Abstract
Some evidence suggests that sex, handedness and disease processes associated with schizophrenia affect the magnitude and/or direction of structural brain asymmetries. There are mixed findings, however, on how these factors influence cerebral torque, when torque is assessed with linear or volumetric measurements. We obtained MRI data from 67 healthy (30 males, 10 non-dextrals) and 84 schizophrenia subjects (60 males; 16 non-dextrals) and applied cortical pattern matching to spatially relate and compare differences in the surface morphology of the two cerebral hemispheres at high spatial resolution. Asymmetry indices, computed at thousands of matched hemispheric locations, were used to examine effects of sex, handedness and schizophrenia on hemispheric shape asymmetries while controlling for age and the other factors. Highly significant and discriminative right-frontal and left parietal–occipital surface expansions and protrusions (petalias) were mapped within groups. Although hemispheric shape asymmetries appeared less pronounced within female non-dextrals, asymmetry indices were not shown to differ significantly across sex, hand preference or diagnosis, or to reveal interactions of handedness with sex or diagnosis. Our 3D maps of spatially detailed anterior and posterior hemispheric shape asymmetries reflect subtle geometric distortions in hemispheric surface morphology that cannot be characterized with 2D or volumetric methods. Inter-individual variations in hemispheric torque appear minimally influenced by sex, dextrality or disease status. Biological factors driving language dominance or other lateralized brain functions dissociable from handedness, may more closely relate to hemispheric shape asymmetries, while the lateralization of other discrete brain regions may be more influenced by sexually dimorphic factors or by schizophrenia pathophysiology.
Introduction
Structural asymmetries are a ubiquitous feature of human brain morphology. Well-documented brain asymmetries identified in postmortem and imaging data include right frontal and left occipital hemispheric protrusions and petalias (LeMay, 1976; Chui and Damasio, 1980; Bear et al., 1986; Kertesz et al., 1990; Zilles et al., 1996), a leftward bias in the volume of the planum temporale, a posterior temporal lobe region and component of Wernicke’s language area (Geschwind and Levitsky, 1968; Geschwind and Galaburda, 1985; Steinmetz, 1996; Shapleske et al., 1999), and hemispheric differences in Sylvian fissure (Galaburda et al., 1978; Ide et al., 1996; Thompson et al., 1998; Narr et al., 2001), planum parietale (Jancke et al., 1994) and parietal operculum (Habib et al., 1995) morphology. Asymmetries are also reported in anterior language-gifted cortices including the pars triangularis (Falzi et al., 1982; Foundas et al., 1998a; Amunts et al., 2003), the approximate site of Broca’s area, the postcentral sulcus that marks primary sensory cortex (Hustler et al., 1998; Thompson et al., 1998; Narr et al., 2001), and motor cortex (Amunts et al., 1996; Zilles et al., 1996; Foundas et al., 1998b; Rademacher et al., 2001). Additionally, tissue density asymmetries in occipital, frontal and temporal regions, including the planum temporale (Good et al., 2001; Watkins et al., 2001), and projections of brain tissue that follow the direction of cerebral torque (a composite of right anterior and left posterior hemispheric protrusions) (Bilder et al., 1994; Mackay et al., 2003; Barrick et al., 2005), have been documented. Laminar thickness has been described as asymmetric in motor cortices, anterior temporal, inferior frontal, superior parietal and occipital regions (Luders et al., 2006).
There is great individual variation in the magnitude, and to a lesser extent, in the direction of structural brain asymmetries, where factors such as handedness, sex and disease processes associated with schizophrenia have been shown to modulate the structural lateralization of the hemispheres. Although less frequently examined, handedness has been shown to influence structural asymmetries in perisylvian (Steinmetz et al., 1991; Jancke et al., 1994), anterior language (Falzi et al., 1982; Foundas et al., 1998a) and motor cortices (Amunts et al., 1996; Amunts et al., 2000) and to affect hemispheric torque (LeMay, 1977; Bear et al., 1986; Zilles et al., 1996). Handedness is linked with language lateralization, and in most right-handers asymmetries generally reflect a leftward bias. However, hand preference and language dominance are only partially correlated. Approximately 95% of right-handers and 60–70% of left-handers are left lateralized for language (Geschwind, 1970; Rasmussen and Milner, 1977; Woods et al., 1988). Further characterization of the relationships between handedness and brain asymmetries may thus help to establish morphological phenotypes that relate to hand preference specifically.
Sex differences and/or interactions between sex and handedness are also found to moderate asymmetries in several cortical areas, most notably in perisylvian regions (Geschwind and Galaburda, 1985; Ide et al., 1996; Paus et al., 1996; Beaton, 1997; Wisniewski, 1998; Amunts et al., 2000) and to influence hemispheric torque (Bear et al., 1986; Zilles et al., 1996). Greater structural lateralization is typically reported in men, although several studies fail to detect sex differences (Foundas et al., 1999; Watkins et al., 2001). Disease-related factors associated with schizophrenia are further found to influence structural asymmetry patterns. Atypical asymmetries of the planum temporale, the superior temporal gyrus, the Sylvian fissure (Hoff et al., 1992; Shenton et al., 1992; Falkai et al., 1995a; Gur and Chin, 1999), the cerebellum (Szeszko et al., 2003), disturbances of cerebral torque (Luchins and Meltzer, 1983; Bilder et al., 1994; Falkai et al., 1995b; DeLisi et al., 1997) and altered hemispheric gyrification patterns (Kulynych et al., 1997; Vogeley et al., 2000; Narr et al., 2001; Narr et al., 2004a) have been documented. A non-trivial number of studies, however, fail to replicate findings of altered structural lateralization in schizophrenia (Harrison, 1999; Narr et al., 2001; Shenton et al., 2001).
