Abstract
Hyaluronic acid (HA) is one of nature's most versatile and fascinating macromolecules. Being an essential component of the natural extracellular matrix (ECM), HA plays an important role in a variety of biological processes. Inherently biocompatible, biodegradable and non-immunogenic, HA is an attractive starting material for the construction of hydrogels with desired morphology, stiffness and bioactivity. While the interconnected network extends to the macroscopic level in HA bulk gels, HA hydrogel particles (HGPs, microgels or nanogels) confine the network to microscopic dimensions. Taking advantage of various scaffold fabrication techniques, HA hydrogels with complex architecture, unique anisotropy, tunable viscoelasticity and desired biologic outcomes have been synthesized and characterized. Physical entrapment and covalent integration of hydrogel particles in a secondary HA network give rise to hybrid networks that are hierarchically structured and mechanically robust, capable of mediating cellular activities through the spatial and temporal presentation of biological cues. This review highlights recent efforts in converting a naturally occurring polysaccharide to drug releasing hydrogel particles, and finally, complex and instructive macroscopic networks. HA-based hydrogels are promising materials for tissue repair and regeneration.
Keywords: Hyaluronic acid, biological functions, hydrogels, hydrogel particles, bulk gels, complex networks, drug delivery, tissue engineering
1. Introduction
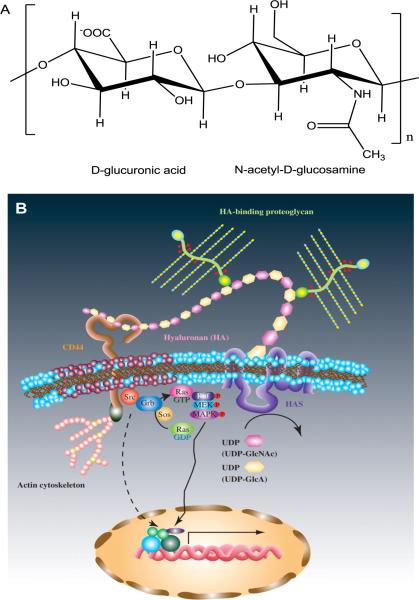
Hyaluronic acid (HA) is a non-sulphated glycosaminoglycan (GAG) in the extracellular matrix (ECM) of many soft connective tissues, composed of alternating units of D-glucuronic acid and N-acetyl-D-glucosamine, linked together via alternating β-1,4 and β-1,3 glycosidic bonds 1 (Figure 1A). It is synthesized at the inner wall of the plasma membrane by HA synthase, and is extruded to the ECM space without any further modifications. 2 In the ECM of most tissues, the high molecular weight HA (up to several million Daltons), along with other structural macromolecules, contributes to the mechanical integrity of the network. 3 HA regulates many cellular processes through its binding with cell surface receptors such as CD44 and RHAMM (Figure 1B). 4, 5 HA can be rapidly degraded in the body by hyaluronidase and reactive oxygen species, with tissue half-lives ranging from minutes in the blood to hours or days in skin and joints. 6
Figure 1.
Basic chemical structure (A) and signaling mechanism (B, Reproduced with permission,163 Copyright 2008, Oxford University Press) of HA. HA is consisted of disaccharide repeats of d-glucoronic acid and N-acetyl-d-glucosamine. HA interacts with cell surface receptors and activates intracellular signaling to direct cell functions.
HA is an attractive building block for the fabrication of artificial matrices for tissue engineering because it is biocompatible, biodegradable, bioactive, non-immunogenic and non-thrombogenic.7 In physiological solutions, HA assumes an expanded random coil structure that occupies a very large domain that facilitates solute diffusion. Although high molecular weight HA at high concentrations in solution (e.g. 5 MDa at >0.1 mg/mL) can form entangled molecular networks that are viscoelastic, solutions of HA do not have long lasting mechanical integrity.8, 9 To afford HA-based hydrogels with tailored mechanical properties and degradation rates, while at the same time maintaining their native biological functions, controlled chemical modification and covalent crosslinking are often necessary. By varying the molecular weight of HA, the degree of modification and the concentration of the reactive HA precursors, hydrogels with varying stiffness, pore size and degradation rate can be readily produced. Additional biological functionality can be incorporated into HA gels via the coupling of different biological moieties, cytokines and therapeutic drugs. Efficient, biocompatible and chemo-selective crosslinking chemistries have enabled the encapsulation of cells during gelation, giving rise to three dimensional (3D) cell/gel constructs with intimate cell-matrix interactions. 10
Traditional HA-based hydrogels are macroscopic networks (or bulk gels) consisting of randomly interconnected HA chains, lacking the structural complexity and functional diversity seen in the natural ECM. Drug molecules encapsulated in the network without any covalent linkage or other specific interactions are released rapidly due to the relatively large pore size. If the crosslinking reaction takes place in a microscopic reaction vessel, HA hydrogel particles (HGPs, microgels or nanogels) can be produced. HA HGPs exhibit tunable size, large surface area, abundant interior space and addressable functional groups.11 When properly designed, HA HGPs can sequester therapeutically active compounds, mediate their release and potentiate their biological functions. Combining well-defined crosslinking chemistries with established fabrication techniques, researchers have successfully introduced nanoscale and microscopic features to the existing HA bulk gels. Nanofibrous HA hydrogels with anisotropic properties have been engineered to foster cell-matrix interactions. Hybrid, multicomponent HA hydrogels containing chemically, morphologically and functionally different building blocks interconnected via chemical or physical means have been engineered.
Over the past few decades, researchers have accumulated significant knowledge on HA as a unique biomacromolecule that is involved in various cell signaling processes,1, 12–16 and at the same time, have created a range of HA-based hydrogel materials with increasing complexity and diverse functions. 17 In this article, an overview of the important biological properties of HA is followed by a summary of chemical approaches for the fabrication of HA-based bulk gels. Methods for producing HA HGPs are then presented, and finally emerging types of HA gels with complex structures and diverse functions are discussed. When rationally designed and properly processed, HA hydrogels have the potential to provide cells with a biologically relevant microenvironment that fosters cell proliferation, migration and ECM production, ultimately leading to the growth of functional tissues. The central theme of this review is materials development. Readers are referred to a recent review by Burdick and Prestwich 17 for relevant biomedical applications of various HA-based hydrogels.
2. The Biology of HA
HA differs from other synthetic polymers (such as poly(ethylene glycol), PEG) in that it is biologically active. HA is primarily found in the connective tissue matrix and it is produced by cells of mesenchymal origin to organize the tissue ECM.3, 18 HA is synthesized by three types of HA synthase (HAS1, HAS2 and HAS3) that are located in the cell membrane 19 and is immediately extruded out of the cell into the ECM space where it interacts with constituents of the ECM to provide mechanical support. 2 The in vivo half life of HA varies from hours to 2–3 days, depending on the types of tissues. 16 HA is partially disintegrated in the ECM by reactive oxygen species or hyaluronidase. The degraded species are either immediately internalized by cells and degraded in lysosomes or first transferred to the circulation, from where they are cleared by the drainage systems (liver, lymph nodes or kidneys). 6
Due to its abundant negative charges, HA can absorb large amounts of water and expand up to 1000 times in volume, forming a loose hydrated network.3 Therefore, HA acts as a space filler, lubricant, and osmotic buffer in the native ECM. The hydrated HA network functions as a sieve, controlling the transport of water and restricting the movement of pathogens, plasma proteins, and proteases.3 The hydrated HA also helps maintain the viscoelasticity of connective tissues such as the vitreous humor,20 cartilage21 and vocal folds. 22 HA interacts with its cell surface receptors (CD44 or RHAMM) to activate various signaling pathways, such as c-Src, Ras and mitogen-activated protein (MAP) kinases as shown in Figure 1B. These signaling pathways direct various cell functions, including cell adhesion, cytoskeletal rearrangement, cell migration, cell proliferation and differentiation. 5, 23, 24 The ability of HA to react with oxygen-derived free radicals imparts HA with antioxidant effects. Finally, HA is a strong inflammation mediator because of its ability to inhibit macrophage migration and aggregation, as well as to prevent the immune complex from adhering to polymorphonuclear cells.3
The presence of HA in the ECM of the injured tissues underscores its relevance in wound healing. HA content usually increases very quickly at scarless fetal wounds due to the reduced expression of proinflammatory cytokines (such as IL-1 and TNF-alpha) that are responsible for the down regulation of HA synthesis. HA expedites the delivery of solutes and nutrients to the wounded tissue because of its high water absorption capacity and its ability to stimulate inflammatory signals for wound healing. A HA-rich wound matrix may also facilitate cell motility and proliferation that are essential for wound repair.13, 25, 26 While the intact HA maintains the tissue in a hydrated state, the degraded HA released into the wound promotes cell proliferation, cell migration 27, 28 and angiogenesis,29 facilitating the scarless wound healing processes. The essential roles of HA in would healing justifies its utility in tissue repair and regeneration. 10
In cartilage, HA binds aggrecan to form large aggregates of the order of 108 Daltons within the collagenous framework, providing compressive resistance to the tissue. Cellular interactions between chondrocytes and HA help organize the cartilage ECM and retain the proteoglycans within the cartilage. 30 HA also stimulates the chondrogenic differentiation of mesenchymal stem cells (MSCs) and the proteoglycan production via its interaction with the chondrocytes.31, 32 Various HA and HA-containing scaffolds have been used to stimulate chondrocytes to produce essential cartilage ECM components.33–35
HA is also present in the ECM of the vocal fold lamina propria, and is differentially enriched in the intermediate layer of the lamina propria.36 In addition to providing shock absorbing properties, HA is a major modulator of the tissue viscosity.22, 37 The presence of HA in human vocal folds is evolutionarily beneficial due to the constant trauma they are subjected to during phonation.38 It has been implied that if there are lower amounts of HA in the most superficial area of the lamina propria, that there is less protection from vibratory trauma and overuse.39 Moreover, the shear-thinning properties of HA create optimum conditions for phonation by decreasing the tissue stiffness while vibrating.22 Finally, the newborn vocal fold is composed of a loose ground substance rich in HA, suggesting the critical role HA plays in vocal fold development and maturation.40 These studies suggest that ECM analogs based on HA may provide useful tissue engineering strategies for the repair and regeneration of functional vocal fold tissues.41, 42
In recent years, tissue engineering principles have been applied to the fabrication of 3D tumor tissues that can be utilized as in vitro models to gain improved understanding of tumor biology and to aid drug discovery.43 HA is highly expressed in tumors and is a requisite component of the microenvironment of cancer cells.44–47 The amount of HA on the cell surface also directs the metastasis of tumor cells.48, 49 HA alters the biological activity of cancer cells by triggering the transforming growth factor β (TGF-β), Rho GTPase, and FAK pathways via the interaction with its cell surface receptors.50–52 In tumor tissues, HA facilitates migration of invasive tumors through the expansion upon hydration and interaction of HA through certain cell surface receptors.53 HA oligomers encourage angiogenesis and induce inflammatory cytokine production, which activates various signaling mechanisms for cancer progression.44 Hence, tumor progression and angiogenesis depend on HA and hyaluronidase levels, as well as the degradation profile of HA.15, 53, 54 High concentrations of HA are sometimes observed at tumor invasion sites, and the HA coating around tumor cells effectively protects these cells against immune surveillance.46
Traditionally, HA is extracted from rooster comb, shark skin, bovine eyeballs or human umbilical cords.20, 55, 56 Such HA products suffer from batch-to-batch variations, lack of control over the molecular weight and molecular weight distribution, and the presence of protein impurities57, 58 that can be potentially immunogenic.56, 59 Modern technology has enabled the production of high molecular weight (usually > 1.0 MDa), well-defined HA in large quantities with high purity by bacterial fermentation.60 Currently, microbially produced HA has been approved for wide applications in various clinical treatments and cosmetic applications.58 Low molecular weight HA can be readily generated by γ irradiation or enzymatic degradation.61, 62 Precise control over molecular size distribution of HA is achieved via chemoenzymatic processes.63, 64
3. HA Bulk Gels
While HA participates in diverse biological events and processes, native HA is not a useful biomaterial due to its susceptibility to degradation and inferior mechanical properties. Covalent crosslinking is necessary to impart stability and to improve functions. HA can be directly crosslinked without any chemical modifications. For example, HA has been crosslinked by bisepoxide65, 66 or divinyl sulfone derivatives67 under alkaline conditions. HA can also be crosslinked by glutaraldehyde,68 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)68, biscarbodiimide69 and multifunctional hydrazides70, 71 under acidic conditions. Compared to the native HA, the crosslinked hydrogels exhibit more robust mechanical properties and are less susceptible to enzymatic degradation.
