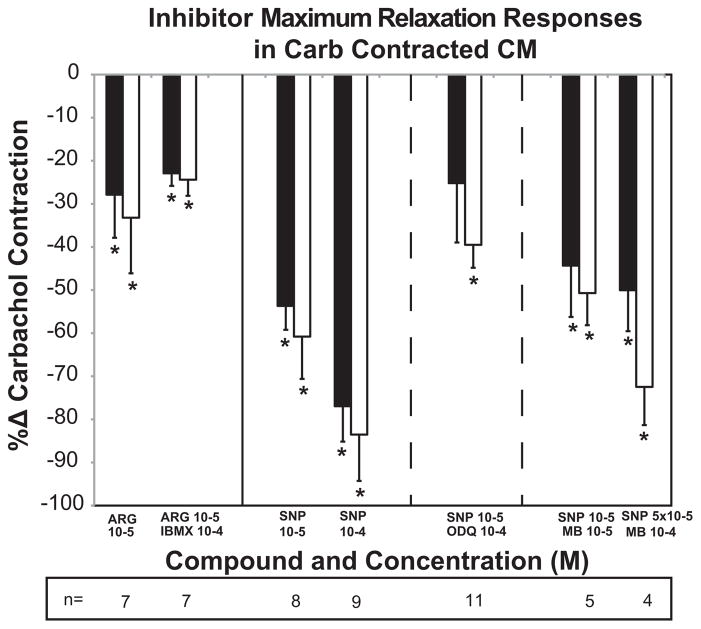
The relaxation response of approximately 30% with 10−5 M L-arg was not further enhanced in the presence of the phosphodiesterase inhibitor IBMX (Fig. 3, Table 3). Subsequent incubation with CARB alone before or after Krebs washout, resulted in a 25–60% enhancement of contraction (not shown) compared to the initial CARB + IBMX contraction force, suggesting some relaxant effect of IBMX alone perhaps as a result of preventing endogenous cGMP breakdown.
Fig. 3.
Effects of inhibitors of phosphodiesterase and nitric oxide synthase on responses to L-arg and SNP in carbachol (CARB)-precontracted monkey ciliary muscle (CM) strips. L-arg and SNP responses without inhibitor are taken from the concentration–response curves in Fig. 2 for reference. Inhibitor data are expressed as the %Δ in the initial CARB contraction force (in the presence of the inhibitor) for each vector (longitudinal = LONG, black bars; circular = CIRC, white bars) versus the concentration of each compound (*p < 0.05, paired t-test). N is the number of different monkey CMs represented. A given CM may have received more than one concentration of a given agent. Maximal relaxation of CM contraction response in the presence of CARB + test agent is shown. See data in Table 3. Continued incubation of the CM with the given agent in the presence of CARB resulted in partial attenuation of the relaxation response or enhancement of contractility response (not shown).
Table 3.
Effects of inhibitors of phosphodiesterase and nitric oxide synthase on responses to L-arg and SNP in carbachol (CARB)-precontracted monkey ciliary muscle (CM) strips.
| Agents, conc (M) | Maximum relaxation response % change in CARB ΔForce
|
n | Comparison | |||
|---|---|---|---|---|---|---|
| LONG
|
CIRC
|
|||||
| mean | sem | mean | sem | |||
| CARB/L-ARG 10−5 | −27.86* | 10.00 | −33.22* | 12.91 | 7 | vs CARB from L-arg concentration–response |
| CARB/IBMX (10−4) + L-ARG (10−5) | −22.91* | 2.93 | −24.39* | 3.74 | 7 | vs CARB + IBMX contraction |
| CARB/SNP 10−5 | −53.68* | 5.54 | −60.79* | 9.82 | 8 | vs CARB from SNP concentration–response data |
| CARB/SNP 10−4 | −76.95* | 8.22 | −83.53* | 10.75 | 9 | vs CARB from SNP concentration–response data |
| CARB/ODQ (10−4) + SNP (10−5) | −36.49* | 8.08 | −37.35* | 8.62 | 6 | vs CARB/ODQ contraction after ODQ pretreat |
| CARB/ODQ (10−4) + SNP (10−5) | −11.64 | 29.33 | −42.09* | 6.42 | 5 | vs CARB/ODQ contraction, simultaneous |
| CARB/ODQ (10−4) + SNP (10−5) | −25.19 | 13.77 | −39.51* | 5.32 | 11 | vs CARB/ODQ contraction; combined data |
| CARB/MB (10−5) + SNP (10−5) | −44.33* | 11.92 | −50.70* | 7.46 | 5 | vs CARB/MB contraction |
| CARB/MB (10−4) + SNP (5 × 10−5) | −50.03* | 9.50 | −72.47* | 8.85 | 4 | vs CARB/MB contraction |
L-arg and SNP responses without inhibitor are taken from the concentration–response curves in Fig. 2 for reference. Inhibitor data are expressed as the %Δ in the initial CARB contraction force (in the presence of the inhibitor) for each vector (longitudinal = LONG; circular = CIRC) versus the concentration of each compound. N is the number of different monkey CMs represented. A given CM may have received more than one concentration of a given agent. Bold values indicate values that are significant.