Many of the structural asymmetries described above reflect shape differences in the morphology of the two hemispheres. Notably, the endocranial cast captures right frontal and left occipital protrusions called petalias, a term also used to describe the protrusions themselves, that constitute a widely described, although relatively subtle, index of cerebral asymmetry. Hemispheric shape asymmetries also encompass geometric distortions referred to as Yakovlevian anticlockwise torque (Bilder et al., 1994), or cerebral torque, that includes right-frontal and left-occipital protrusions, but further describes a bending of the interhemispheric fissure towards the right, an extension of the occipital pole across midline and shifts of morphology on the lateral aspects of the hemispheres, including the perisylvian region (Toga et al., in press). In earlier computerized tomography (CT) studies, cerebral torque was typically measured by examining differences in hemispheric widths and lengths obtained from arbitrary brain slices, while MRI studies have additionally assessed volumetric differences obtained from anterior and posterior brain regions in each hemisphere. Cerebral shape, however, is highly variable among individuals (Woods et al., 1998) and similarly, shape differences vary considerably in pattern, spatial extent, magnitude and in direction across hemispheres. 2D or volumetric measures may thus fail to sufficiently characterize subtle differences in the morphology of the two cerebral hemispheres.
To characterize individual differences in hemispheric shape as associated with cerebral torque, we set out to employ a rigorous 3D approach capable of measuring subtle differences in hemispheric morphology at high spatial resolution. Although prior evidence suggests that in general, structural asymmetries are less pronounced in left – as compared to right – handers and in females compared to males, while disturbances in structural lateralization have been documented in schizophrenia (Toga et al., in press), findings relating to cerebral torque and/or petalias, specifically, have been mixed and sometimes contradictory. Thus, an important goal of our investigation was to extend and to clarify mixed evidence concerning the effects of sex, handedness, and schizophrenia on spatially detailed cerebral torque patterns in a relatively large sample.
Methods
Subjects
Subjects included 84 patients experiencing their first episode of schizophrenia (60 males, 12 non-dextral; 24 females, 4 non-dextral) and 67 healthy comparison subjects (30 males, 7 non-dextral; 37 females, 3 non-dextral) similar in age (patients [mean± SD]: 24.26±4.6 years; controls: 28.3±7.1 years). Handedness was determined using a modified 20-item Edinburgh Handedness Inventory (Oldfield, 1971). Subjects performed each activity while an examiner observed and recorded whether either a ‘right’, ‘left’ or ‘ambiguous’ response was made for each item. Laterality quotient (LQ) scores were obtained using the following formula: (the total number of items performed with the right hand – the total number of items performed with the left hand) / the total number of items performed by the left and right hand. LQ calculations were made only on items for which unambiguous responses were recorded, and thus in rare occasions the denominator was <20. LQ scores have a distribution of −1 (extremely sinistral) to +1 (extremely dextral). LQ scores of >0.70 were used to separate dextrals from non-dextrals (Bilder et al., 1994). Study participants overlapped with subjects included in prior investigations of schizophrenia (Narr et al., 2005a,b), but were not identical, since handedness information was not available for all subjects studied previously. The diagnostic status of patients was confirmed with the Schedule for Affective Disorders and Schizophrenia (Endicott and Spitzer, 1978) and the Structured Clinical Interview for Axis I DSM-IV Disorders (First et al., 1997).
Healthy comparison subjects were recruited from local newspaper advertisements and through word of mouth in the community. To meet inclusion criteria, healthy comparison subjects were determined to have no history of psychiatric illness as assessed by clinical interview using the SCID-NP. Exclusion criteria for all subjects included serious neurological or endocrine disorders, any medical condition or treatment known to affect the brain, or meeting DSM-IV criteria for mental retardation. The North Shore–Long Island Jewish Health System Institutional Review Board (IRB) approved all procedures and informed written consent was obtained from all subjects. Additional approval for image processing and analysis was received from the UCLA IRB.
Image acquisition and preprocessing
High-resolution 3D SPGR MR images were obtained on a 1.5 Tesla scanner (General Electric, Milwaukee, WI) as a series of 124 contiguous 1.5 mm coronal brain slices (256×256 matrix, 0.86 mm×0.86 mm in-plane resolution). Image volumes were prepared for analysis by removing non-brain tissue (inter-rater reliability for scalp editing procedures, rI=0.99), correcting for intensity non-uniformity due to magnetic field inhomogeneities (Zijdenbos and Dawant, 1994; Sled and Pike, 1998), and by reorienting each volume into the standard position of the ICBM-305 average brain (Mazziotta et al., 1995) using a six-parameter rigid-body transformation with no scaling (Sowell et al., 1999; Narr et al., 2004b) to correct for head tilt and alignment. Hemispheric surfaces comprising of 65,536 surface points were extracted (MacDonald et al., 1994) after manually separating the right from the left hemisphere. Twenty-nine sulcal and gyral landmarks were then landmarked on each hemispheric surface using validated anatomic delineation protocols (Ballmaier et al., 2004; Narr et al., 2005a,b) (Fig. 1). Inter-rater reliability estimates demonstrated less than a 2 mm root mean square (rms) difference in the matched 3D locations of sulcal landmarks traced on six test brains relative to a gold standard arrived at by a consensus of raters.
