Abstract
Lung sonography has rapidly emerged as a reliable technique in the evaluation of various thoracic diseases. One important, well-established application is the diagnosis of a pneumothorax. Prompt and accurate diagnosis of a pneumothorax in the management of a critical patient can prevent the progression into a life-threatening situation. Sonographic signs, including ‘lung sliding’, ‘B-lines’ or ‘comet tail artifacts’, ‘A-lines’, and ‘the lung point sign’ can help in the diagnosis of a pneumothorax. Ultrasound has a higher sensitivity than the traditional upright anteroposterior chest radiography (CXR) for the detection of a pneumothorax. Small occult pneumothoraces may be missed on CXR during a busy trauma scenario, and CXR may not always be feasible in critically ill patients. Computed tomography, the gold standard for the detection of pneumothorax, requires patients to be transported out of the clinical area, compromising their hemodynamic stability and delaying the diagnosis. As ultrasound machines have become more portable and easier to use, lung sonography now allows a rapid evaluation of an unstable patient, at the bedside. These advantages combined with the low cost and ease of use, have allowed thoracic sonography to become a useful modality in many clinical settings.
Keywords: Emergency medicine, pneumothorax, diagnosis, thoracic ultrasonography
INTRODUCTION
The use of ultrasound (US) in the diagnosis and treatment of patients is a well-established modality that has existed for many years. Thoracic sonography is fairly new in comparison to other accepted ultrasound applications, and is still rapidly evolving. The use of thoracic ultrasound has gained slow acceptance due to the traditional teaching that the air-filled lungs are not ultrasound friendly. Poor imaging is a result of the confinement of air between the lung and the chest wall, preventing diffusion of the ultrasound beam into the parietal pleura and deep lung structures, leading to a production of artifacts. Over the past decade, bedside lung sonography has developed an established role in literature for the diagnosis of thoracic diseases. This development is based on an improved understanding and appreciation of the sonographic artifacts created by the interplay of air and fluid in the lungs. The first reported use of ultrasound to detect pneumothorax in humans was by Wernecke et al., in 1987.[1] The Focused Assessment with Sonography in Trauma (FAST) examination has now been modified to include lung imaging as part of the evaluation in a trauma patient. The application has been renamed as the E-FAST examination, with ‘E’ standing for extended, including the standard lung views.
A pneumothorax can be divided into two broad categories: traumatic (including iatrogenic) or atraumatic. Atraumatic pneumothorax can further be categorized as primary spontaneous or secondary spontaneous. Pneumothorax is commonly associated with both blunt and penetrating chest injury and is a leading cause of preventable morbidity and mortality. Traumatic pneumothorax, the most common life-threatening outcome in blunt chest trauma, occurs in over 20% of patients with blunt injuries and about 40% with penetrating chest injuries.[2]
The diagnosis of a pneumothorax is usually made with a combination of clinical signs and symptoms, which may be subtle, and plain chest radiography. Regardless of its presentation, the early detection and treatment of a pneumothorax is critical. Small- (10% or less) or medium (11 to 40%)-sized pneumothoraces are generally not life-threatening and their management varies.[3–5] However, a delay in the diagnosis and treatment, especially in those who are mechanically ventilated, may lead to the progression of a pneumothorax and resultant hemodynamic instability.[6,7] In these critical situations where a subtle pneumothorax may be missed, a quick bedside lung ultrasound may expedite the diagnosis, treatment, and resuscitation of a patient who may have otherwise decompensated.
Ultrasound has a well-known established role in the diagnosis of a traumatic pneumothorax. In one prospective study a hand-held ultrasound device was used by trauma surgeons to perform the E-FAST examination in patients with blunt or penetrating trauma.[8] The utility of thoracic ultrasound for diagnosing a pneumothorax was compared to chest x-ray (CXR) alone, a composite standard (CXR, chest, and abdomen Computed tomography (CT) scans, clinical course, and invasive interventions), and to the gold standard CT scan (CT only). Their results showed that the E-FAST examination had a sensitivity of 58.9% with a positive likelihood ratio of 69.7 and a specificity of 99.1% when compared to the composite standard. The E-FAST was also compared to CXR, using CT scan as the gold standard, showing that ultrasound had a higher sensitivity than CXR, 48.8 and 20.9%, respectively, and a similar specificity of 99.6 and 98.7%, respectively. In addition, they noted that 63% of all pneumothoraces diagnosed were occult. Traditionally, these would end up getting diagnosed later on a CT scan. Although CT scan remains the gold standard, they concluded that ultrasound was more sensitive in identifying occult traumatic pneumathoraces compared to CXR.