Significantly different from 0.0, paired t-test:
p < 0.05, minimum. Initial CARB contraction forces (mean ± S.E.M.) were as follows: L-arg, 66.6 ± 9.8 mg LONG, 40.2 ± 6.7 mg CIRC; L-arg + IBMX, 80.9 ± 5.1 mg LONG, 42.9 ± 5.6 mg CIRC; SNP, 80.0 ± 9.2 mg LONG, 43.8 ± 6.4 mg CIRC; SNP + ODQ (pretreat), 79.4 ± 12.2 mg LONG, 36.7 ± 6.6 mg CIRC; SNP + ODQ (simultaneous), 91.0 ± 33.0 mg LONG, 66.8 ± 29.3 mg CIRC; SNP + MB (10−5 M), 56.8 ± 13.2 mg LONG, 47.8 ± 9.9 mg CIRC; SNP + MB(10−4 M), 72.8 ± 13.6 mg LONG, 42.2 ± 9.0 mg CIRC.
ODQ pretreat, CM was incubated in ODQ alone prior to the addition of CARB + ODQ. ODQ + CARB simultaneous, ODQ and CARB were added to the CM simultaneously without ODQ preincubation.
Using the unpaired t-test: there was no difference between results with ODQ pretreat vs simultaneous (p > 0.39 for any combination of peak, trough, CIRC, LONG); there was borderline significance comparing combined ODQ data vs SNP 10−5 (0.05 < p < 0.1 for maximum relaxation in both LONG and CIRC vectors).
There was no difference between results with ODQ pretreatment vs no pretreatment for any combination of CIRC or LONG data, therefore the data were combined. Selective inhibition of guanylate cyclase with ODQ only partially reversed the relaxation response to 10−5 M SNP (Fig. 3, Table 3).
Methylene blue (10−5 M) had very little, if any effect on the relaxation response to SNP (10−5 M) in CARB-contracted ciliary muscle. Similar results were obtained with SNP (10−4 M) + CARB when compared to SNP (5 × 10−5 M) + methylene blue (10−4 M) + CARB (5 × 10−5 M SNP was not done as part of the concentration–response curve, therefore the comparison was made to 10−4 M SNP + CARB).
4. Discussion
Data from both cynomolgus and rhesus ciliary muscle strips were utilized. The number of cynomolgus samples was small (7) relative to the number of rhesus samples (56), so it is difficult to evaluate any species differences that may be present. However, the CARB contraction forces for cynomolgus ciliary muscles were within the same range of values as for rhesus ciliary muscles (LONG, 22.5–147.5 mg; CIRC, 12.5–72.5 mg). In vivo, physiologic responses such as accommodation and outflow facility of rhesus and cynomolgus eyes to pilocarpine are robust and similar in magnitude (citations for examples include (Gabelt et al., 1991, Gabelt and Kaufman, 1992; Kiland et al., 1997b, Okka et al., 2004, Takagi et al., 2004)), so there is no reason to suspect that the relative ciliary muscle responses might be different although this needs to be investigated.