Fig. 1.

Sulcal and gyral curves, which are subsequently used as anchors in warping procedures to match anatomy between hemispheres and subjects, are shown on the extracted left hemisphere cortical surfaces of three individuals. Detailed anatomical protocols for defining sulcal/gyral landmarks are available at: http://www.loni.ucla.edu/~esowell/edevel/proto.html.
Cortical pattern matching
To compare shape differences between the hemispheres at high-spatial resolution, previously detailed cortical pattern matching methods (Thompson et al., 2000, 2004) were first employed to spatially relate homologous regions of cortical surface morphology between subjects (Narr et al., 2005a,b). Briefly, for matching procedures, a surface-warping algorithm computes a 3D vector deformation field that records the amount of x, y, and z co-ordinate shift (or deformation) to associate the same cortical surface locations in each subject with reference to the average anatomical pattern of the entire study group, using the manually derived sulcal/gyral curves as landmarks. Notably, the average anatomical pattern represents anatomy from both hemispheres where the right hemisphere is flipped across horizontal axis before averaging to further ensure matching of co-ordinate locations across hemispheres. The cortical pattern matching algorithms thus reparameterize (regrid) each hemispheric surface, without imposing any scaling, to spatially relate homologous anatomical regions between hemispheres and across subjects.
Hemispheric shape estimates
To describe hemispheric shape, we used a previously validated ‘distance from the center’ or DFC measure that has been used to describe local patterns of brain growth during brain maturation and to characterize cerebral shape differences in children with fetal alcohol syndrome (Sowell et al., 2001, 2002). This measure was adapted to assess regional shape asymmetries within and across groups defined by sex, handedness and schizophrenia. First, a central point, the anterior commissure set at x=0, y=0, z=0, was located in each brain volume. To ensure that the definition of midline was not biased to the left or to the right, this central point was obtained after averaging the left with the right hemisphere flipped along the horizontal axis. Radial distances (mm) were then computed from this central point to corresponding co-ordinate locations on the lateral surface of the left and right hemispheres, respectively, in each brain volume. DFC measures obtained at each hemispheric surface location, spatially matched using the cortical pattern matching methods described above, were then compared to provide quantitative measures of relative differences of hemispheric shape within subjects at high spatial resolution. Since the central point is defined at midline, DFC measures were not computed for medial hemispheric surface points where distances represent shape differences in one axis only. Fig. 2 shows slice views, DFC or radial distance measurements (middle row), and asymmetry indices computed from DFC measures, from two individuals exhibiting typical (left), and two individuals exhibiting atypical patterns of cerebral torque (right).
Fig. 2.
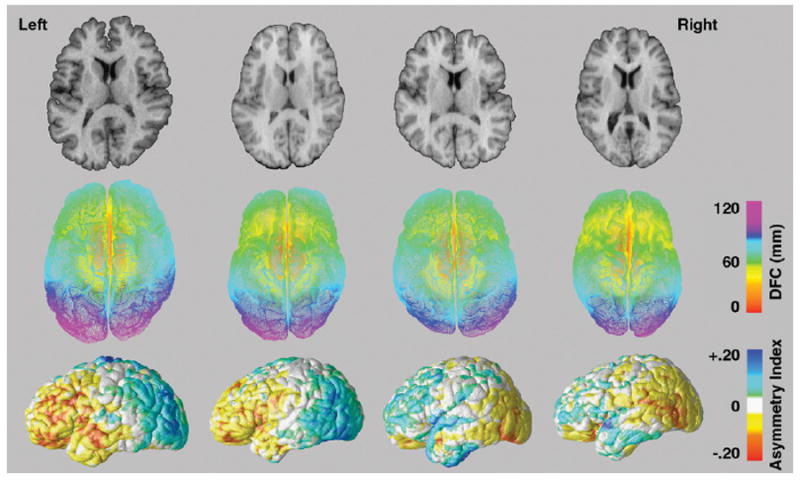
The top row shows axial brain slices from two brain volumes exhibiting the typical patterns of right frontal and left parieto-occipital cerebral torque (left) and brain slices from two brain volumes that show reversals in the typical patterns of cerebral torque (right). The second row shows radial distance or DFC measurements encoded in color at thousands of locations on the left and right hemispheric surfaces corresponding to each brain volume above. The last row shows asymmetry indices of DFC measures projected onto the average surface of the left and right hemisphere flipped along the x-axis for each respective brain volume. Warm colors indicate right hemisphere biased surface expansions while cool colors index left hemisphere biased hemispheric torque.