Similarly, a prospective study by Ball et al., noted that up to 76% of all traumatic pneumothoraces were missed by the standard supine AP chest film when interpreted by the trauma team.[9] This number was much higher than their prior retrospective study (55%), where image interpretation relied on radiologists. This stressed the poor sensitivity of CXR in a rushed trauma scenario and the utility of performing a rapid bedside ultrasound, to possibly aid in the diagnosis, prior to sending a patient for a CT scan.[10]
Several other studies highlight the utility of ultrasound compared to CXR for the diagnosis of pneumothorax in the Emergency Department.[11–14] The sensitivity of ultrasound in certain studies has been similar to that found in CT scan, which is still considered to be the gold standard for the detection of a pneumothorax.[11,15] Lichtenstein et al., have shown that ultrasound has a sensitivity of 95.3% and a specificity of 91.1% for detecting pneumothorax in intensive care unit (ICU) patients.[16] However, in this particular article, the authors cite that the underlying lung process may have affected the accuracy of ultrasound, resulting in both false-positive and false-negative cases.
Relevant English language articles and case reports were searched by using PubMed and Google Scholar (1984–2011). The following search terms were used: ‘Ultrasonography’, ‘chest sonography,’ ‘bedside lung ultrasound,’ ‘pneumothorax,’ and ‘emergency medicine.’
Probe selection and equipment
The bedside sonographic diagnosis of pneumothorax can be performed with most ultrasound machines without the need of any sophisticated functions. Most machines are now portable and can be brought to the bedside, which is especially helpful in the critically ill and hemodynamically unstable patient, as it obviates the need for transport. Also, the physician performing the scan can interpret the results of the bedside ultrasound immediately. A straight linear array high frequency probe (5–13 MHz) may be most helpful in analyzing superficial structures such as the pleural line and providing better resolution.[17] A microconvex or curvilinear array probe may be more suitable for deeper lung imaging as it provides better penetration (1–8 MHz), at the cost of less resolution. Finally, some advocate the use of the phased array probe, generally used in cardiac imaging (2–8 MHz), as its flat and smaller footprint is better suited for imaging in between the ribs.
Technique and normal anatomy
A pneumothorax contains air and no fluid, and therefore, will rise to the least dependent area of the chest. In a supine patient this area corresponds to the anterior region of the chest at approximately the second to fourth intercostal spaces in the mid-clavicular line. This location will identify the majority of significant pneumothoraces in the supine patient, which makes it the recommended initial area for investigation in a trauma.[17,18] In contrast, air will accumulate in an apicolateral location in an upright patient.[19]
Based on the above, patients are scanned in a supine or near-to-supine position. The probe should be placed in a sagittal position (indicator pointing cephalad) on the anterior chest wall at about the second intercostal space, in the mid-clavicular line [Figure 1]. The sonographer should first identify the landmarks of two ribs with posterior shadowing behind them and visualize the pleural line in between them. This is typically called ‘the bat sign’ where the periosteum of the ribs represents the wings and the bright hyperechoic pleural line in between them represents the bats’ body [Figures 2a and 2b].[20] If the ribs are not visualized the probe should be slowly moved in a caudal direction (inferiorly) until two ribs appear on the screen. It is in between these two rib landmarks that the two layers of pleura, parietal and visceral, are seen sliding across one another. As stated earlier, air will rise to the anterior chest wall, and therefore a pneumothorax that is large enough to require a chest tube will appear with this simple technique.
Figure 1.