Nitric oxide generating compounds were effective in relaxing CARB precontracted monkey ciliary muscle in vitro. The most effective agent was the nonnitrate, SNP, which produced nearly complete relaxation. The spontaneous release of nitric oxide from SNP may contribute to the magnitude of its effect as compared to the other agents investigated. Incomplete inhibition of SNP-induced relaxation following methylene blue treatment led us to utilize the more specific inhibitor, ODQ. However, incomplete inhibition of the initial relaxation response in the presence of ODQ suggests that other mechanisms may contribute to the SNP-induced relaxation (see below). Continued incubation with ODQ did result in nearly complete attenuation (not shown) of the relaxation produced by SNP, indicating that sustained relaxation was the result of cGMP production. The contributions of calcium channel blockade, K+ channel activation cAMP- or prostanoid (Mollace et al., 2005) -mediated mechanisms were not investigated in the current study. Alternatively, differential sensitivity to ODQ inhibition of relaxation by nitric oxide donors has been reported (Tseng et al., 2000). Organic nitrates such as ISDN require bio-activation for the release of nitric oxide, which could limit the levels of nitric oxide available as compared to SNP [reviewed in (Mollace et al., 2005)].
The endogenous substrate, L-arg, produced approximately half the relaxation response compared to SNP, suggesting that the capacity for stimulating relaxation via this pathway is limited by the amount of nitric oxide synthase present. Inhibition of phosphodiesterase with IBMX did not further enhance the initial relaxation response but may have contributed to sustaining the relaxation upon continued incubation. This lack of enhancement of the L-arg relaxation response in the presence of IBMX also supports hypothesis that nitric oxide synthesis, and not cGMP hydrolysis, may be the limiting factor in the magnitude of the relaxation response to L-arg.
However, another nitric oxide signaling pathway that may confound all of the nitric oxide responses described thus far is S-nitrosylation of cysteine groups in proteins (Torta et al., 2008). S-nitrosylation has been reported to desensitize soluble guanylyl cyclase (Sayed et al., 2007) and to decrease G-actin polymerization (Dalle-Donne et al., 2000). These additional actions by nitric oxide could possibly contribute to the magnitude of the relaxation with SNP (actin depolymerization contribution), the transient relaxation responses with ISDN and L-arg as well as the lack of enhancement of relaxation by IBMX (contribution of guanylyl cyclase desensitization). However, these speculations need to be further investigated and will also need to be considered when interpreting data from other ocular physiology studies involving nitric oxide generating agents.
The lesser magnitude of response following 8-Br cGMP as compared to SNP suggests that either cGMP is not the only mediator of the relaxation response, or that 8-Br cGMP does not penetrate the cells as well as nitric oxide.
Nonspecific inhibition of nitric oxide synthase with L-NAME produced an initial relaxation response. The enhancement of CARB contraction in the LONG vector with continued incubation in the presence of L-NAME indicates that there is endogenous production of nitric oxide that modulates the contraction response to CARB. However, alkyl esters of arginine, such as L-NAME, have been reported to also act as muscarinic antagonists (Buxton et al., 1993). Therefore the biphasic response of monkey ciliary muscle contraction in the presence of L-NAME can be explained by both the competitive antagonism of CARB contraction which could be responsible for the apparent initial relaxation and, subsequently, by the inhibition of nitric oxide synthase causing a reduction in nitric oxide levels. The reduction in nitric oxide levels when combined with the continued presence of CARB and competition at the muscarinic receptor, may result in enhanced contraction, at least in the LONG vector. These differential results in CIRC and LONG vectors in response to L-NAME possibly suggests that CIRC vector fibers may contain less nitric oxide synthase or that the number or affinity of muscarinic receptors may be different from those in LONG muscle fibers. Initial contraction responses to CARB were generally less in CIRC than LONG vectors (see legends in Tables 2 and 3). Differential accommodative and outflow facility responses in vivo to cholinomimetic drugs has been reported in humans (Fechner et al., 1975, Keren and Treister, 1980; Lieberman and Leopold, 1967) and in monkeys (Erickson-Lamy and Schroeder, 1990) but are not likely due to differences in muscarinic receptor subtypes (Gabelt and Kaufman, 1992, 1994; Poyer et al., 1994).