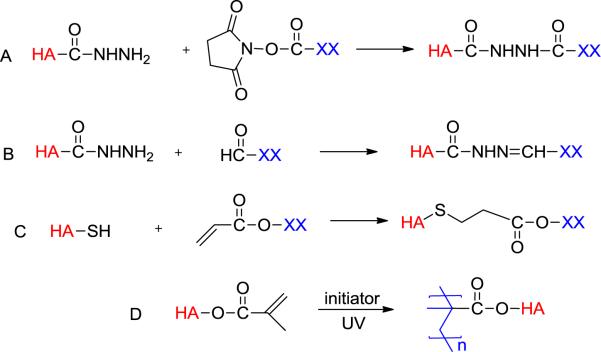
Covalent crosslinking of native HA requires toxic reagents and harsh conditions that are not suitable for cell and protein encapsulation. For tissue engineering applications, chemistries adopted for the synthesis of HA hydrogels should be chemo-selective, and can occur under physiological conditions without generating any toxic by-products or causing severe HA degradation. The gelation kinetic should be fast enough to allow for in situ cell encapsulation for the fabrication of 3D cell/gel constructs or in vivo hydrogel formation in a minimally invasive injectable manner. A variety of chemistries have been adapted to the synthesis of HA-based hydrogels using distinct functional groups on HA as the reactive handles (Figure 2).70, 72–74
Figure 2.
Common crosslinking schemes for the synthesis of HA hydrogels. XX represents PEG, HA or other multifunctional crosslinkers.
3.1. Crosslinking using hydrazide-functionalized HA
Hydrazide functionalized HA has been successfully synthesized by reacting HA with a large excess of adipic acid dihydrazide (ADH) in the presence of EDC and 1-hydroxybenzotriazole (HOBt) at pH 6.875 or EDC alone at pH 4.75.76 Adipic acid dihydrazide-derivitized HA (HAADH) has been crosslinked using active ester- (Figure 2A) or aldehyde-mediated reactions (Figure 2B).75, 77, 78 Aldehyde-functionalized HA (HAALD) has been synthesized by periodate oxidation of HA; this reaction inevitably leads to HA degradation.77 Using a hetero-bifunctional reagent containing a protected aldehyde in the form of an acetal and an acyl hydrazide, HAALD has been prepared by EDC/HOBt-mediated coupling reaction, followed by a mild acid treatment.75 Simple mixing of HAADH with HAALD resulted in elastic gels, with water being the only by-product.
Ossipov et al.79 recently developed a new approach for the introduction of unique reactive handles into HA. Amidation of HA with homobifunctional reagents containing a divalent disulfide-based protecting group, followed by dithiothreitol (DTT) treatment that eliminated the generated 2-thioethoxycarbonyl moiety, afforded free amine-type functionality, such as hydrazide, aminooxy, and carbazate. The same methodology was used to graft serine residues to the HA backbone, which were subsequently oxidized into aldehyde groups without causing severe HA chain scission. A series of new hydrogel materials was prepared by mixing the new HA aldehyde derivative with different HA-nucleophile counterparts.
Hydrazide-derivatized HA facilitates the preparation of hydrogel materials containing biologically active factors such as drugs, growth factors and cytokines. Hydrogels prepared from HAADH and PEG bis(succinimidyl propionate) showed excellent cell infiltration and chondroosseous differentiation when loaded with bone morphogenetic protein-2 (BMP-2). The synergistic action of insulin-like growth factor-1 (IGF-1) with BMP-2 promoted cartilage formation, while the addition of TGF-β and BMP-2 led to rapid replacement of the matrix by bone.75 When loaded with bupivacaine, HAADH/HAALD gels were found to prolong the sciatic nerve blockade in a rat model without a statistically significant increase in myotoxicity.77 When covalently conjugated with dexamethasone, the same hydrogels were effective in preventing postoperative peritoneal adhesions.80
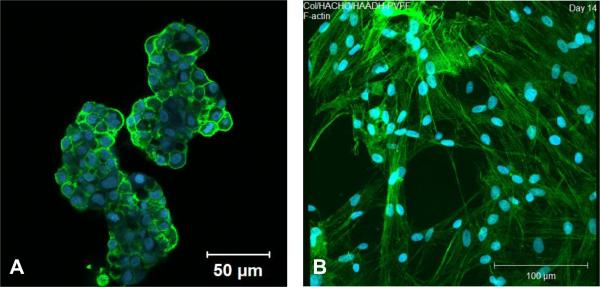
The role of HA in cancer metastasis motivated us to test the efficacy of HA gels in establishing a 3D prostate cancer tumor model that can be used to test the efficacy of anti-cancer drugs. Bone metastatic prostate cancer cells (C4-2B cells) were successfully encapsulated in HA gels with minimal cell death by mixing the cell pellets with HAADH and HAALD. This hydrogel culture system maintains cell growth and viability; cancer cells within the HA hydrogel form distinct aggregated structures (Figure 3A) reminiscent of real tumors. The HA hydrogel system was used to test the efficacy of several anti-cancer drugs (camptothecin, docetaxel and rapamycin) in terms of specificity, dose and time responses, alone and in combination. We discovered that cells in 3D are more susceptible to the drug treatment than those in 2D, possibly due to the enhanced cell-cell communication in HA hydrogels.81
Figure 3.
Confocal images of C4-2B cells cultured in HAALD/HAADH hydrogel for 2 days (A, Reproduced with permission,81 Copyright 2009, Elsevier) and PVFFs cultured in HAALD/HAADH/collagen gels for 14 days (B, Reproduced with permission,82 Copyright 2010, Mary Ann Liebert). The F-actin was stained green with phalloidin and the cell nucleus was stained blue with Draq5.
The orthogonal nature of the hydrazone chemistry, combined with the rapid gelation kinetics, permits the facile incorporation of structural proteins in the HAADH/HAALD gels. A composite hydrogel containing self-assembled collagen fibrils interpenetrated in an amorphous, covalently cross-linked HA matrix was synthesized using HAADH, HAALD and acid-solublized collagen. Primary porcine vocal fold fibroblasts (PVFFs) encapsulated in the matrix adopted a fibroblastic morphology (Figure 3B), proliferated readily and expressed genes related to important ECM proteins. Applying the torsional wave analysis, we found that the elastic modulus of the cell/gel constructs increased moderately over time, reaching a value close to that of pig vocal fold lamina propria at day 28. It is postulated that PVFFs residing in gels alter the matrix organization, chemical compositions and viscoelasticity through cell-mediated remodeling processes.82
3.2. Crosslinking using thiolated HA
Using dihydrazide reagents containing a disulfide bond in the middle, Prestwich and colleagues successfully introduced thiol groups to HA.83, 84 Alternatively, thiolated HA can be prepared by reacting hydrazide-derivatized HA with Traut's reagent.85 A biocompatible gelation method was developed by crosslinking thiolated HA with multifunctional electrophiles, such as PEG diacrylate (PEGDA), through the Michael-type addition reaction (Figure 2C).17, 74 Although the Michael addition reaction is kinetically much faster than the disulfide bond formation, stress sweep tests on short-term cured hydrogels revealed the simultaneous, but gradual, formation of disulfide crosslinks in the hydrogels.86 The EDC-mediated coupling reaction can be readily applied to gelatin and heparin (HP) to afford the respective thiolated precursors. Synthetic ECM (sECM) was prepared by mixing thiol-functionalized HA, thiol-functionalized gelatin and PEGDA under physiological conditions. The elastic moduli of the resultant gels can span three orders of magnitude, from 11 to 3500 Pa by varying the: (1) molecular weight of starting HA employed; (2) percentage of thiol modification on HA; (3) concentration of thiol-modified HA in the hydrogel; (4) molecular weight of PEGDA; and (5) ratio of thiols to acrylates.87 By incorporating disulfide bonds in the backbone of the PEGDA crosslinker,88 cells trapped in these synthetic ECM were released by incubating the cell/gel constructs with N–acetylcysteine or glutathione.