Statistical analyses
To identify relative differences in the surface morphology of the hemispheres, paired Student’s t-tests were used to compare DFC measures obtained at thousands of equivalent surface points between the left and right hemisphere. Results were mapped within groups defined by hand preference, sex and diagnosis. However, since cell sizes for non-dextral subjects were small, interhemispheric effects were examined by pooling subjects across sex and separately, across diagnosis.
To examine between group differences in hemispheric shape, an asymmetry index [L − R/(0.5(L+R))] was computed for DFC measures obtained at spatially equivalent hemispheric locations in each subject. Using asymmetry indices as dependent measures, the General Linear Model (GLM) was employed using the statistical package R (http://www.r-project.org/) to examine effects of schizophrenia, sex and handedness while controlling for all other factors in the model as well as for age. Two-way interactions between handedness and sex, and between handedness and diagnosis were also examined. Three-way interactions were not assessed given that the cell sizes for non-dextrals defined by sex and diagnosis were considered too small to provide sufficient statistical power. Statistical mapping results, indexed in color, were projected onto the 3D group-averaged hemispheric surface models where an uncorrected two-tailed alpha level of p<0.01 was used as the threshold for interpreting results.
Since statistical tests were performed at thousands of homologous cortical surface co-ordinate points, permutation testing was used to test the overall significance of within and between group (diagnosis, sex and handedness) effects and interactions. For within-group analyses, the number of surface points showing significant hemispheric shape asymmetries at a statistical threshold of p<0.01 was compared with the number of significant surface points that occurred by chance when the left and flipped right hemispheres were randomly assigned labels of left and right, while keeping the numbers of left and right labels the same. For between-group analyses of shape asymmetry indices, residuals from the reduced model for each effect were permuted, enabling the significance of each factor of interest and interaction to be assessed while controlling for the other terms included in the model (Freedman and Lane, 1983; Anderson and Legendre, 1999; Anderson and Braak, 2003). For between-group permutation testing, the number of surface points that were significant for partial regression coefficients obtained for each factor or interaction of interest using an alpha level of p<0.05, was compared to the number of significant surface points that occurred by chance when subjects were randomly assigned to groups in 1000 new randomized analyses.
Results
Statistical maps in Fig. 3 show significant differences in the relative shapes of the left and right hemispheres within (1) dextral healthy male and female subjects (top left); (2) dextral male and female patients with schizophrenia (bottom left); and within (3) non-dextral male and female subjects irrespective of diagnosis (top right); and (4) non-dextral schizophrenia and healthy subjects collapsed across sex (bottom right). The significance and the direction of regional hemispheric shape asymmetries are indexed by the color bars shown at the bottom of Fig. 3.
Fig. 3.

Significant differences in the relative shapes of the left and right hemispheres are shown within dextral healthy male and female subjects (top left), dextral male and female patients with schizophrenia (bottom left), non-dextral male and female subjects collapsed across diagnosis (top right), and non-dextral schizophrenia and healthy subjects collapsed across sex (bottom right). The significance and the direction of regional hemispheric shape asymmetries are indexed by the color bar.
Highly significant surface expansions of the frontal lobe were observed in the right compared to the left hemisphere in dextral subjects irrespective of diagnosis; these reflect right-frontal hemispheric protrusions or petalias. Right frontal shape asymmetries extended towards the posterior limits of the Sylvian fissure and included ventral portions of the postcentral gyrus and rostral portions of the temporal lobe. A similar pattern of results was observed in male non-dextrals, and in non-dextral subjects defined by diagnostic group. However, right-frontal shape asymmetries were less pronounced and less spatially diffuse in female non-dextrals (n=7).
In posterior parietal–occipital regions, radial surface expansions were greater in the left compared to the right hemisphere in dextral subjects, reflecting the expected presence of left occipital protrusions related to cerebral torque. Leftward shape asymmetries, however, appeared slightly more pronounced in males than in females and in dextral subjects. Permutation testing confirmed the overall significance of shape asymmetries in all dextral groups (corrected p-values: p<0.05 for schizophrenia females and p<0.001 for all other groups), and in non-dextrals (corrected p-values: p<0.01 for non-dextral male subjects, and p<0.05 for non-dextral patients and controls) with the exception of female non-dextrals (corrected p-value: p>0.05).
Fig. 4 shows uncorrected statistical mapping results after modeling differences in shape asymmetry indices between groups while controlling for all other factors and age. When comparing patients with first episode schizophrenia and healthy comparison subjects (controlling for sex, handedness and age) (Fig. 4, top left), regional differences in hemispheric shape asymmetries were largely below the threshold of statistical significance. Significant differences in shape asymmetries between individuals defined according to hand preference (Fig. 4, center) revealed some localized increases in shape asymmetries in the vicinity of the occipital pole in dextral compared to non-dextral subjects. Shape asymmetries appeared marginally larger in the vicinity of primary motor cortices in males compared to females (Fig. 4, top right).
Fig. 4.