Relaxation responses in CARB-contracted ciliary muscle were similar in the LONG compared to the CIRC vectors with SNP and with L-arg. There were tendencies toward greater relaxation in the LONG vector with ISDN and in the CIRC vector with 8-Br cGMP. However, previous studies in intact monkey eyes, did not detect regional differences in NADPH-diaphorase staining, representative of nitric oxide synthase activity (Chen et al., 1998).
Bovine ciliary muscle strips at resting tension were reported to relax further in the presence of nitric oxide donors (Wiederholt et al., 1994). No further relaxation of resting tension was produced by nitric oxide donors in cat ciliary muscle (Goh et al., 1995). Relaxation responses in resting monkey ciliary muscle were not investigated in the current study. However, in vivo, monkey ciliary muscle may exist in a partially contracted state even at accommodative rest (night or anesthetic myopia, tonic accommodation, etc.) (Kiland et al., 1997a) so that modulation by enhanced nitric oxide generation could be effective in fine tuning outflow through both ciliary muscle- and trabecular meshwork-associated pathways. Alternatively, due to the intimate association of LONG ciliary muscle fibers with the trabecular meshwork, (Rohen et al., 1967) relaxation of the ciliary muscle could potentially decrease outflow facility. However, the effects of nitric oxide on trabecular meshwork relaxation may be the predominating response (see below). The magnitude of an IOP response to ciliary muscle relaxation in vivo may be small and of short duration.
Stimulating ocular nitric oxide/cGMP production decreased IOP and also increased outflow facility in monkeys in vivo (Schuman et al., 1994) and in human organ-cultured anterior segments (Schneemann et al., 2002). Also, in monkeys in vivo, intracameral 8-Br cGMP increased outflow facility and intravitreal dosing decreased aqueous humor flow rate (Kee et al., 1994). Nitric oxide modulators produced greater relaxation in bovine trabecular meshwork compared to ciliary muscle in vitro (Wiederholt et al., 1994). This suggests that the trabecular meshwork, as compared to the ciliary muscle, may be the more likely therapeutic target for nitric oxide-induced IOP effects. IOP was significantly reduced in ocular normotensive humans during i.v. L-arginine infusion (10 min) but recovered rapidly after infusion ceased. No changes in pupil diameter or accommodative amplitude were found (Chuman et al., 2000). This would be in keeping with the finding that nitric oxide synthase activity is more prevalent in the LONG portion of the ciliary muscle in humans. Conversely, no reduction in IOP was found after topical nitroglycerin administration to normal and glaucomatous monkeys (Wang and Podos, 1995).
The nitric oxide system could potentially be targeted to enhance of aqueous outflow and lower IOP in glaucoma. Since nitric oxide synthase levels appear to be diminished in glaucomatous eyes, (Chen et al., 1998, Nathanson and McKee, 1995a) pharmacotherapy would have to bypass this part of the nitric oxide pathway. Recently, a nitric oxide -releasing prostaglandin analog was shown to produce a larger IOP reduction compared to latanoprost alone in ocular hypertensive rabbits, dogs and monkeys (Borghi et al., 2010). Long-term nitric oxide therapy might also contribute to enhanced neuroprotective properties as a result of nitric oxide -mediated inhibition of oxidative stress, pro-inflammatory mediators, and cytokine production (Neufeld, 1999). Alternatively, gene therapy (Kaufman et al., 2000) could potentially be used to restore or elevate nitric oxide synthase levels in target tissues.
Relaxation of the CIRC vector by the nitric oxide compounds used in the current study, also supports the use of these types of compounds to prevent myopia (Beauregard et al., 2001). Intrinsic nitric oxide synthase -positive nerve cells concentrated in the inner parts of the human ciliary muscle indicate a physiological role of nitric oxide for disaccommodation or fine adjustment of focus during accommodation (Tamm et al., 1995). Most recently, in human ciliary muscle, an intrinsic network of proprioceptive nerve terminals was identified that, in part, surrounds the nitrergic neurons indicating that contraction of the CIRC muscle can be modulated locally via a self-contained reflex arc (Flugel-Koch et al., 2009).
The current study demonstrates the large capacity of the ciliary muscle to respond to nitric oxide regulation which may potentially be utilized in glaucoma and presbyopia therapy.