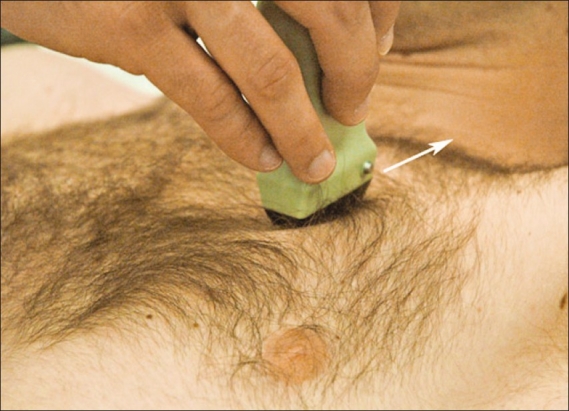
Correct probe positioning in the initial evaluation of a pneumothorax. The probe is placed on the anterior chest wall in a sagittal orientation, pointing toward the patient's head at approximately the second intercostal space in the mid-clavicular line
Figure 2.
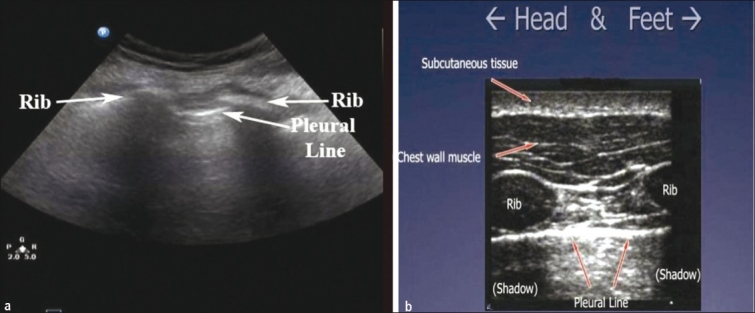
(a) ‘The bat sign.’ Two ribs with posterior shadowing represents the wings of the bat, and the hyperechoic pleural line, its body (b) A sagittal scan at the upper intercostal spaces depicting normal anatomy
The presence of pleural sliding is the most important finding in normal aerated lung. The sonographer should visualize the hyperechoic pleural line in between two ribs moving or shimmering back and forth. Lung sliding corresponds to the to-and-fro movement of the visceral pleura on the parietal pleura that occurs with respiration. It is a dynamic sign and can be identified on ultrasound as horizontal movement along the pleural line.[21] Sliding is best seen at the lung apex in a supine patient.[21] The use of M-mode, which detects motion over time, provides more evidence that the pleural line is sliding. It is beneficial in patients where sliding may be subtle, such as, in the elderly or in patients with poor pulmonary reserve, who are not taking large breaths. The M-mode cursor is placed over the pleural line and two different patterns are displayed on the screen: The motionless portion of the chest above the pleural line creates horizontal ‘waves,’ and the sliding below the pleural line creates a granular pattern, the ‘sand’ [Figure 3]. The resultant picture is one that resembles waves crashing in onto the sand and is therefore called the ‘seashore sign’ and is present in normal lung.[13,22,23]
Figure 3.