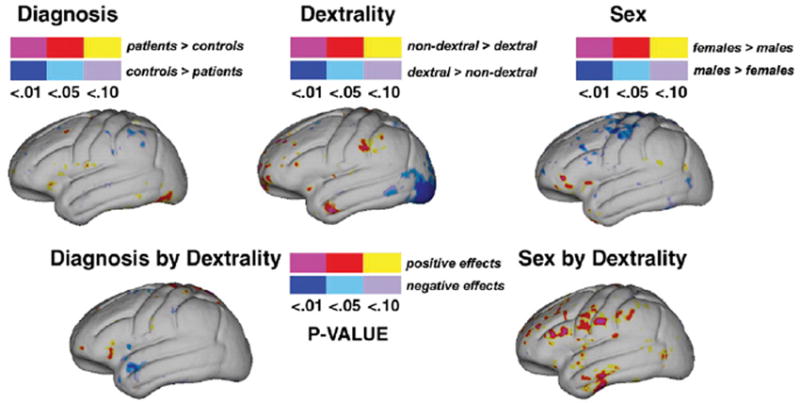
Statistical maps show significant effects (uncorrected) of diagnosis (top left), dextrality (top center), and sex (top right) for hemispheric shape asymmetry indices compared at thousands of hemispheric surface locations. The color bars encode the probability value and the direction of regional group effects. Effects showing significant regional interactions between diagnosis and dextrality (bottom left) and sex and dextrality (bottom right) are mapped below.
Effects of handedness did not differ significantly across diagnostic groups (Fig. 4, bottom left). Finally, some evidence for significant hand preference by sex interactions were observed across the frontal lobe and anterior aspects of the temporal lobe indicating decreases of shape asymmetry in female non-dextrals relative to the other groups (Fig. 4, bottom right). However, permutation testing failed to confirm significant global differences in interhemispheric shape asymmetries between groups defined by diagnosis, handedness or sex, or the presence of diagnosis by handedness and sex by handedness interactions (corrected p-value>0.05).
Discussion
Statistical mapping results showed highly significant and regionally distinct differences in the shapes of the two cerebral hemispheres. The patterns of shape asymmetries were similar across sex and diagnosis and were not significantly influenced by handedness, sex or by diagnostic group interactions with hand preference, as determined by permutation testing. Hemispheric shape asymmetries demonstrated features of cerebral torque that are consistent with the well-described patterns of right-frontal and left-occipital hemispheric protrusions documented in earlier imaging and postmortem studies (LeMay, 1976; Chui and Damasio, 1980; Bear et al., 1986; Koff et al., 1986; Zilles et al., 1996). Specifically, radial distance measures were larger in right compared to left hemisphere frontal regions, and larger in left compared to right occipital regions, in line with CT observations that frontal aspects of the right hemispheres are typically wider and longer than the left, and that parietal–occipital regions are wider and longer in the left hemisphere (LeMay, 1976; Chui and Damasio, 1980; Koff et al., 1986). CT studies using visual categorizations and/or linear measurements from arbitrary brain slices to estimate cerebral torque, however, frequently report that right-biased frontal asymmetries are less frequent and distinct as compared to left-biased occipital asymmetries (LeMay, 1976; Chui and Damasio, 1980; Bear et al., 1986; Koff et al., 1986). Our 3D results show pronounced rightward biases of hemispheric shape across the majority of the frontal lobes, encompassing rostral temporal and perisylvian regions, that are strikingly similar to the patterns of frontal and parietal–occipital torque asymmetries observed using a 3D voxel-based, as opposed to a surface-based shape metric, in a smaller sample of male subjects (Zilles et al., 1996). Our results thus highlight the utility of employing spatially detailed 3D measurement approaches to capture subtle geometric features of hemispheric anatomy to describe shape asymmetries in both anterior and posterior brain regions.
The theory of cerebral dominance proposed by Geschwind and Galaburda (1985) suggests that there are complex relationships between handedness, sex and neuropathology. Specifically, variations in testosterone levels linked with sex were proposed to differentially influence the maturation rates of the two cerebral hemispheres (but see Bryden and Bulman-Fleming, 1994, and McManus and Bryden, 1992 for a critical review of this hypothesis). Furthermore, abnormal neurodevelopmental events interfering with left hemisphere development are posited to alter the organization of brain lateralization and to increase the likelihood of left-handedness and language-related disorders, particularly in males. Indeed, lateralized brain insults occurring early during neurodevelopment have been shown to influence the direction of speech lateralization and handedness (Woods et al., 1988), where harmful environmental events in utero appear twice as likely to affect the left hemisphere (Geschwind et al., 2002). There is also wide agreement that neurodevelopmental disturbances induced by harmful genetic or environmental events contribute to schizophrenia pathophysiology (Harrison, 1999; Bilder, 2001) with some evidence suggesting a preponderance of left hemisphere pathology (Nopoulos et al., 1995; Gur, 1999; Petty, 1999). Particularly, it has been posited that disturbances in lateralization constitute a genetic and evolutionary basis for the disease, which is hypothesized to have developed in concert with hemispheric specialization for language via a gene influencing the direction and magnitude of cerebral dominance (Crow, 1997, 2000). A separate thesis, however, proposes that a single ‘right-shift’ gene (RS+) accounts for speech lateralization, which is associated with handedness, and that a genetic mutation of the right-shift gene contributes towards schizophrenia pathophysiology (Annett, 1999).