Acknowledgments
Support: NIH/NEI R01 EY002698, R01 EY018567, P30 EY016665, P51 RR000167; Research to Prevent Blindness, Inc, New York, NY, unrestricted departmental and Physician-Scientist awards; Ocular Physiology Research and Education Foundation; Walter Helmerich Chair from the Retina Research Foundation.
Rebecca James, Meredith Cohler, Pan San Chan, Hannah Schmidt and Jessie Bisgrove assisted with experiments and data compilation.
Contributor Information
B’Ann T. Gabelt, Email: btgabelt@wisc.edu.
Paul L. Kaufman, Email: kaufmanp@mhub.ophth.wisc.edu.
Carol A. Rasmussen, Email: crasmussen@wisc.edu.
References
- Beauregard C, Liu Q, Chiou GC. Effects of nitric oxide donors and nitric oxide synthase substrates on ciliary muscle contracted by carbachol and endothelin for possible use in myopia prevention. J Ocul Pharmacol Ther. 2001;17:1–9. doi: 10.1089/108076801750125577. [DOI] [PubMed] [Google Scholar]
- Becquet F, Courtois Y, Goureau O. Nitric oxide in the eye: multifaceted roles and diverse outcomes. Surv Ophthalmol. 1997;42:71–82. doi: 10.1016/s0039-6257(97)84043-x. [DOI] [PubMed] [Google Scholar]
- Bill A. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus) Exp Eye Res. 1967;6:120–125. doi: 10.1016/s0014-4835(67)80062-9. [DOI] [PubMed] [Google Scholar]
- Borghi V, Bastia E, Guzzetta M, Chiroli V, Toris CB, Batugo MR, Carreiro ST, Chong WKM, Gale DC, Kucera DJ, Jia L, Prasanna G, Ongini E, Krauss AHP, Impagnatiello F. A novel nitric oxide releasing prostaglandin analog, ncx 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther. 2010;26:125–131. doi: 10.1089/jop.2009.0120. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. No-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Carreiro S, Anderson S, Gukasyan HJ, Krauss A, Prasanna G. Correlation of in vitro and in vivo kinetics of nitric oxide donors in ocular tissues. J Ocul Pharmacol Ther. 2009;25:105–112. doi: 10.1089/jop.2008.0091. [DOI] [PubMed] [Google Scholar]
- Carter-Dawson L, Crawford ML, Harwerth RS, Smith EL, Feldman R, Shen FF, Mitchell CK, Whitetree A. Vitreal glutamate concentration in monkeys with experimental glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2633–2637. [PubMed] [Google Scholar]
- Chen Z, Gu Q, Kaufman PL, Cynader MS. Histochemical mapping of NADPH-diaphorase in monkey and human eyes. Curr Eye Res. 1998;17:370–379. doi: 10.1080/02713689808951217. [DOI] [PubMed] [Google Scholar]
- Chuman H, Chuman T, Nao-i N, Sawada A. The effect of L-arginine on intraocular pressure in the human eye. Curr Eye Res. 2000;20:511–516. [PubMed] [Google Scholar]
- Dalle-Donne I, Milzani A, Guistarini D, De Simplicio P, Colombo R, Rossi R. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J Muscle Res Cell Motil. 2000;21:171–181. doi: 10.1023/a:1005671319604. [DOI] [PubMed] [Google Scholar]
- Doganay S, Evereklioglu C, Turkoz Y, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002;12:44–48. doi: 10.1177/112067210201200109. [DOI] [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder A. Dissociation between the effect of aceclidine on outflow facility and accommodation. Exp Eye Res. 1990;50:143–147. doi: 10.1016/0014-4835(90)90224-i. [DOI] [PubMed] [Google Scholar]
- Fechner PU, Teichman KD, Weyrauch W. Accommodative effects of ace-clidine in the treatment of glaucoma. Am J Ophthalmol. 1975;79:104–106. doi: 10.1016/0002-9394(75)90464-x. [DOI] [PubMed] [Google Scholar]