Co-crosslinking thiolated HA with thiolated heparin creates an immobilized heparin that acts as a mimic of heparan sulphate proteoglycan for growth factor sequestration and release. Hydrogels composed of crosslinked, chemically modified HA, gelatin and heparin were preloaded with vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), keratinocyte growth factor (KGF) or platelet derived growth factor (PDGF) either individually or in combination with VEGF and implanted into the Balb/c mouse ear pinna. It was found that the introduction of dual cytokines, one to initiate angiogenesis and the other to induce maturation, produced microvessel networks that were more mature than those produced by administration of either cytokine independently. Further, the inclusion of heparin had a cytokine-specific effect, promoting microvascular maturity for co-delivery of VEGF + KGF, but inhibiting maturity for delivery of VEGF + Ang-1 (Figure 4). The ability to elicit microvasculature with sustained levels of maturity is an important pre-requisite for effective application of the gelation technique towards regenerative therapeutic strategies.89
Figure 4.
Representative images of microvasculature stained for smooth muscle α-actin (α-SMA, brown) and von Willebrand factor (vWF, red) at 7 days post-surgery. The contralateral ear (A) exhibited mature vasculature (microvessels indicated by long arrows). HA:HP-VEGF treated tissue exhibited the least mature vasculature at 7 days post-surgery (B, hyperfused vessels indicated by short arrows). HA:HP-VEGF+KGF treated tissue (C) exhibited the most mature vasculature of all 7 day experimental cases (vessels indicated by long arrows). Magnification: 400×. Reproduced with permission,89 Copyright 2008, Elsevier.
Additional information regarding the applications of this particular sECM system in cell therapy, growth factor delivery and the regeneration of healthy bladder, bone, cartilage, sinus, spinal cord and vocal fold tissues and the creation of disease models can be found in a recent review article.17 In addition to PEGDA, thiolated HA has been crosslinked by haloacetate,90 aminoethyl methacrylated HA,91 (meth)acrylate bearing, physically associated triblock copolymer gels92 and gold nanoparticles.93
3.3. Crosslinking using (meth)acrylated HA
(Meth)acrylate groups have been introduced to HA by reacting HA with a large excess of glycidyl methacrylate (GMA),94, 95 or methacrylic anhydride in aqueous media.17 The resultant HA derivatives (macromers) were converted into elastic hydrogels by radical polymerization using a redox initiator pair or a photoinitiator in the presence of light. (Figure 2)96 Photoinitiated radical crosslinking permits temporal and spatial control over hydrogel geometry and properties by light-triggered polymerization. The properties of the resultant networks can be tailored by modification of the HA molecular weight, the degree of methacrylation, and the concentration of the macromer.97 The hydrogel properties can be further modulated by grafting a synthetic polymer to HA prior to the methacrylation reaction.95 Cells and protein molecules can be mixed with the macromer solution and be encapsulated in situ upon UV irradiation in the presence of a biocompatible initiator. Mechanically robust and cell adhesive interpenetrating networks have been produced by incorporating collagen in a radically crosslinked HA network.98 Photocrosslinkable fibronectin has been covalently integrated into HAGAM gels to enhance cell adhesion.99 (Meth)acrylated HA has been extensively investigated for use in device fabrication, drug delivery and tissue engineering.17, 96
In addition to the crosslinking density, the specific chemistry employed for (meth)acrylation determines the degradation kinetics of radically crosslinked HA hydrogels.100 For example, the conjugation of GMA to HA can be achieved either through the transesterification reaction at low pH (transfer of only the methacrylate group) or through the ring opening reaction of epoxide at high pH (leading to the presence of an additional glyceryl spacer). Because the ester bond is susceptible to hydrolysis while the ether bond is not, different reaction conditions led to hydrogels with varying stability in aqueous condition.100, 101 When hydrolytically degradable lactic acid or caprolactone repeats were purposefully introduced between the HA backbone and the methacrylate group,102 the mesh size of the resultant hydrogels evolve over time due to crosslink degradation. Compared to the static HA scaffolds, the new hydrogels are more conducive to neotissue formation.103
In addition to radical polymerization, (meth)acrylated HA can participate in Michael-type addition reactions. Acrylated HA synthesized by reacting HAADH with N-acryloxysuccinimide has been crosslinked with a matrix metalloproteinase (MMP) degradable peptide crosslinker. Mouse MSCs encapsulated in the gels spread when both cell adhesive RGD peptide and MMP degradation sites were present in the hydrogel. Unfortunately, such gels degrade rapidly within a week.104 Acrylated HA crosslinked by tetrathiolated PEG was used as a scaffold for BMP-2 and MSCs for rat calvarial defect regeneration.105
3.4. Other crosslinking methods
HA hydrogels were prepared using pyridyl disulfide derived HA and α, ω-thiolated PEG via the thiol-disulfide exchange reaction at pH 7.4, releasing pyridine-2-thione as the by-product. The chemistry allows for in situ cell and growth factor encapsulation.106 A photoresponsive drug delivery system was developed using HA conjugated with PEG-bound anthracene. The ability of anthracene to dimerize at λ>300 nm and dissociate at λ<300 nm permits the facile adjustment of gel properties and the release profiles of the encapsulated drugs.107 Furan-modified HA derivatives were synthesized and crosslinked by PEG dimaleimide via the Diels Alder reaction.108 These HA crosslinked hydrogels were shown to be cytocompatible and may represent a promising material for soft tissue engineering. Enzymatically crosslinkable HA has been synthesized by coupling tyramine to HA, directly109, 110 or via the grafted dextran.111 Crosslinking was initiated by the addition of hydrogen peroxide and horseradish peroxidase (HRP). Although interesting from the materials chemistry perspective, the translational aspects of these new hydrogels have not been addressed.
4. HA-based hydrogel particles (HGPs, microgels or nanogels)
The HA hydrogels discussed above are bulk gels in which the individual polyanionic chains are interconnected up to the macroscopic level. If the crosslinked network is reduced to the microscopic scale, microgels and nanogels (or hydrogel particles, HGPs) are obtained. These hydrophilic colloidal particles are ideal drug delivery vehicles due to their tunable sizes, colloidal stability, low cytotoxicity, large surface area and protection from enzymatic degradation. The nanosized, water-filled interior space not only prevents severe aggregation of the drug payload but also is suitable for the physical association and covalent conjugation of therapeutic agents.11 Various processing techniques have been adopted for the production of HA HGPs.
4.1. HA HGPs by inverse emulsion crosslinking
The majority of reported HA HGPs were prepared by a water-in-oil (w/o) emulsion crosslinking process, where the reaction occurs within the aqueous droplets of HA or its derivatives that are stably dispersed in a continuous organic phase with the aid of oil-soluble surfactants.112 The chemical reaction rate affects both the nucleation and growth process, thus also affecting the particle size and size distribution.113, 114 Control over the particle size and size distribution can be achieved by establishing uniform micellar dispersion and efficient cross-linking, at the same time avoiding immature micelle collision and ripening.
Microgels of HA/chitosan glutamate with an average diameter of 29 μm were prepared in an inverse emulsion of water/mineral oil/Span 80 at 40 °C, possibly through charge complexation. Owing to the rapid dissolution of the particles, over 60% of a model drug, gentamicin sulphate, loaded into the particles was released in less than an hour.115 To improve the particle stability, a two-step crosslinking process was developed.116 In the first step, HA was crosslinked by ADH in the presence of EDC within Span 80-stablized water droplets dispersed in mineral oil. In the second step, the collected particles were subjected to the same reaction in organic media. The resultant HGPs (5–20 μm) were capable of sustained release of physically incorporated plasmid-DNA. Separately, polyaspartylhydrazide (PAHy) was employed in place of ADH as a crosslinker for the production of HA submicrometer HGPs (Figure 5A and 5B). The inverse emulsion system is composed of an aqueous solution of HA and PAHy at pH 7.5 dispersed in an oil phase of propylene carbonate, ethyl acetate, and Span 85. Upon addition of EDC and N-hydroxysulfosuccinimide sodium salt (in that order), stably crosslinked, enzymatically degradable HA particles were produced. A model drug, 5-fluorouracil, entrapped in the HA microgels was released from the particles via a Fickian diffusion.117
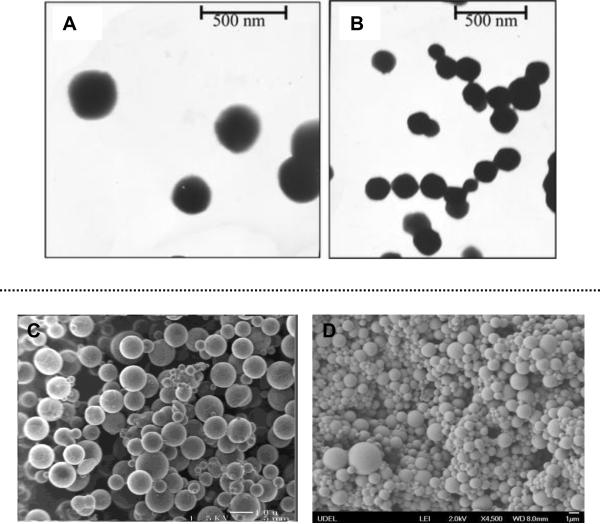
Figure 5.