Sex effects
Although much research has been dedicated to understanding how sex, handedness and schizophrenia influence structural lateralization, results remain relatively mixed, particularly at the regional level (Beaton, 1997; Shapleske et al., 1999; Toga et al., in press). Nonetheless, empirical evidence supporting presence of greater structural asymmetries in men compared to women are relatively well replicated in perisylvian brain regions (Wada et al., 1975; Witelson and Kigar, 1992; Kulynych et al., 1994; Good et al., 2001; Knaus et al., 2004), where sex effects have also been shown to be influenced by handedness (Witelson and Kigar, 1992; Jancke et al., 1994; Kulynych et al., 1994). Some evidence also suggests that sex influences hemispheric torque where greater degrees of right-frontal and left-occipital width asymmetries have been observed in men compared to women using CT data (Bear et al., 1986). Other CT findings, however, fail to support the presence of sex differences in right-frontal and left-occipital width asymmetries or in hemispheric length asymmetries (Chui and Damasio, 1980; Koff et al., 1986). Similarly, sex-related changes in hemispheric shape asymmetries were largely below the threshold of significance in our investigation. Furthermore, although the magnitude and spatial extent of shape asymmetries within female non-dextrals were less pronounced relative to the other groups, handedness by sex interactions were not determined as significant after permutation testing.
Handedness effects
In spite of a paucity of data, relationships between handedness and some structural brain asymmetries are implicated, particularly with regard to planum temporale asymmetries that are often reported as reduced or more frequently reversed in left-handers (Steinmetz et al., 1991; Foundas et al., 1995; Habib et al., 1995; Shapleske et al., 1999; Foundas et al., 2002; Herve et al., 2006). The influences of hand preference on cerebral torque asymmetries are less conclusive. In left-compared to right-handers determined by self-report, LeMay (1976) noted that right-frontal and left-occipital hemispheric widths, and skull indentations reflecting cortical protrusions tended to be more symmetric and/or more frequently reversed. In partial agreement, Bear et al. (1986) revealed that left-biased occipital, but not right-biased frontal, hemispheric width and length asymmetries were reduced in non right-handers compared to right-handers. Conversely, Zilles et al. (1996) showed that frontal as opposed to occipital regions were largely symmetric in left-compared to right-handers; the method for determining hand preference was not described. Chui and Damasio (1980) failed to detect changes in brain torque measures between right-handers and non-right-handers or between strongly left-handed and ambidextrous individuals. Similarly, results from a large study by Koff et al. (1986) (right-handers: 146; left-handers: 26) using positive and negative handedness scores to dichotomize hand preference, failed to support handedness differences in frontal and occipital hemispheric width and length measures. Although our uncorrected statistical maps suggested reductions in shape asymmetries within occipital regions in non-dextrals relative to dexrals, as most consistent with the 2D findings of Bear et al. (1986) who used the same criteria to classify hand preference, regional effects did not survive permutation testing. Interestingly, cerebral torque of hemispheric protrusions and lengths were found reversed in otherwise healthy subjects with situs inversus (asymmetric internal organs are on the opposite side of the body) (n=3), even though individuals were strongly right-handed (Kennedy et al., 1999). These findings and our results thus suggest that cerebral torque asymmetries occur through mechanisms dissociable from those affecting handedness.
Approximately 10% of the population is left-handed (Gilbert and Wysocki, 1992; Perelle and Ehrman, 2005) and in research studies, the sizes of study groups defined by hand preference are almost always disparate. Likewise, in our investigation substantially fewer subjects were determined as non-dextral, where sample sizes were smallest for female non-dextrals. Since findings for right-handers are less disputed, and our within-group observations of reduced shape asymmetries in female non-dextrals did not translate into significant handedness by sex interactions, reductions in statistical power associated with including fewer left-handed or non-dextral study participants may account for results as well as previous discrepancies across studies. However, differences in handedness categorization schemes may also contribute to inconsistencies in findings, as a historical debate concerning how best to dichotomize handedness remains unresolved (Annett, 1970; Peters and Murphy, 1992; Corey et al., 2001; Byrne et al., 2004; Dragovic, 2004). In spite of an absence of unanimity, observations that left-handers are more inconsistent with regard to hand preference measures than right-handers, that both left- and mixed-handers possess higher incidences of bihemispheric language lateralization (Rasmussen and Milner, 1977; Woods et al., 1988; Knecht et al., 2000), and based on theories concerning the origins of handedness (Annett, 1999), there appears to be a biological basis for categorizing right-handers separately from non-consistent right-handers (Rasmussen and Milner, 1977; Witelson, 1985; Schachter et al., 1987; Witelson and Kigar, 1992). Notwithstanding, group differences associated with hand preference may be underestimated when separating dextral from non-dextral subjects and therefore by categorizing purely left- and mixed-handers together. Differences in prior results concerning the influence of handedness on cerebral torque measures, however, do not appear solely attributable to handedness categorization schemes.