- Flugel-Koch C, Neuhuber WL, Kaufman PL, Lutjen-Drecoll E. Morphologic indication for proprioception in the human ciliary muscle. Invest Ophthalmol Vis Sci. 2009;50:5529–5536. doi: 10.1167/iovs.09-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelt BT, Crawford K, Kaufman PL. Outflow facility and its response to pilocarpine decline in aging rhesus monkeys. Arch Ophthalmol. 1991;109:879–882. doi: 10.1001/archopht.1991.01080060143044. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Inhibition of outflow facility, accommodative, and miotic responses to pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J Pharmacol Exp Ther. 1992;263:1133–1139. [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL. Inhibition of aceclidine-stimulated outflow facility, accommodation and meiosis by muscarinic receptor subtype antagonists in rhesus monkeys. Exp Eye Res. 1994;58:623–630. doi: 10.1006/exer.1994.1057. [DOI] [PubMed] [Google Scholar]
- Galassi F, Renieri G, Sodi A, Ucci F, Vannozzi L, Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004;88:757–760. doi: 10.1136/bjo.2003.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Y, Hotehama Y, Mishima HK. Characterization of ciliary muscle relaxation induced by various agents in cats. Invest Ophthalmol Vis Sci. 1995;36:1188–1192. [PubMed] [Google Scholar]
- Hannappel E, Pankow G, Grassl F, Brand K, Naumann GO. Amino acid pattern in human aqueous humor of patients with senile cataract and primary open-angle glaucoma. Ophthalmic Res. 1985;17:341–343. doi: 10.1159/000265398. [DOI] [PubMed] [Google Scholar]
- Inomata H, Bill A, Smelser GK. Unconventional routes of aqueous humor outflow in cynomolgus monkey (Macaca irus) Am J Ophthalmol. 1972;73:893–907. doi: 10.1016/0002-9394(72)90459-x. [DOI] [PubMed] [Google Scholar]
- Kamikawatoko S, Tokoro T, Ishida A, Masuda H, Hamasaki H, Sato J, Azuma H. Nitric oxide relaxes bovine ciliary muscle contracted by carbachol through elevation of cyclic gmp. Exp Eye Res. 1998;66:1–7. doi: 10.1006/exer.1997.0408. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Tian B, Gabelt BT, Liu X. Outflow enhancing drugs and gene therapy in glaucoma. In: Weinreb R, Krieglstein G, Kitazawa Y, editors. Glaucoma in the 21st Century. Chapter 17 Harcourt-Mosby; London: 2000. pp. 117–128. [Google Scholar]
- Kee C, Kaufman PL, Gabelt BT. Effect of 8-Br-cGMP on aqueous humor dynamics in monkeys. Invest Ophthalmol Vis Sci. 1994;35:2769–2773. [PubMed] [Google Scholar]
- Keren G, Treister G. Effect of aceclidine (+) isomer and pilocarpine on the intraocular pressure decrease and the miosis in glaucomatous eyes. Effect on accommodation in normal eyes of young subjects. Opthalmologica. 1980;180:181–187. doi: 10.1159/000308972. [DOI] [PubMed] [Google Scholar]
- Kiland JA, Croft MA, Gabelt BT, Kaufman PL. Atropine reduces but does not eliminate the age-related decline in perfusion outflow facility in monkeys. Exp Eye Res. 1997a;64:831–835. doi: 10.1006/exer.1997.0283. [DOI] [PubMed] [Google Scholar]
- Kiland JA, Peterson JA, Gabelt BT, Kaufman PL. Effect of DMSO and exchange volume on outflow resistance washout and response to pilocarpine during anterior chamber perfusion in monkeys. Curr Eye Res. 1997b;16:1215–1220. doi: 10.1076/ceyr.16.12.1215.5026. [DOI] [PubMed] [Google Scholar]
- Lieberman TW, Leopold IH. The use of aceclidine in the treatment of glaucoma. Its effect on intraocular pressure and facility of aqueous humor outflow as compared to that of pilocarpine. Am J Ophthalmol. 1967;64:405–415. [PubMed] [Google Scholar]
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and mitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- Nathanson JA, McKee M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest Ophthalmol Vis Sci. 1995a;36:1774–1784. [PubMed] [Google Scholar]
- Nathanson JA, McKee M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest Ophthalmol Vis Sci. 1995b;36:1765–1773. [PubMed] [Google Scholar]
- Neufeld AH. Nitic oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthalmol. 1999;43:S129–S135. doi: 10.1016/s0039-6257(99)00010-7. [DOI] [PubMed] [Google Scholar]