Top: transmission electron micrographs of HA–PAHy nanoparticles at a calibrated magnification of (A) 36000× and (B) 30000×. Reproduced with permission,117 Copyright 2008, American Chemical Society. Bottom: scanning electron micrographs of HA HGP10 (C, Reproduced with permission,120 Copyright 2006, American Chemical Society) and HGP0.9 (D, Reproduced with permission,114 Copyright 2009, American Chemical Society).
Chemically modified HA provides a more efficient means for the production of HA HGPs. For example, radical polymerization of HAGMA in an inverse emulsion of water in hexanes containing Span 80 and Tween resulted in nanogels of ~30 nm in size with an estimated swelling ratio of ~10.118 Disulfide crosslinked HA HGPs (200–500 nm) were prepared by ultrasonically dispersing the aqueous solution of thiolated HA in hexanes containing a surfactant mixture (Span 65, Span 80, and Tween 80). The HA hydrogel particles loaded with siRNA were selectively endocytosed by cells expressing HA-specific CD44 receptors on the surface. The release of siRNA was triggered by an intracellular reducing agent and the released siRNA was effective in silencing the target genes.119
Our group has developed techniques for the production of HA-based, covalently crosslinked HGPs of micron to submicron dimensions. HA HGPs with an average diameter of 10 μm (HGP10, Figure 5C) were prepared by crosslinking HA derivatives carrying hydrazide (HAADH) and aldehyde (HAALD) groups within the inverse emulsion droplets of water/mineral oil/Span 80.120 HA HGPs with an average diameter of ~0.9 μm (HGP0.9, Figure 5D) and a narrow particle size distribution were synthesized via the chemical crosslinking of HA with DVS using a sodium bis(2-ethylhexyl)sulfosuccinate) (AOT)/isooctane reverse micelle system in the presence of 1-heptanol.121 While HGP10 exhibit residual functional groups (aldehyde and hydrazide) after the synthesis, sodium periodate oxidation was used to introudce aldehyde groups to the HGP0.9, at the same time, reducing the particle diameter to ~0.5 μm (oxHGP0.5). Alternatively, photocrosslinkable, HA HGPs were synthesized by chemical modification of HA HGP0.9 with GMA (HGP0.9-GMA) without changing the average particle size or porosity.122 These functional groups can be used as reactive handles for covalent conjugation of therapeutic molecules. These HA particles are highly porous and enzymatically more stable compared to their macroscopic counterparts. HGP0.9 and HGP0.9-GMA have an average mesh size of approximately 5.5–7.0 nm while HGP10 contains pores greater than 12 nm. These particles are non-toxic to the cultured primary fibroblasts, chondrocytes and MSCs.
The HA-based HGPs are intended not just as passive fillers; rather they are engineered to exhibit defined biological functions. The presence of nanoscale pores within the HA HGPs makes them ideal candidates as release depots for protein-based, biomacromolecular drugs, such as growth factors. To improve the bioactivity of HA HGPs, perlecan domain I (PlnDI), with heparan sulphate chains for binding of heparin binding growth factors, was conjugated to the particles (HGP-P1) through the core protein via a flexible PEG linker using reducative amination reaction. Compared to HGPs without PlnDI immobilization, HGP-P1 exhibited a higher BMP-2 binding capacity and a better control over BMP-2 release (Figure 6). More importantly, the released BMP-2 effectively induced the chondrogenic differentiation of MSCs, as evidenced by the strong alcian blue staining and the positive immunohistochemical staining for collagen II and aggrecan (Figure 6). Similarly, a sustained release of biologically active BMP-2 was achieved using HA HGPs with covalently integrated heparin. By varying the amount of heparin incorporated in HGPs, BMP-2 release kinetics are readily tuned.124 Ultimately, these HGPs could be used as injectable materials for cartilage repair.
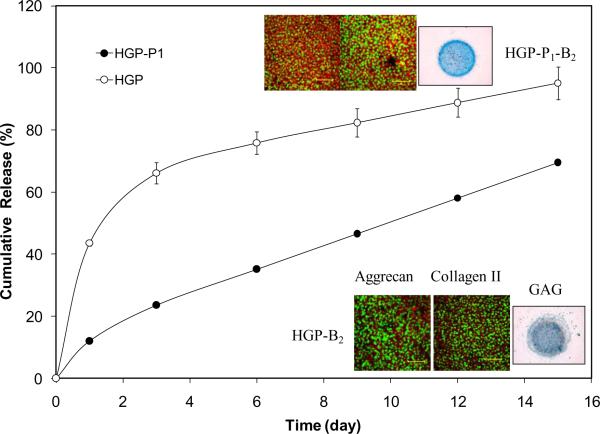
Figure 6.
Cumulative release of BMP-2 from PlnDI conjugated HGPs and the control HGPs. Inserts show the immunostaining for aggrecan and collagen II and Alcian blue staining for GAG expressed by C3H10T1/2 cells cultured in the presence of various particles. HGP: HA hydrogel particles without PlnDI conjugation; HGP-B2: BMP-2 loaded HGPs; HGP-P1: PlnDI conjugated HGPs; HGP-P1-B2: HGP-P1 loaded with BMP-2. Reproduced with permission,123 Copyright 2009, Elsevier.
4.2. HA HGPs by spray drying
Spray drying is a convenient technique for the preparation of pharmaceutical microparticles. In this process, solutions or suspensions of drugs, polymers, and/or particles are atomized to fine droplets, and the solvent is evaporated from the droplets by the stream of hot air. The dry solid is usually collected in a drum or cyclone.125 Spray drying can be combined with colloidal templating to produce highly porous HA particles. Specifically, HA and polystyrene latex (PSL) particles were mixed in an aqueous solution and spray-dried using a two-fluid nozzle system. The composite particles were collected and washed with an organic solvent to dissolve the PSL templating agent. The porosity and pore size of the resulting particles were easily controlled by changing the initial mass ratio of precursor to templating agent and by altering the size of the PSL template particles.126 Because HA was not crosslinked in the particles, exposure to aqueous media can lead to the rapid dissolution of the particles. In fact, when preparing the particles via spray drying, HA is frequently mixed with other polymers (PEG127 or Eudragit128), lipid or surfactant molecules to stablize the particles and to modulate the drug release. Relatively well defined HA HGPs were prepared by reactive spray drying of a dilute HA-SH precursor solution containing sodium tetrathionate. The mean particle size was to 2.3 μm and the water content after spray drying was ~14%. Erythropoietin (EPO) was encapsulated in the microgels without degradation. Elevated plasma concentration of EPO was maintained up to 7 days in vivo using this HA formulation.85
4.3. Other particle fabrication techniques
Hydrophobically modified HA can self-assemble in water to form nanogels owing to its amphiphilic nature.129 These particles have been used to deliver small molecule drugs or proteins that are chemically or physically bound to HA.130, 131 The systems are considered in terms of intracellular delivery to different cultured cells that express HA-specific receptors differently.132,133, 134 This topic is beyond the scope of this review and the readers are referred to recent opinion papers135, 136 for more information. Alternatively, a micromolding approach was employed for the preparation of shape-controlled, harvestable HA HGPs. In the process, a hydrogel precursor solution of methacrylated HA and a photoinitiator in water was deposited onto plasma-cleaned hydrophilic PDMS patterns and then photocrosslinked via the exposure to the UV light. The resulting microgels were removed, hydrated, and then harvested. When cells are included in the precursor liquid, cell-laden microgels are prepared. Disk-shaped HA particles over 200 × 200 μm2 were prepared by this method.137
5. HA Hydrogels with complex structure and improved functions
It is well known that the structural characteristics of hydrogels, such as the mesh size and the matrix morphology, have profound effects on cellular functions including cell migration, proliferation, phenotype and metabolism.138, 139 Traditional HA-based hydrogels are macroscopic gels consisting of nanoscale pores defined by the soluble polymer precursors that are randomly interconnected, lacking the structural complexity and functional diversity seen in the natural ECM.140 These materials exhibit homogeneous bulk properties and are unable to induce cells to regenerate architecturally complex healthy tissue. On the other hand, the natural ECM has to be able to accommodate cells that are typically ~10 μm in size, yet exhibit features at all length scales from the macro- down to the molecular to allow cells to respond, maintain and remodel their environment as they go through various cell cycles and different stages of development.139 Thus, there is a need for methods that can create structural complexity within HA hydrogel scaffolds to guide tissue regeneration.141, 142
5.1. Complex HA gels by templating or patterning
Photocrosslinking is a versatile technique well suited for the fabrication of 3D hydrogels with complex geometry and structures with spatial resolution. The macromer liquid can fill the space around a template, and light-induced polymerization will fix the gel structure. Light can be blocked by a mask so that the light triggered gelation only occurs in the exposed areas. Schmidt and colleagues developed HA hydrogels with anisotropic swelling via the combination of chemical crosslinks and patterned photocrosslinks. In this method, an unswollen chemically crosslinked hydrogel substrate was spatially patterned with photocrosslinks that restricted swelling at selected sites. The resulting dual-crosslinked hydrogel swelled anisotropically because of differential crosslink densities between the photopatterned and non-photopatterned regions. Anisotropic swelling permitted the hydrogel to contort and evolve a shape different from that of the unswollen hydrogel.143
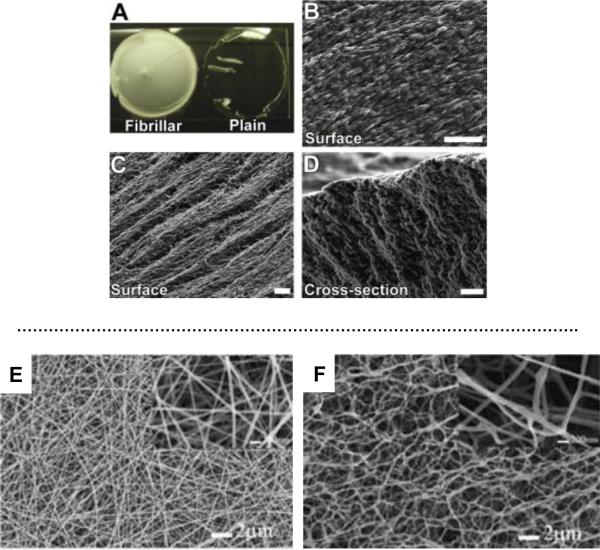
Using a simple and inexpensive “crystal templating” technique, Zawko et al. fabricated HA scaffolds with space-filling dendritic pore networks and fibrillar microtopography. Specifically, dendritic urea crystals with fibrillar microstructure were grown in solution cast films of HAGMA that was subsequently photocrosslinked around the crystal network to lock in the dendritic configuration. After the crystals were dissolved, porous fibrillar HA scaffolds were obtained (Figure 7, A–D). These nanostructure hydrogels may be applicable as regenerative patches for skin and other tissues.144
Figure 7.