Schizophrenia effects
Many studies have observed schizophrenia-related reductions (or reversals) in asymmetric perisylvian regions, but negative findings are common, for review see (Toga et al., in press; Harrison, 1999; Petty, 1999; Shapleske et al., 1999; Shenton et al., 2001; Sommer et al., 2001). Similarly, some CT studies report significant differences in left-biased occipital (Luchins et al., 1979; Falkai et al., 1995b) and right-biased frontal hemispheric width asymmetries (Falkai et al., 1995b), but several investigators have failed to replicate these findings in schizophrenia (Andreasen et al., 1982; Jernigan et al., 1982; Luchins and Meltzer, 1983; Crow et al., 1989). A meta-analysis of five CT studies examining the proportion of individuals exhibiting typical versus reduced/reversed torque-related asymmetries, rather than the heterogeneous asymmetry measures themselves, however, concluded that disturbances in the typical asymmetry patterns occur more frequently in schizophrenia relative to normal (Sommer et al., 2001). One MRI study appears to support this conclusion reporting reductions of occipital width asymmetries in first episode schizophrenia patients, although hemispheric lengths were found significantly more asymmetric in patients relative to controls (DeLisi et al., 1997). A second MRI study failed to find differences in hemispheric width or length asymmetry indices between schizophrenia patients and controls (Guerguerian and Lewine, 1998).
In our investigation, cerebral shape asymmetries appeared undisturbed in first episode schizophrenia compared to normal. These results contrast with our earlier findings suggesting reductions of cerebral torque in an independent sample of first episode patients compared to healthy subjects (Bilder et al., 1994). Prior measurements, however, were based on volumetric asymmetries for large parcellated regions comprising both gray and white matter, as opposed to shape measures, and torque was quantified using a composite asymmetry index summing volume asymmetries across different regions (Bilder et al., 1994). These results were replicated in another sample of patients with schizophrenia and in their first degree relatives, suggesting these findings are related to genetic risk for schizophrenia (Sharma et al., 1999). But using the same approach to quantify volumetric torque asymmetries, results of Bilder et al. (1994) were not replicated in a smaller schizophrenia study (Guerguerian and Lewine, 1998). Likewise, differences in cerebral torque, estimated using a 3D method to extract and measure local volume asymmetries, were not shown to differ significantly between patients with schizophrenia and healthy subjects (Mackay et al., 2003). Inconsistencies in findings concerning disturbances of torque-related asymmetries in schizophrenia are perhaps not surprising given that measurements used to describe cerebral torque have varied substantially across studies. Since the area or volume of an object can be independent of that object’s shape, it seems reasonable to hypothesize that the volume and shape of the cerebral hemispheres are only moderately related. Since cerebral torque more closely describes 3D changes of hemispheric morphology rather than of volume, volume asymmetry indices may result from different underlying biological factors. In our investigation, main effects of schizophrenia were determined after correcting for all other factors including handedness, and diagnosis by handedness effects were not determined as significant. Thus, it is unlikely that increases in left- and/or mixed-handedness as have been reported in schizophrenia (Satz and Green, 1999; Dragovic and Hammond, 2005) influenced the results.
Conclusion
Subtle geometric distortions of hemispheric surface morphology manifest as right-frontal and left-occipital shape asymmetries that characterize cerebral torque and are consistent with the patterns of petalias. Although the magnitude, spatial extent and directions of hemispheric shape asymmetries vary substantially between individuals, factors including sex, hand preference or a diagnosis for schizophrenia are not shown to measurably alter cerebral torque. Thus, hemispheric shape differences may be more closely associated with language dominance and/or other lateralized functions in the brain that are at least partially independent of hand preference. Alternatively, processes separate from those determining functional lateralization of language or handedness, but which affect anatomic asymmetries of the viscera, may influence cerebral torque (Kennedy et al., 1999). The failure to replicate disturbances of cerebral torque in schizophrenia may reflect differences in measurement techniques previously used to define such asymmetries. However, it may still be plausible that disease processes in schizophrenia are lateralized resulting in disproportionate tissue loss in one hemisphere with respect to the other, although whether localized tissue loss relates to changes in brain shape remains to be determined. Finally, our failure to confirm differences in hemispheric shape asymmetries between men and women does not exclude the presence of sex effects on perisylvian asymmetries, but implies that some non-overlapping biological mechanisms account for these asymmetries.
Acknowledgments
This work was generously supported by research grants from the National Center for Research Resources (P41 RR13642), the National Institute of Mental Health (RO1 MH60374), the NIH Roadmap Initiative (P20 RR020750), the National Library of Medicine (R01 LM05639), the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB), a NARSAD Young Investigator Award and Career Development Award (K01 MH073990-01A1, to KLN). Algorithm development was also supported by grants R21 EB01651, R21 RR019771, and AG016570 (to PT).
Footnotes
Publisher's Disclaimer: This article was originally published in a journal published by Elsevier, and the attached copy is provided by Elsevier for the author’s benefit and for the benefit of the author’s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues that you know, and providing a copy to your institution’s administrator.