- Okka M, Tian B, Kaufman PL. Effects of latrunculin B on outflow facility, intraocular pressure, corneal thickness, and miotic and accommodative responses to pilocarpine in monkeys. Trans Am Ophthalmol Soc. 2004;102:251–259. [PMC free article] [PubMed] [Google Scholar]
- Poyer JF, Gabelt BT, Kaufman PL. The effect of muscarinic agonists and selective receptor subtype antagonists on the contractile response of the isolated rhesus monkeys ciliary muscle. Exp Eye Res. 1994;59:729–736. doi: 10.1006/exer.1994.1159. [DOI] [PubMed] [Google Scholar]
- Poyer JF, Kaufman PL, Flügel C. Age does not affect contractile responses of the isolated rhesus monkey ciliary muscle to muscarinic agonists. Curr Eye Res. 1993;12:413–422. doi: 10.3109/02713689309024623. [DOI] [PubMed] [Google Scholar]
- Poyer JF, Millar JC, Kaufman PL. Prostaglandin F2a effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci. 1995;36:2461–2465. [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Rohen JW, Lütjen E, Bárány E. The relation between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1967;172:23–47. doi: 10.1007/BF00577152. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Gabelt BT, Tian B, Kaufman PL, Geiger B. H-7 effects on structure and fluid conductance of monkey trabecular meshwork. Arch Ophthalmol. 2000;118:955–962. [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–246. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the no receptor, by s-nitrosylation. Proc Natl Acad Sci. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann A, Dijkstra BG, van den Berg TJ, Kamphuis W, Hoyng PFJ. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:936–941. doi: 10.1007/s00417-002-0559-7. [DOI] [PubMed] [Google Scholar]
- Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intra-ocular pressure and outflow facility in monkeys. Exp Eye Res. 1994;58:99–105. doi: 10.1006/exer.1994.1199. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Yap M, To CH. Cyclic gmp, sodium nitroprusside and sodium azide reduce aqueous humour formation in the arterially perfused pig eye. Br J Pharmacol. 2005;145:84–92. doi: 10.1038/sj.bjp.0706156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Nakajima T, Shimazaki A, Kageyama M, Matsugi T, Matsumura Y, Gabelt BT, Kaufman PL, Hara H. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid fp receptor agonist, as an ocular hypotensive drug. Exp Eye Res. 2004;78:767–776. doi: 10.1016/j.exer.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Koch TA, Mayer B, Stefani FH, Lutjen-Drecoll E. Innervation of myofibroblast-like scleral spur cells in human and monkey eyes. Invest Ophthalmol Vis Sci. 1995;36:1633–1644. [PubMed] [Google Scholar]
- Torta F, Usuelli V, Malgaroli A, Bachi A. Proteomic analysis of protein s-nitrosylation. Proteomics. 2008;8:4484–4494. doi: 10.1002/pmic.200800089. [DOI] [PubMed] [Google Scholar]
- Tseng CML, Tabrizi-Fard MA, Fung HL. Differential sensitivity among nitric oxide donors toward odq-mediated inhibition of vascular relaxation. J Pharmacol Exp Ther. 2000;292:737–742. [PubMed] [Google Scholar]
- Wamsley S, Gabelt BT, Dahl DB, Case GL, Sherwood RW, May CA, Hernandez MR, Kaufman PL. Vitreous glutamate concentration and axon loss in monkeys with experimental glaucoma. Arch Ophthalmol. 2002;123:64–70. doi: 10.1001/archopht.123.1.64. [DOI] [PubMed] [Google Scholar]
- Wang R-F, Podos SM. Effect of the topical application of nitroglycerin on intraocular pressure in normal and glaucomatous monkeys. Exp Eye Res. 1995;60:337–339. doi: 10.1016/s0014-4835(05)80116-2. (letter) [DOI] [PubMed] [Google Scholar]
- Wiederholt M, Sturm A, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994;35:2515–2520. [PubMed] [Google Scholar]
- Zamora DO, Keil JW. Episcleral venous pressure responses to topical nitroprusside and n-nitro-l-arginine methyl ester. Invest Ophthalmol Vis Sci. 2010;51:1614–1620. doi: 10.1167/iovs.09-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]