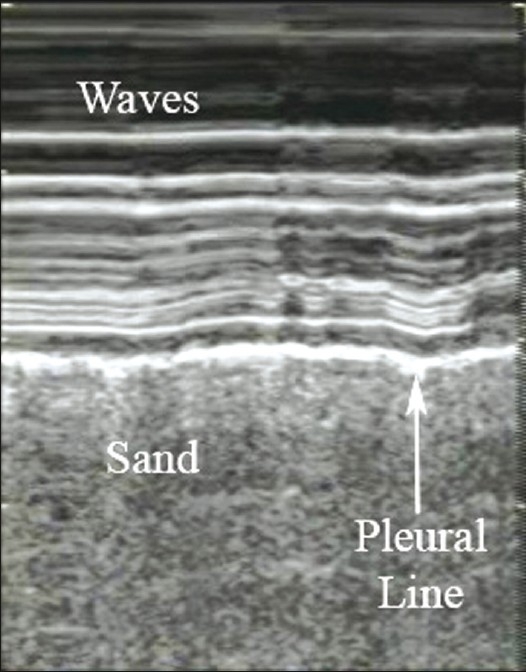
M-mode illustrating the ‘seashore sign.’ The pleural line divides the image in half: The motionless portion above the pleural line creates horizontal ‘waves,’ and the sliding line below it creates granular pattern, the ‘sand’
‘B-lines’ or ‘comet-tail artifacts’ are reverberation artifacts that appear as hyperechoic vertical lines that extend from the pleura to the edge of the screen without fading [Figure 4]. ‘Comet-tail artifacts’ move synchronously with lung sliding and respiratory movements.[21] A few visualized ‘B-lines’ in dependent regions are expected in normal aerated lung and are visualized moving along with the sliding pleura. These artifacts are seen in normal lung due to the acoustic impedance differences between water and air.[22] Excessive ‘B-lines’, especially in the anterior lung, are abnormal and are usually indicative of interstitial edema.
Figure 4.
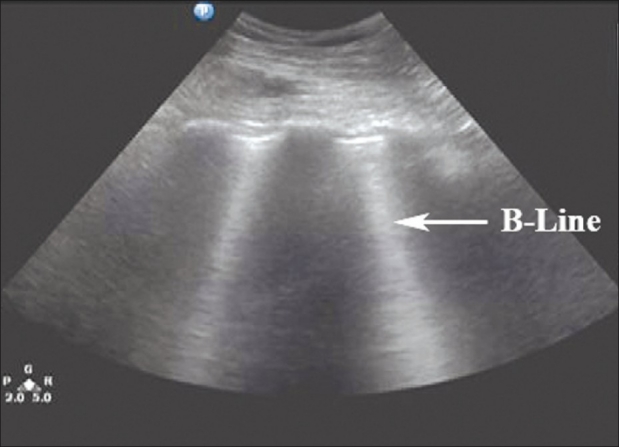
‘B-lines’ or ‘comet-tail artifacts’ are seen originating from the bright white hyperechoic pleural line, extending vertically to the edge of the screen. ‘B-lines’ move in synchrony with the sliding pleura in a normal well-aerated lung
The average time to perform this examination varies from two to three minutes; less than one minute to rule out a pneumothorax and several minutes to rule it in.[8,12]
Sonographic signs of pneumothorax
Absence of lung sliding
In a pneumothorax, there is air present that separates the visceral and parietal pleura and prevents visualization of the visceral pleura. In this situation, lung sliding is absent. This lack of lung sliding can be visualized by identifying the landmarks discussed earlier. Two ribs should be identified with the pleural line in between them. The typical to-and-fro movement or shimmering of the pleural line will not be present. The same technique using M-mode can be used to confirm a lack of sliding. The resultant M-mode tracing in a pneumothorax will only display one pattern of parallel horizontal lines above and below the pleural line, exemplifying the lack of movement. This pattern resembles a ‘barcode’ and is often called the ‘stratosphere sign’ [Figure 5].[22,23]
Figure 5.

M-mode and the absence of lung sliding are shown as the ‘stratosphere sign’: Parallel horizontal lines above and below the pleural line, resemble a ‘barcode.’ This sign indicates a pneumothorax at this intercoastal space
The negative predictive value for lung sliding is reported as 99.2–100%, indicating that the presence of sliding effectively rules out a pneumothorax.[11,16,24] However, the absence of lung sliding does not necessarily indicate that a pneumothorax is present. Lung sliding is abolished in a variety of conditions other than pneumothorax, including acute respiratory distress syndrome (ARDS), pulmonary fibrosis, large consolidations, pleural adhesions, atelectasis, right mainstem intubation, and phrenic nerve paralysis.[21,25,26] Specificity values range from 60–99% depending on the patient population, with higher values in the general population and lower values in the Intensive Care Unit and in those with ARDS.[11,16,21,24] Although the absence of lung sliding is not specific for pneumothorax, the combination of this with other signs improves the accuracy of the diagnosis.