Fibrillar HA hydrogels. Top: (A) Comparison of a fibrillar GMHA hydrogel (left) with a plain GMHA hydrogel (right); (B) Scanning electron micrograph depicting the surface of a urea-templated HA hydrogel that resembles the fibrillar microstructure of the basement membrane. (C) The surface of the fibrillar hydrogel reveals pores among the fibers. (D) A cross-section perpendicular to the direction of fiber length that shows that the fibrillar microstructure is present throughout the thickness of the hydrogel. Scale bars: 20 μm. Reproduced with permission,144 Copyright 2010, Elsevier. Bottom: Scanning electron micrographs of HA-DTPH/PEO blend scaffolds before PEO (E) and after PEO (F) extraction. Reproduced with permission,155 Copyright 2006, Wiley.
Photocroslinking chemistry has been combined with Michael addition reaction to form 3D patterns to regionally control cell behavior. A primary addition reaction was applied to introduce protease degradable peptide crosslinks. Subsequently, a radical reaction was introduced for spatial control via the formation of non-degradable kinetic chains. These differential network structures either permitted (primary crosslinking only) or inhibited (sequential crosslinking) cellular remodeling. In addition, network structures were found to dictate MSC fate decisions, with spatial control, by controlling the spreading of the encapsulated MSCs.145, 146
5.2. Nanofibrous HA hydrogels by electrospinning and templating
Over the past decade, electrospinning147 has emerged as a highly promising process for producing tissue engineering constructs since the resulting scaffolds possess many of the desired properties, such as a high surface-to-volume ratio, high porosity and an interconnected 3D porous network.148, 149 A wide range of natural or synthetic materials have been electrospun into fibrous scaffolds with desirable morphology and tissue-like mechanical properties.150 Electrospinning of HA, however, can be challenging because of the hydrophilic nature of HA, the high viscosity and the high surface tension of an aqueous HA solution.151, 152 HA fibrous meshes could be produced by blowing-assisted electrospinning or using a mixed solvent system.153, 154 To prevent complete dissolution, covalent crosslinking, during or after the spinning process, is necessary.151 Reactive electrospinning has been applied to the fabrication of hydrogel nanofibers using thiolated HA and PEGDA.155 The cross-linking reaction occurs simultaneously during the electrospinning process using a dual-syringe mixing technique. Poly(ethylene oxide) (PEO) is added into the spinning solution as a viscosity modifier to facilitate the fiber formation and is selectively removed with water after the electrospinning process. The nanfibrous HA hydrogels have a 3D fibrous structure with a fiber diameter of 110 nm (Figure 7E, F). When adsorbed with fibronectin, the 3D scaffolds facilitate the migration of NIH 3T3 fibroblasts into the scaffolds.
A common problem in the design of tissue engineered scaffolds using electrospun scaffolds is the poor cellular infiltration into the structure. To tackle this issue, electrospun fibrous meshes with defined gradients throughout the thickness of the scaffolds were fabricated using methacrylated HA. Specifically, mechanical and adhesive gradients were created using methacrylated HA and RGD-conjugated HA by mixing two solutions (one ramping up, one ramping down) prior to electrospinning and fiber collection. UV irradiation of the scaffolds led to inter- and intrafiber crosslinking, giving rise to a fibrous hydrogel scaffold with enhanced cell infiltration.156 An alternative approach to enhance cell infiltration and attachment is to introduce macropores to the nanofibrous scaffolds.157 A 3D macroporous and nanofibrous HA scaffold was fabricated by an electrospinning process combined with a salt leaching technique. By the simultaneous deposition of salt particulates as a porogen during electrospinning and subsequent chemical crosslinking and salt leaching, a water-swellable HA-based scaffold retaining a macroporous and nanofibrous geometry was produced. Chondrocytes were found to attach and proliferate readily in this matrix, maintaining the chondrocytic morphology.
5.3. HA hydrogels containing embedded HGPs
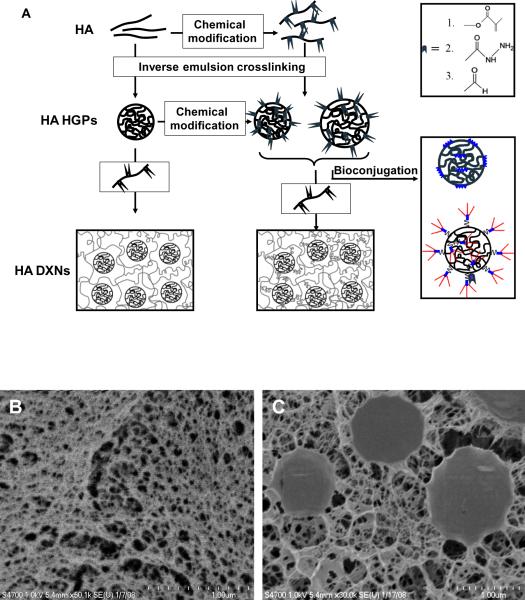
As the field of materials science continues to evolve, sophisticated hydrogel scaffolds with hybrid composition, defined bioactivities, and tunable viscoelasticity are being developed. Artificial scaffolds were employed not only to create a 3D structure that mimics the architecture and function of tissue, but also provide a delivery vehicle for cells or to manipulate the environment of cells in vitro.141, 158 Porous hydrogel matrices embedded with HGPs of micro- to nano- dimensions provide tailored viscoelasticity and structural integrity that are suitable for use as tissue engineering scaffolds. The HGPs, irrespective of their chemical nature, shape or sizes, can be engineered as means for physical anchorage and structural reinforcement. In addition, they can be designed as controlled release depots for spatio-temporal presentation of biologically active compounds that ultimately elicit desired cellular activities. To accomplish these goals, the particles have been physically trapped, covalently bound or co-assembled in the matrices.140 When both the particles and the secondary network are covalently crosslinked, the hybrid gels are referred to as doubly crosslinked networks (Figure 8).
Figure 8.
Synthetic procedures for the fabrication of HA HGPs and DXNs (A) and cryogenic scanning electron micrographs of HA bulk gel (B) and DXN (C). Reproduced with permission,114 Copyright 2009, American Chemical Society.
Hirakura et al. reported a159 hybrid HA hydrogel encapsulating nanogels assembled from cholesteryl group-bearing pullulan (CHP). HA modified with 2-aminoethyl methacrylate was cross-linked via Michael addition reaction in the presence of CHP nanogels. Therapeutic peptides and proteins, such as glucagon-like peptide-1, insulin and erythropoietin, were spontaneously trapped in the CHP nanogels in the HA gel just by immersing hybrid hydrogels into the drug solutions. CHP/protein complex nanogels were released from the hybrid hydrogels in a sustained manner both in vitro and in vivo. The release was controlled by the cross-linking density and the degradability of the HA gel, modulated by the initial gelation condition. The synergy between the CHP nanogel as a drug reservoir and the HA gel as a nanogel-releasing matrix of the hybrid hydrogel system simultaneously achieved both simple drug loading and controlled release with no denaturation of the protein drugs.
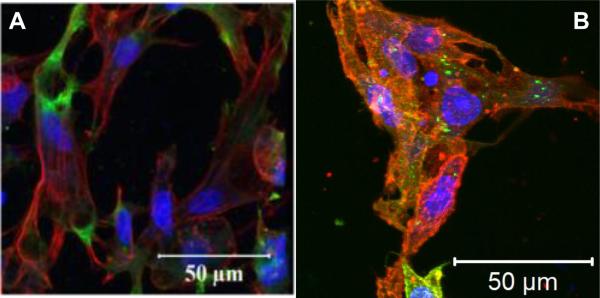
Our group has created a new type of HA-based, cell-adhesive hydrogels that direct the initial attachment and the subsequent differentiation of human MSCs into pre-osteoblasts without osteogenic supplements. Doubly crosslinked networks (DXNs) were engineered by embedding gelatin conjugated HA HGPs (gHGPs) in a secondary network established by HAGMA (Figure 8A). MSCs readily attached to the resultant DXNs, adopting a stellated cell morphology with extended filopodia by day 7 (Figure 9A). Moreover, cells had migrated deep into the matrix, forming a three dimensional, branched and interconnected cell community. After 28 days of culture, Type I collagen production and mineral deposition were detected in the absence of osteogenic supplements, suggesting induction of osteogenic differentiation.160 Using the same matrix, but replacing gelatin with a collagen-like peptide (HA-clpHGP), we observed integrin-mediated cellular responses including adhesion, spreading and proliferation (Figure 9B) on the composite DXNs161 Overall, this HA composite matrix has great promise for directing the osteogenic differentiation of MSCs by providing an adaptable environment through the spatial presentation of cell adhesive modules.