Comet tail artifacts or ‘B-lines’
Ultrasound demonstrates the loss of ‘comet-tail artifacts’ in patients with a pneumothorax. These reverberation artifacts are lost due to air accumulating within the pleural space, which hinders the propagation of sound waves and eliminates the acoustic impedance gradient.[20] In addition, ‘comet-tail’ artifacts are generated by the visceral pleura, which is not visualized in a pneumothorax, therefore, these artifacts are not generated.[24] The negative predictive value for this artifact is high, reported at 98–100%, such that visualization of even one comet-tail essentially rules out the diagnosis of a pneumothorax.[21,24,27]
‘A-lines’ are other important thoracic artifacts that can help in the diagnosis of a pneumothorax. These are also reverberation artifacts appearing as equally spaced repetitive horizontal hyperechoic lines reflecting off of the pleura [Figure 6]. The space in between each A-line corresponds to the same distance between the skin surface and the parietal pleura. In the normal patient, when ‘B-lines’ are present, they extend from the pleural line and erase ‘A-lines’, as they emanate out to the edge of the screen. ‘A-lines’ will be present in a patient with a pneumothorax, but ‘B-lines’ will not. If lung sliding is absent with the presence of ‘A-lines’, the sensitivity and specificity for an occult pneumothorax is as high as 95 and 94%, respectively.[24]
Figure 6.
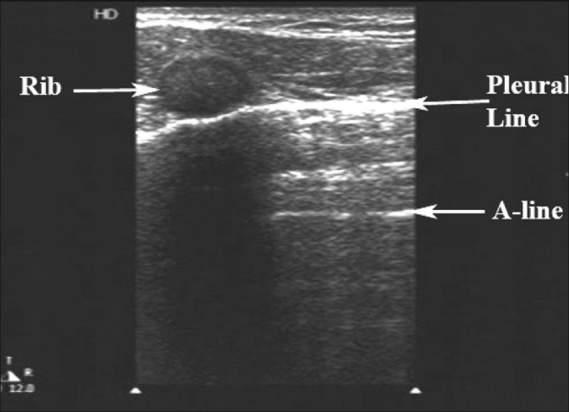
‘A-lines’, a type of reverberation artifact, are horizontal, equally spaced lines seen originating from the bright white hyperechoic pleural line. If ‘B-lines’ are present, they extend out from the pleural line and erase the ‘A-lines’ in their path
Lung-point sign
The ‘lung-point sign’ occurs at the border of a pneumothorax. It is due to sliding lung intermittently coming into contact with the chest wall during inspiration and is helpful in determining the actual size of the pneumothorax. This sign can further be delineated using M-mode where alternating ‘seashore’ and ‘stratosphere’ patterns are depicted over time [Figure 7]. The ‘lung-point sign’ is 100% specific for pneumothorax and defines its border.[21,28] The location of the lung point is beneficial in determining the size of the pneumothorax. If a lack of lung sliding is visualized anteriorly, the probe can progressively be moved to more lateral and posterior positions on the chest wall searching for the location of the lung-point. The more lateral or posterior the ‘lung-point sign’ is identified, the larger the pneumothorax. Therefore, if the ‘lung-point sign’ is seen in an anterior location on the chest wall, the sonographer can be assured that the pneumothorax is relatively small.[11,24,29] Although the specificity is high, the sensitivity of the ‘lung-point sign’ is relatively low (reported at 66%) and is not seen in cases of total lung collapse.[28] Studies have shown concordance between pneumothorax size on ultrasound and CT scan, reportedly within 1.9–2.3 cm.[15,26,29] The determination of the size of a pneumothorax is important for clinical decision-making, as larger pneumothoraces are more likely to require thoracostomy.[11,24]
Figure 7.
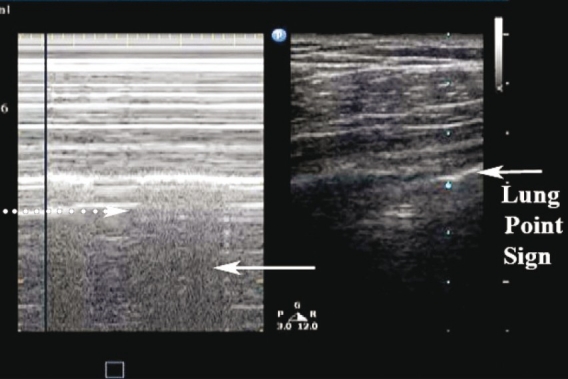
‘Lung point sign.’ (Right) B-mode depicting the lung point: Sliding lung touching the chest wall. (Left) The ‘seashore sign’ (white arrow) and the ‘stratosphere sign’ (dotted arrow) as the lung intermittently contacts the chest wall
Other signs
The ‘Power Slide’ refers to the use of power (angiography) Doppler to help identify lung sliding. Power Doppler is very sensitive and picks up subtle flow and movement. If there is lung sliding present, power Doppler will light up the sliding pleural line with color flow [Figure 8]. This technique can be helpful in cases of subtle sliding when direct visualization may be difficult. The disadvantage of this type of Doppler is that due to its increased sensitivity, the probe needs to be held in a steady manner and the patient has to be motionless in order to prevent artifact and erroneous color flow over the pleural line, when sliding is actually absent.[23,30]
Figure 8.
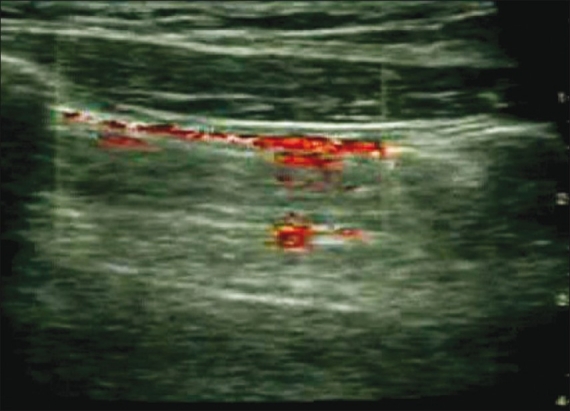
‘Power slide’ in normal sliding lung. Power (angiography) Doppler is used at the pleural line, which is visualized lighting up with color flow as subtle sliding is detected. The probe must be steady to avoid unwanted color artifacts
The ‘lung pulse’ refers to the rhythmic movement of the pleura in synchrony with the cardiac rhythm. It is best viewed in areas of the lung adjacent to the heart, at the pleural line. The ‘lung pulse’ is a result of cardiac vibrations being transmitted to the lung pleura in poorly aerated lung. Cardiac activity is essentially detected at the pleural line when there is absent lung sliding. In normal well-aerated lung, the ‘lung pulse’ is not present, as lung sliding becomes dominant and resistant to cardiac vibrations.[21]
CONCLUSIONS
Thoracic sonography for the detection of pneumothorax has become a well-established modality in the acute care setting. It is indispensible in the blunt or penetrating chest trauma patient, where the identification of a pneumothorax can prevent life-threatening consequences. The ease of use and portability of newer machines, combined with the improved training among physicians has allowed thoracic ultrasound to become a useful bedside tool in patients with respiratory complaints. The traditional upright AP radiograph has become less important due to its poor sensitivity in diagnosing a pneumothorax compared to ultrasound. Although CT scan remains the gold standard and may still catch smaller occult pneumothoraces that ultrasound misses, its disadvantages are becoming more apparent. Bedside ultrasound obviates the need for patient transport in unstable situations, it eliminates radiation exposure, it is quicker to perform and is immediately interpreted at the bedside without unnecessary delays. In addition, it is more cost-effective and can be repeated multiple times during a resuscitation.
In addition, ultrasound is the perfect modality in the emergency and critical care setting after performing certain procedures, such as a thoracentesis or the placement of a central line, to quickly confirm the presence of lung-sliding and to rule out an iatrogenic pneumothorax. It has also been found to be beneficial in the post-intubation scenario, where a confirmation of bilateral lung sliding rules out a right mainstem intubation.