Figure 9.
Confocal fluorescence images of MSCs cultured on HA-gHGP (A) and HA-clpHGP (B, day 7). Cells were fixed and stained for actin stress fibers (TRITC-phalloidin; red), nuclei (Draq5; blue) and vinculin (FITC-anti-vinculin; green). Reproduced with permission,160, 161 Copyright 2011, Elsevier.
Our discussion so far on HA-based DXNs has been limited to composite hydrogel systems containing physically entrapped HGPs. The absence of specific linkages between the particles and the surrounding matrix gives rise to a depleted interphase that can result in inferior mechanical properties and limit the tunability of the overall drug release profile of the composite gels.140 Using HA-based HGPs as the constituent building blocks and the microscopic crosslinkers, we have engineered HA-based hydrogels containing covalently integrated HA particles. This type of HA DXNs (Figure 8A) contains highly crosslinked HA HGPs in a loosely crosslinked secondary HA network, both of which are nanoporous. For example, simply mixing HGPs containing reactive aldheyde (HGP10 or oxHGP0.9) with a soluble HA derivative carrying complementary functional gorups (HAADH) results in a hierachically structured, elastic hydrogel within 5 mins.114, 120 A close inspection of the cryoSEM image (Figure 8D) for DXNs indicates a diffuse interphase between individual oxHGPs and the matrix, which proves that the secondary network originates from the particle surface. As a result, the secondary network can exert mechanical constraints on the HGPs, leading to the deformation of HGP through the covalent hydrazone linkage between the secondary network and the HGP surface. The viscoelastic properties of the matrix can be readily modulated by varying the particle size, surface functional group, inter-particle and intra-particle crosslinking.
Separately, macroscopic HA hydrogels containing covalently integrated hydrogel particles (HA-c-HGP) were prepared by radical polymerization of HAGMA in the presence of crosslinkable methacrylated HGPs (HGP0.9-GMA).122 Again, the covalent linkages between the hydrogel particles and the secondary HA matrix resulted in the formation of a diffuse, fibrilar interface around the particles. The composite network exhibited superior mechanical properties than the traditional bulk gels derived from HAGMA, and is conducive to the maintenance of the chondrocytic phenotype. In addition, BMP-2 loaded into the immobilized HGPs was released from the HA-c-HGP gels in a controlled manner with reduced initial burst over prolonged periods of time. We believe that the HA-based DXN systems, with the built-in topological features, temporal/spatial release of growth factors and tunable viscoelasticity, recapture the essential structural, morphological and functional characteristics of the natural ECM. Cells residing in the matrix will receive specific biological cues based on the particle chemistry and the local gradient of the morphogenic factors. These HA-based DXNs are attractive candidates for the repair and regeneration of soft tissues.
Covalent integration of well-defined poly(oligo (ethylene oxide) monomethyl ether methacrylate) nanogels (110–120 nm) into a 3D, photocrosslinked HA (HAGMA) matrix resulted in a nanostructured HA matrix.162 The nanogels with pendant hydroxyl groups were prepared by activators generated electron transfer atom transfer radical polymerization (AGET ATRP) in cyclohexane inverse miniemulsion. Hydroxyl-containing nanogels were functionalized with methacrylated groups to generate photoreactive nanospheres. The introduction of disulfide moieties into the polymerizable groups resulted in a controlled release of nanogels from cross-linked HAGMA hydrogels under a reducing environment. It is shown that swelling and nanogel content are independent of scaffold mechanics. In vitro assays showed the nanostructured hybrid hydrogels were cytocompatible and the GRGDS contained in the nanogel structure promoted cell-substrate interactions within 4 days of incubation. These nanostructured hydrogels have potential as an artificial ECM impermeable to low molecular weight biomolecules and with controlled pharmaceutical release capability. Moreover, the nanogels can control drug or biomolecule delivery, while HA-based hydrogels can act as a macroscopic scaffold for tissue regeneration and regulator for nanogel release.
6. Conclusion and Outlook
Over the past few decades, HA has evolved from a simple, ubiquitous polysaccharide to a macromolecule with complex biological functions ranging from matrix organization, cell adhesion and migration, angiogenesis and morphologensis, wound healing and inflammatory responses to cancer metastasis. Meanwhile, new crosslinking methods, combined with novel processing and fabrication techniques, have enabled the development of HA hydrogels with tunable structural, mechanical and biological properties. HA-based hydrogel biomaterials have been transformed from passive supporting scaffolds with simple and random structures to instructive matrices with hierarchical organization, anisortropic properties and improved viscoelasticity. While traditional HA-based hydrogels are composed of the polysaccharide chains randomly interconnected at the crosslinking points established by covalent bonds, HA-based hybrid hydrogels consisting of chemically, morphologically and functionally distinct modules interconnected via chemical or physical means have been engineered. Incorporation of microgel or nanogels into existing hydrogels may improve their mechanical properties as well as their biological functions. The embedded particles not only provide physical anchorage and structural reinforcement but also allow for spatio-temporal release of biologically active compounds capable of stimulating desired cell functions locally within the hybrid hydrogel matrix. The nanogels can also serve as chaperones to protect the encapsulated protein. When designing HA-based materials, researchers have started to embrace the unique roles HA plays in signal transduction, and to incorporate its biological functions into biomaterial products. As insight continues to be gleaned from developmental biology and other biology disciplines, more intelligent and complex hydrogel materials will likely emerge as conducive matrices.
Acknowledgments
Work in the authors' laboratories has been funded by grants from the National Institutes of Health (R01 DC008965, XJ; P20 RR017716, XJ; P01 CA098912, MFC).
References
- 1.Garg HG, Hales CA. Chemistry and biology of hyaluronan. Elsevier Ltd.; Oxford: 2004. [Google Scholar]
- 2.Prehm P. Biochem. J. 1984;220:597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laurent TC, Fraser JRE. Faseb Journal. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 4.Turley EA, Austen L, Vandeligt K, Clary C. Journal of Cell Biology. 1991;112:1041–1047. doi: 10.1083/jcb.112.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turley EA, Noble PW, Bourguignon LYW. Journal of Biological Chemistry. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 6.Lepperdinger G, Fehrer C, Reitinger S. Chemistry and Biology of Hyaluronan. Elsevier Ltd.; 2004. [Google Scholar]
- 7.Laurent TCE. The Chemistry, Biology, and Medical Applications of Hyaluronan and Its Derivatives. Portland Press; Miami: 1998. [Google Scholar]
- 8.Zhu W, Mow VC, Rosenberg LC, Tang LH. J Biomech. 1994;27:571–579. doi: 10.1016/0021-9290(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 9.Hardingham TE. In: Chemistry and biology of hyaluronan. Garg HG, Hales CA, editors. Elsevier Ltd; 2004. pp. 1–20. [Google Scholar]
- 10.Allison DD, Grande-Allen KJ. Tissue Eng. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 11.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. Prog. Polym. Sci. 2008;33:448–477. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent T. Ciba Found Symp. 1989;143:1–20. [PubMed] [Google Scholar]
- 13.Chen WYJ, Abatangelo G. Wound Repair and Regeneration. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 14.Toole BP. Journal of Internal Medicine. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 15.Stern R. Seminars in Cancer Biology. 2008;18:237. doi: 10.1016/j.semcancer.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Spicer AP. Current Opinion in Cell Biology. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 17.Burdick JA, Prestwich GD. Adv. Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent TC, Laurent UB, Fraser JR. Immunol Cell Biol. 1996;74:A1–7. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 19.Weigel PH, Hascall VC, Tammi M. Journal of Biological Chemistry. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 20.Meyer K, Palmer JW. Journal of Biological Chemistry. 1934;107:629–634. [Google Scholar]
- 21.Holmes MWA, Bayliss MT, Muir H. Biochemical Journal. 1988;250:435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan RW, Gray SD, Titze IR. Otolaryngology-Head and Neck Surgery. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 23.Knudson CB, Knudson W. Faseb J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- 24.Day RM, Mascarenhas MM. Chemistry and Biology of Hyaluronan. Elsevier Ltd.; 2004. [Google Scholar]