The increasing portability of newer ultrasound machines makes them easier to use in first responder and disaster settings, wilderness medicine, air medical transport, rural medicine, and even space explorations. Studies indicate that the recognition of key artifacts in thoracic ultrasound is readily teachable to both physicians as well as non-physician health care providers and its uses continue to expand in the out-of-hospital setting.[31,32]
This article offers a review of the current evidence for the use of thoracic ultrasound in the diagnosis of a pneumothorax, reviews the proper techniques used, and highlights its clinical utility.
We conducted a literature search for the latest scientific evidence on this topic in English language articles and case studies (1984 - 2011) using PubMed and Google Scholar.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wernecke K, Galanski M, Peters PE, Hansen J. Pneumothorax: Evaluation by ultrasound-preliminary results. J Thorac Imaging. 1987;2:76–8. [PubMed] [Google Scholar]
- 2.Di Bartolomeo S, Sanson G, Nardi G, Scian F, Michelutto V, Lattuada L. A population-based study on pneumothorax in severely traumatized patients. J Trauma. 2001;51:677–82. doi: 10.1097/00005373-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 3.British Thoracic Society Fitness to Dive Group, Subgroup of the British Thoracic Society Standards of Care Committee. British thoracic society guidelines on respiratory aspects of fitness for diving. Thorax. 2003;58:3–13. doi: 10.1136/thorax.58.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax: An american college of chest physicians delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 5.de Leyn P, Lismonde M, Ninane V, Noppen M, Slabbynck H, van Meerhaeghe A, et al. Guidelines belgian society of pneumology.Guidelines on the management of spontaneous pneumothorax. Acta Chir Belg. 2005;105:265–7. doi: 10.1080/00015458.2005.11679714. [DOI] [PubMed] [Google Scholar]
- 6.Enderson BL, Abdalla R, Frame SB, Casey MT, Gould H, Maull KI. Tube thoracostomy for occult pneumothorax: A prospective randomized study of its use. J Trauma. 1993;35:726–30. [PubMed] [Google Scholar]
- 7.Bridges KG, Welch G, Silver M, Schinco MA, Esposito B. CT detection of occult pneumothorax in multiple trauma patients. J Emerg Med. 1993;11:179–86. doi: 10.1016/0736-4679(93)90517-b. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG, et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: The extended focused assessment with sonography for trauma (EFAST) J Trauma. 2004;57:288–95. doi: 10.1097/01.ta.0000133565.88871.e4. [DOI] [PubMed] [Google Scholar]
- 9.Ball CG, Ranson K, Dente CJ, Feliciano DV, Laupland KB, Dyer D, et al. Clinical predictors of occult pneumothoraces in severely injured blunt polytrauma patients: A prospective observational study. Injury. 2009;40:44–7. doi: 10.1016/j.injury.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Ball CG, Kirkpatrick AW, Laupland KB, Fox DI, Nicolaou S, Anderson IB, et al. Incidence, risk factors, and outcomes for occult pneumothoraces in victims of major trauma. J Trauma. 2005;59:917–25. doi: 10.1097/01.ta.0000174663.46453.86. [DOI] [PubMed] [Google Scholar]
- 11.Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12:844–9. doi: 10.1197/j.aem.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Dulchavsky SA, Schwarz KL, Kirkpatrick AW, Billica RD, Williams DR, Diebel LN, et al. Prospective evaluation of thoracic ultrasound in the detection of pneumothorax. J Trauma. 2001;50:201–5. doi: 10.1097/00005373-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Liu ZH, Yang JX, Gan JX, Xu SW, You XD, et al. Rapid detection of pneumothorax by ultrasonography in patients with multiple trauma. Crit Care. 2006;10:R112. doi: 10.1186/cc5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tocino IM, Miller MH, Frederick PR, Bahr AL, Thomas F. CT detection of occult pneumothorax in head trauma. AJR Am J Roentgenol. 1984;143:987–90. doi: 10.2214/ajr.143.5.987. [DOI] [PubMed] [Google Scholar]