- 25.West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. International Journal of Biochemistry & Cell Biology. 1997;29:201–210. doi: 10.1016/s1357-2725(96)00133-1. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan EP, Longaker MT, Lorenz HP, Gregory SM. Advances in Clinical Chemistry. Volume 48. Elsevier; 2009. pp. 137–161. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 28.Etscheid M, Beer N, Dodt J. Cell Signal. 2005;17:1486–1494. doi: 10.1016/j.cellsig.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 29.West DC, Kumar S. Ciba Found Symp. 1989;143:187–201. doi: 10.1002/9780470513774.ch12. discussion 201–187, 281–185. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda K, Dan H, Takayama M, Kumano F, Saitoh M, Tanaka S. Journal of Pharmacology and Experimental Therapeutics. 1996;277:1672–1675. [PubMed] [Google Scholar]
- 31.Solursh M, Hardingham TE, Hascall VC, Kimura JH. Dev Biol. 1980;75:121–129. doi: 10.1016/0012-1606(80)90148-7. [DOI] [PubMed] [Google Scholar]
- 32.Toole BP, Turner RE, Banerjee SD. Prog Clin Biol Res. 1993;383B:437–444. [PubMed] [Google Scholar]
- 33.Chang CH, Liu HC, Lin CC, Chou CH, Lin FH. Biomaterials. 2003;24:4853–4858. doi: 10.1016/s0142-9612(03)00383-1. [DOI] [PubMed] [Google Scholar]
- 34.Goodstone NJ, Cartwright A, Ashton B. Tissue Eng. 2004;10:621–631. doi: 10.1089/107632704323061979. [DOI] [PubMed] [Google Scholar]
- 35.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. J. Biomed. Mater. Res. Part A. 2006;77A:518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond TH, Zhou RX, Hammond EH, Pawlak A, Gray SD. Journal of Voice. 1997;11:59–66. doi: 10.1016/s0892-1997(97)80024-0. [DOI] [PubMed] [Google Scholar]
- 37.Chan RW, Titze IR. The Journal of the Acoustical Society of America. 1999;106:2008–2021. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 38.Gray SD. Otolaryngologic Clinics of North America. 2000;33:679–697. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 39.Butler JE, Hammond TH, Gray SD. Laryngoscope. 2001;111:907–911. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Hirano M, Nakashima T. Annals of Otology Rhinology and Laryngology. 2001;110:417–424. doi: 10.1177/000348940111000505. [DOI] [PubMed] [Google Scholar]
- 41.Kutty JK, Webb K. Tissue Eng Part B Rev. 2009;15:249–262. doi: 10.1089/ten.TEB.2008.0588. [DOI] [PubMed] [Google Scholar]
- 42.Long JL. Curr Opin Otolaryngol Head Neck Surg. 2010;18:521–525. doi: 10.1097/MOO.0b013e32833febf2. [DOI] [PubMed] [Google Scholar]
- 43.Prestwich GD. Accounts Chem. Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 44.Franzmann EJ, Schroeder GL, Goodwin WJ, Weed DT, Fisher P, Lokeshwar VB. Int. J. Cancer. 2003;106:438–445. doi: 10.1002/ijc.11252. [DOI] [PubMed] [Google Scholar]
- 45.Kosaki R, Watanabe K, Yamaguchi Y. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 46.Lokeshwar VB, Obek C, Soloway MS, Block NL. Cancer Res. 1997;57:773–777. [PubMed] [Google Scholar]
- 47.Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M, Lokeshwar BL. Journal of Biological Chemistry. 2001;276:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- 48.Zhang LR, Underhill CB, Chen LP. Cancer Res. 1995;55:428–433. [PubMed] [Google Scholar]
- 49.Naor D, Sionov RV, IshShalom D. Advances in Cancer Research, Vol 71. vol. 71. 1997. pp. 241–319. [DOI] [PubMed] [Google Scholar]
- 50.Bourguignon LYW, Singleton PA, Zhu HB, Diedrich F. Journal of Biological Chemistry. 2003;278:29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- 51.Bourguignon LYW, Singleton PA, Zhu HB, Zhou B. Journal of Biological Chemistry. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- 52.Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, Kishi H, Hiwasa T, Koda K, Nakajima N, Harigaya K. FEBS Lett. 2002;528:101–108. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- 53.Toole BP, Hascall VC. American Journal of Pathology. 2002;161:745–747. doi: 10.1016/S0002-9440(10)64232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stern R. Seminars in Cancer Biology. 2008;18:275–280. doi: 10.1016/j.semcancer.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Mendichi R, Soltes L. Inflammation Research. 2002;51:115–116. doi: 10.1007/pl00012388. [DOI] [PubMed] [Google Scholar]
- 56.Kogan G, Soltes L, Stern R, Gemeiner P. Biotechnology Letters. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 57.Oregan M, Martini I, Crescenzi F, Deluca C, Lansing M. International Journal of Biological Macromolecules. 1994;16:283–286. doi: 10.1016/0141-8130(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 58.Chong BF, Blank LM, McLaughlin R, Nielsen LK. Applied Microbiology and Biotechnology. 2005;66:341–351. doi: 10.1007/s00253-004-1774-4. [DOI] [PubMed] [Google Scholar]
- 59.Shiedlin A, Bigelow R, Christopher W, Arbabi S, Yang L, Maier RV, Wainwright N, Childs A, Miller RJ. Biomacromolecules. 2004;5:2122–2127. doi: 10.1021/bm0498427. [DOI] [PubMed] [Google Scholar]
- 60.Lapcik L, De Smedt S, Demeester J, Chabrecek P. Chemical Reviews. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 61.Rehakova M, Bakos D, Soldan M, Vizarova K. International Journal of Biological Macromolecules. 1994;16:121–124. doi: 10.1016/0141-8130(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 62.Tawada A, Masa T, Oonuki Y, Watanabe A, Asari Y. Matsuzaki and A. Glycobiology. 2002;12:421–426. doi: 10.1093/glycob/cwf048. [DOI] [PubMed] [Google Scholar]
- 63.DeAngelis PL. Curr. Pharm. Biotechnol. 2008;9:246–248. doi: 10.2174/138920108785161550. [DOI] [PubMed] [Google Scholar]
- 64.Jing W, DeAngelis PL. Journal of Biological Chemistry. 2004;279:42345–42349. doi: 10.1074/jbc.M402744200. [DOI] [PubMed] [Google Scholar]
- 65.Laurent TC, Gelotte B, Hellsing K. Acta Chem. Scand. 1964;18:274. [Google Scholar]
- 66.Segura T, Anderson BC, Chung PH, Webber RE, Shull KR, Shea LD. Biomaterials. 2005;26:359–371. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 67.Hahn SK, Jelacic S, Maier RV, Stayton PS, Hoffman AS. Journal of Biomaterials Science-Polymer Edition. 2004;15:1111–1119. doi: 10.1163/1568562041753115. [DOI] [PubMed] [Google Scholar]
- 68.Tomihata K, Ikada Y. Journal of Polymer Science Part a-Polymer Chemistry. 1997;35:3553–3559. [Google Scholar]
- 69.Kuo JW, Swann DA, Prestwich GD. Bioconjugate Chem. 1991;2:232–241. doi: 10.1021/bc00010a007. [DOI] [PubMed] [Google Scholar]
- 70.Prestwich GD, Marecak DM, Marecek JF, Vercruysse KP, Ziebell MR. Journal of Controlled Release. 1998;53:93–103. doi: 10.1016/s0168-3659(97)00242-3. [DOI] [PubMed] [Google Scholar]
- 71.Vercruysse KP, Marecak DM, Marecek JF, Prestwich GD. Bioconjugate Chemistry. 1997;8:686–694. doi: 10.1021/bc9701095. [DOI] [PubMed] [Google Scholar]
- 72.Schante CE, Zuber G, Herlin C, Vandamme TF. Carbohydr. Polym. 2011;85:469–489. [Google Scholar]
- 73.Shu XZ, Prestwich GD. In: Chemistry and biology of hyaluronan. 1st edn. Garg HG, Hales CA, editors. Elsevier Ltd.; Oxford: 2004. pp. 475–504. ch. 22. [Google Scholar]
- 74.Kuo G. D. Prestwich and J. W. Curr. Pharm. Biotechnol. 2008;9:242–245. doi: 10.2174/138920108785161523. [DOI] [PubMed] [Google Scholar]
- 75.Aeschlimann P. Bulpitt and D. J. Biomed. Mater. Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 76.Pouyani T, Harbison GS, Prestwich GD. Journal of the American Chemical Society. 1994;116:7515–7522. [Google Scholar]
- 77.Jia XQ, Colombo G, Padera R, Langer R, Kohane DS. Biomaterials. 2004;25:4797–4804. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Luo Y, Kirker KR, Prestwich GD. J. Control. Release. 2000;69:169–184. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 79.Ossipov DA, Piskounova S, Varghese OP, Hilborn J. Biomacromolecules. 2010;11:2247–2254. doi: 10.1021/bm1007986. [DOI] [PubMed] [Google Scholar]
- 80.Ito T, Fraser IP, Yeo Y, Highley CB, Bellas E, Kohane DS. Biomaterials. 2007;28:1778–1786. doi: 10.1016/j.biomaterials.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Gurski LA, Jha AK, Zhang C, Jia XQ, Farach-Carson MC. Biomaterials. 2009;30:6076–6085. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farran AJE, Teller SS, Jha AK, Jiao T, Hule RA, Clifton RJ, Pochan DP, Duncan RL, Jia XQ. Tissue Engineering Part A. 2010;16:1247–1261. doi: 10.1089/ten.tea.2009.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shu XZ, Liu YC, Palumbo F, Prestwich GD. Biomaterials. 2003;24:3825–3834. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 84.Shu XZ, Liu YC, Palumbo FS, Lu Y, Prestwich GD. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 85.Hahn SK, Kim JS, Shimobouji T. J. Biomed. Mater. Res. Part A. 2007;80A:916–924. doi: 10.1002/jbm.a.30997. [DOI] [PubMed] [Google Scholar]
- 86.Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, Clark RAF. Biomacromolecules. 2005;6:2857–2865. doi: 10.1021/bm050361c. [DOI] [PubMed] [Google Scholar]
- 87.Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Macromol. Biosci. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang JX, Skardal A, Prestwich GD. Biomaterials. 2008;29:4521–4531. doi: 10.1016/j.biomaterials.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hosack LW, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Biomaterials. 2008;29:2336–2347. doi: 10.1016/j.biomaterials.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serban MA, Prestwich GD. Biomacromolecules. 2007;8:2821–2828. doi: 10.1021/bm700595s. [DOI] [PubMed] [Google Scholar]
- 91.Yang JA, Kim H, Park K, Hahn SK. Soft Matter. 2011;7:868–870. [Google Scholar]
- 92.Censi R, Fieten PJ, di Martino P, Hennink WE, Vermonden T. Macromolecules. 2010;43:5771–5778. doi: 10.1016/j.jconrel.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 93.Skardal A, Zhang JX, McCoard L, Oottamasathien S, Prestwich GD. Adv. Mater. 2010;22:4736. doi: 10.1002/adma.201001436. [DOI] [PubMed] [Google Scholar]