- 15.Soldati G, Testa A, Sher S, Pignataro G, La Sala M, Silveri NG. Occult traumatic pneumothorax: Diagnostic accuracy of lung ultrasonography in the emergency department. Chest. 2008;133:204–11. doi: 10.1378/chest.07-1595. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill.Lung sliding. Chest. 1995;108:1345–8. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 17.Tocino IM, Miller MH, Fairfax WR. Distribution of pneumothorax in the supine and semirecumbent critically ill adult. AJR Am J Roentgenol. 1985;144:901–5. doi: 10.2214/ajr.144.5.901. [DOI] [PubMed] [Google Scholar]
- 18.Ball CG, Kirkpatrick AW, Laupland KB, Fox DL, Litvinchuk S, Dyer DM, et al. Factors related to the failure of radiographic recognition of occult posttraumatic pneumothoraces. Am J Surg. 2005;189:541–6. doi: 10.1016/j.amjsurg.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Weissberg D, Refaely Y. Pneumothorax: Experience with 1,199 patients. Chest. 2000;117:1279–85. doi: 10.1378/chest.117.5.1279. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein D, Meziere G, Biderman P, Gepner A. The ‘comet-tail artifact’: An ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25:383–8. doi: 10.1007/s001340050862. [DOI] [PubMed] [Google Scholar]
- 21.De Luca C, Valentino M, Rimondi M, Branchini M, Baleni MC, Barozzi L. Use of chest sonography in acute-care radiology. J Ultrasound. 2008;11:125–34. doi: 10.1016/j.jus.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barillari A, Kiuru S. Detection of spontaneous pneumothorax with chest ultrasound in the emergency department. Intern Emerg Med. 2010;5:253–5. doi: 10.1007/s11739-010-0347-z. [DOI] [PubMed] [Google Scholar]
- 23.Johnson A. Emergency department diagnosis of pneumothorax using goal-directed ultrasound. Acad Emerg Med. 2009;16:1379–80. doi: 10.1111/j.1553-2712.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenstein DA, Meziere G, Lascols N, Biderman P, Courret JP, Gepner A, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231–8. doi: 10.1097/01.ccm.0000164542.86954.b4. [DOI] [PubMed] [Google Scholar]
- 25.Murphy M, Nagdev A, Sisson C. Lack of lung sliding on ultrasound does not always indicate a pneumothorax. Resuscitation. 2008;77:270. doi: 10.1016/j.resuscitation.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Ball CG, Kirkpatrick AW, Feliciano DV. The occult pneumothorax: What have we learned? Can J Surg. 2009;52:E173–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Soldati G, Testa A, Pignataro G, Portale G, Biasucci DG, Leone A, et al. The ultrasonographic deep sulcus sign in traumatic pneumothorax. Ultrasound Med Biol. 2006;32:1157–63. doi: 10.1016/j.ultrasmedbio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: An ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434–40. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 29.Soldati G, Testa A, Sher S, Pignataro G, La Sala M, Silveri NG. Occult traumatic pneumothorax: Diagnostic accuracy of lung ultrasonography in the emergency department. Chest. 2008;133:204–11. doi: 10.1378/chest.07-1595. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham J, Kirkpatrick AW, Nicolaou S, Liu D, Hamilton DR, Lawless B, et al. Enhanced recognition of “lung sliding” with power color Doppler imaging in the diagnosis of pneumothorax. J Trauma. 2002;52:769–71. doi: 10.1097/00005373-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 31.Noble VE, Lamhaut L, Capp R, Bosson N, Liteplo A, Marx JS, et al. Evaluation of a thoracic ultrasound training module for the detection of pneumothorax and pulmonary edema by prehospital physician care providers. BMC Med Educ. 2009;9:3. doi: 10.1186/1472-6920-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.32 Monti JD, Younggren B, Blankenship R. Ultrasound detection of pneumothorax with minimally trained sonographers: A preliminary study. J Spec Oper Med. 2009;9:43–6. doi: 10.55460/9GWU-MQO4. [DOI] [PubMed] [Google Scholar]