- 94.Leach JB, Schmidt CE. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Jia XQ, Burdick JA, Kobler J, Clifton RJ, Rosowski JJ, Zeitels SM, Langer R. Macromolecules. 2004;37:3239–3248. [Google Scholar]
- 96.Ifkovits JL, Burdick JA. Tissue Eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 97.Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brigham MD, Bick A, Lo E, Bendali A, Burdick JA, Khademhosseini A. Tissue Engineering Part A. 2009;15:1645–1653. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seidlits SK, Drinnan CT, Petersen RR, Shear JB, Suggs LJ, Schmidt CE. Acta Biomater. 2011;7:2401–2409. doi: 10.1016/j.actbio.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 100.Patterson J, Siew R, Herring SW, Lin ASP, Guldberg R, Stayton PS. Biomaterials. 2010;31:6772–6781. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bencherif SA, Srinivasan A, Horkay F, Hollinger JO, Matyjaszewski K, Washburn NR. Biomaterials. 2008;29:1739–1749. doi: 10.1016/j.biomaterials.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 102.Sahoo S, Chung C, Khetan S, Burdick JA. Biomacromolecules. 2008;9:1088–1092. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chung C, Beecham M, Mauck RL, Burdick JA. Biomaterials. 2009;30:4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lei YG, Gojgini S, Lam J, Segura T. Biomaterials. 2011;32:39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K. Biomaterials. 2007;28:1830–1837. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 106.Choh SY, Cross D, Wang C. Biomacromolecules. 2011;12:1126–1136. doi: 10.1021/bm101451k. [DOI] [PubMed] [Google Scholar]
- 107.Wells LA, Furukawa S, Sheardown H. Biomacromolecules. 2011;12:923–932. doi: 10.1021/bm101233m. [DOI] [PubMed] [Google Scholar]
- 108.Nimmo CM, Owen SC, Shoichet MS. Biomacromolecules. 2011;12:824–830. doi: 10.1021/bm101446k. [DOI] [PubMed] [Google Scholar]
- 109.Darr A, Calabro A. J. Mater. Sci.-Mater. Med. 2009;20:33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 110.Kim KS, Park SJ, Yang JA, Jeon JH, Bhang SH, Kim BS, Hahn SK. Acta Biomater. 2011;7:666–674. doi: 10.1016/j.actbio.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 111.Jin R, Teixeira LSM, Dijkstra PJ, van Blitterswijk CA, Karperien M, Feijen J. Biomaterials. 2010;31:3103–3113. doi: 10.1016/j.biomaterials.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Oh JK, Lee DI, Park JM. Prog. Polym. Sci. 2009;34:1261–1282. [Google Scholar]
- 113.Dios M. de, Barroso F, Tojo C, Blanco MC, Lopez-Quintela MA. Colloid Surface A. 2005;270:83–87. [Google Scholar]
- 114.Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL, Pochan DJ, Jia XQ. Macromolecules. 2009;42:537–546. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim ST, Martin GP, Berry DJ, Brown MB. Journal of Controlled Release. 2000;66:281–292. doi: 10.1016/s0168-3659(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 116.Yun YH, Goetz DJ, Yellen P, Chen WL. Biomaterials. 2004;25:147–157. doi: 10.1016/s0142-9612(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 117.Pitarresi G, Craparo EF, Palumbo FS, Carlisi B, Giammona G. Biomacromolecules. 2007;8:1890–1898. doi: 10.1021/bm070224a. [DOI] [PubMed] [Google Scholar]
- 118.Prata JE, Barth TA, Bencherif SA, Washburn NR. Biomacromolecules. 2010;11:769–775. doi: 10.1021/bm901373x. [DOI] [PubMed] [Google Scholar]
- 119.Lee H, Mok H, Lee S, Oh YK, Park TG. Journal of Controlled Release. 2007;119:245–252. doi: 10.1016/j.jconrel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 120.Jia XQ, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, Zeitels SM, Langer R. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 121.Sahiner N, Jha AK, Nguyen D, Jia XQ. J. Biomater. Sci.-Polym. Ed. 2008;19:223–243. doi: 10.1163/156856208783432462. [DOI] [PubMed] [Google Scholar]
- 122.Jha AK, Malik MS, Farach-Carson MC, Duncan RL, Jia XQ. Soft Matter. 2010;6:5045–5055. doi: 10.1039/C0SM00101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jha AK, Yang WD, Kirn-Safran CB, Farach-Carson MC, Jia XQ. Biomaterials. 2009;30:6964–6975. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu X, Jha AK, Duncan RL, X. J. Acta Biomater. 2011 doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shoyele SA, Cawthorne S. Adv. Drug Deliv. Rev. 2006;58:1009–1029. doi: 10.1016/j.addr.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 126.Iskandar F, Nandiyanto ABD, Widiyastuti W, Young LS, Okuyama K, Gradon L. Acta Biomater. 2009;5:1027–1034. doi: 10.1016/j.actbio.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 127.Huh Y, Cho HJ, Yoon IS, Choi MK, Kim JS, Oh E, Chung SJ, Shim CK, Kim DD. Eur. J. Pharm. Sci. 2010;40:9–15. doi: 10.1016/j.ejps.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 128.Domnina YA, Yeo Y, Tse JY, Bellas E, Kohane DS. J. Biomed. Mater. Res. Part A. 2008;87A:825–831. doi: 10.1002/jbm.a.31741. [DOI] [PubMed] [Google Scholar]
- 129.Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, Kwon IC, Jeong SY. J. Mater. Chem. 2009;19:4102–4107. [Google Scholar]
- 130.Coradini D, Pellizzaro C, Miglierini G, Daidone MG, Perbellini A. Int. J. Cancer. 1999;81:411–416. doi: 10.1002/(sici)1097-0215(19990505)81:3<411::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 131.Lee H, Lee K, Park TG. Bioconjugate Chemistry. 2008;19:1319–1325. doi: 10.1021/bc8000485. [DOI] [PubMed] [Google Scholar]
- 132.Toole BP, Wight TN, Tammi MI. Journal of Biological Chemistry. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 133.Knudson W, Chow G, Knudson CB. Matrix Biology. 2002;21:15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 134.Hua Q, Knudson CB, Knudson W. Journal of Cell Science. 1993;106:365–375. doi: 10.1242/jcs.106.1.365. [DOI] [PubMed] [Google Scholar]
- 135.Raemdonck K, Demeester J, De Smedt S. Soft Matter. 2009;5:707–715. [Google Scholar]
- 136.Ossipov DA. Expert Opin. Drug Deliv. 2010;7:681–703. doi: 10.1517/17425241003730399. [DOI] [PubMed] [Google Scholar]
- 137.Yeh J, Ling YB, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Langer R, Khademhosseini A. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 138.Stevens B, Yang YZ, MohandaS A, Stucker B, Nguyen KT. J. Biomed. Mater. Res. Part B. 2008;85B:573–582. doi: 10.1002/jbm.b.30962. [DOI] [PubMed] [Google Scholar]
- 139.Stevens MM, George JH. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 140.Jia XQ, Kiick KL. Macromol. Biosci. 2009;9:140–156. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lutolf MP, Gilbert PM, Blau HM. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Zawko SA, Suri S, Truong Q, Schmidt CE. Acta Biomater. 2009;5:14–22. doi: 10.1016/j.actbio.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 144.Zawko SA, Schmidt CE. Acta Biomater. 2010;6:2415–2421. doi: 10.1016/j.actbio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 145.Khetan S, Burdick JA. Soft Matter. 2011;7:830–838. [Google Scholar]
- 146.Khetan S, Burdick JA. Biomaterials. 2010;31:8228–8234. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 147.Reneker DH, Chun I. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 148.Mauck RL, Baker BM, Nerurkar NL, Burdick JA, Li WJ, Tuan RS, Elliott DM. Tissue Eng. Part B-Rev. 2009;15:171–193. doi: 10.1089/ten.teb.2008.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goldberg M, Langer R, Jia Journal of Biomaterials Science-Polymer Edition. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khademhosseini A, Vacanti JP, Langer R. Sci Am. 2009;300:64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 151.Lee KY, Jeong L, Kang YO, Lee SJ, Park WH. Adv. Drug Deliv. Rev. 2009;61:1020–1032. doi: 10.1016/j.addr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 152.Li J, He A, Zheng J, Han CC. Biomacromolecules. 2006;7:2243–2247. doi: 10.1021/bm0603342. [DOI] [PubMed] [Google Scholar]
- 153.Li JX, He AH, Han CC, Fang DF, Hsiao BS, Chu B. Macromol. Rapid Commun. 2006;27:114–120. [Google Scholar]
- 154.Um IC, Fang DF, Hsiao BS, Okamoto A, Chu B. Biomacromolecules. 2004;5:1428–1436. doi: 10.1021/bm034539b. [DOI] [PubMed] [Google Scholar]
- 155.Ji Y, Ghosh K, Li BQ, Sokolov JC, Clark RAF, Rafailovich MH. Macromol. Biosci. 2006;6:811–817. doi: 10.1002/mabi.200600132. [DOI] [PubMed] [Google Scholar]
- 156.Sundararaghavan HG, Burdick JA. Biomacromolecules. 2011;12:2344–2350. doi: 10.1021/bm200415g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim TG, Chung HJ, Park TG. Acta Biomater. 2008;4:1611–1619. doi: 10.1016/j.actbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 158.Biondi M, Ungaro F, Quaglia F, Netti PA. Adv. Drug Deliver Rev. 2008;60:229–242. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 159.Hirakura T, Yasugi K, Nemoto T, Sato M, Shimoboji T, Aso Y, Morimoto N, Akiyoshi K. Journal of Controlled Release. 2010;142:483–489. doi: 10.1016/j.jconrel.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 160.Jha AK, Xu XA, Duncan RL, Jia XQ. Biomaterials. 2011;32:2466–2478. doi: 10.1016/j.biomaterials.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Krishna OD, Jha AK, Jia XQ, Kiick KL. Biomaterials. 2011;32:6412–6424. doi: 10.1016/j.biomaterials.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bencherif SA, Siegwart DJ, Srinivasan A, Horkay F, Hollinger JO, Washburn NR, Matyjaszewski K. Biomaterials. 2009;30:5270–5278. doi: 10.1016/j.biomaterials.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]