All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier’s permissions site at: http://www.elsevier.com/locate/permissionusematerial
References
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K. Asymmetry in the human motor cortex and handedness. NeuroImage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000;38:304–312. doi: 10.1016/s0028-3932(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca’s region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Braak CJ. Permutation tests for multi-factorial analysis of variance. J Stat Comput Simul. 2003;73:85–113. [Google Scholar]
- Anderson MJ, Legendre P. An empirical comparison of permutation methods for tests of partial regression coefficients in a linear model. J Stat Comput Simul. 1999;62:271–303. [Google Scholar]
- Andreasen NC, Dennert JW, Olsen SA, Damasio AR. Hemispheric asymmetries and schizophrenia. Am J Psychiatry. 1982;139:427–430. doi: 10.1176/ajp.139.4.427. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br J Psychology. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Annett M. The theory of an agnostic right shift gene in schizophrenia and autism. Schizophr Res. 1999;39:177–182. doi: 10.1016/s0920-9964(99)00072-9. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, O’Brien JT, Burton EJ, Thompson PM, Rex DE, Narr KL, McKeith IG, DeLuca H, Toga AW. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer’s disease using cortical pattern matching: diagnosis and gender effects. NeuroImage. 2004;23:325–335. doi: 10.1016/j.neuroimage.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Mackay CE, Prima S, Maes F, Vandermeulen D, Crow TJ, Roberts N. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. NeuroImage. 2005;24:678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Bear D, Schiff D, Saver J, Greenberg M, Freeman R. Quantitative analysis of cerebral asymmetries. Fronto-occipital correlation, sexual dimorphism and association with handedness. Arch Neurol. 1986;43:598–603. doi: 10.1001/archneur.1986.00520060060019. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: a review of the evidence. Brain Lang. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Bilder RM. Schizophrenia as a neurodevelopmental disorder. Curr Opin Psychiatry. 2001;14:9–15. [Google Scholar]
- Bilder RM, Wu H, Bogerts B, Degreef G. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- Bryden MP, Bulman-Fleming MB. Evaluating the empirical support for the Geschwind–Behan–Galaburda model of cerebral lateralization. Brain Cogn. 1994;26:103–167. doi: 10.1006/brcg.1994.1045. [DOI] [PubMed] [Google Scholar]
- Byrne M, Clafferty RA, Cosway R, Grant E, Hodges A, Lawrie SM, Johnstone EC. Measurement of lateral preferences and schizophrenia: results of the Edinburgh High-Risk Study and methodological issues. Psychiatry Res. 2004;125:205–217. doi: 10.1016/j.psychres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Chui HD, Damasio AR. Human cerebral asymmetries evaluated by computerized tomography. J Neurol Neurosurg Psychiatry. 1980;43:873–878. doi: 10.1136/jnnp.43.10.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DM, Hurley MM, Foundas AL. Right and left handedness defined: a multivariate approach using hand preference and hand performance measures. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:144–152. [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that Homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000;31:118–129. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Colter N, Frith CD, Johnstone EC. Developmental arrest of cerebral asymmetries in early onset schizophrenia. Psychiatry Res. 1989;29:247–253. doi: 10.1016/0165-1781(89)90053-x. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Kushner M, Finer DL, Hoff AL, Crow TJ. Anomalous cerebral asymmetry and language processing in schizophrenia. Schizophr Bull. 1997;23:255–271. doi: 10.1093/schbul/23.2.255. [DOI] [PubMed] [Google Scholar]
- Dragovic M. Categorisation and validation of handedness using Latent Class Analysis. Acta Neuropsychiatr. 2004;16:212–218. doi: 10.1111/j.0924-2708.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G. Handedness in schizophrenia: a quantitative review of evidence. Acta Psychiatr Scand. 2005;111:410–419. doi: 10.1111/j.1600-0447.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Schneider T, Greve B. Disturbed planum temporale asymmetry in schizophrenia: a quantitative post-mortem study. Schizophr Res. 1995a;14:161–176. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- Falkai P, Schneider T, Greve B, Klieser E, Bogerts B. Reduced frontal and occipital lobe asymmetry on the CT-scans of schizophrenic patients. Its specificity and clinical significance. J Neural Transm Gen Sect. 1995b;99:63–77. doi: 10.1007/BF01271470. [DOI] [PubMed] [Google Scholar]
- Falzi G, Perrone P, Vignolo LA. Right–left asymmetry in anterior speech region. Arch Neurol. 1982;39:239–240. doi: 10.1001/archneur.1982.00510160045009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition. State Psychiatric Institute; New York, NY: 1997. [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca’s area: the pars triangularis and pars opercularis. Brain Lang. 1998a;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Hong K, Leonard CM, Heilman KM. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry Neuropsychol Behav Neurol. 1998b;11:65–71. [PubMed] [Google Scholar]
- Foundas AL, Faulhaber JR, Kulynych JJ, Browning CA, Weinberger DR. Hemispheric and sex-linked differences in Sylvian fissure morphology: a quantitative approach using volumetric magnetic resonance imaging. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:1–10. [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: do right- and left handers differ? Brain Lang. 2002;83:403–424. doi: 10.1016/s0093-934x(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphologic cerebral asymmetries and handedness. Arch Neurol. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983;1:292–298. [Google Scholar]
- Galaburda AM, Sanides F, Geschwind N. Human brain. Cytoarchitectonic left–right asymmetries in the temporal speech region. Arch Neurol. 1978;35:812–817. doi: 10.1001/archneur.1978.00500360036007. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci U S A. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization: biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Arch Neurol. 1985;42:521–552. doi: 10.1001/archneur.1985.04060060019009. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left–right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Gilbert AN, Wysocki CJ. Hand preference and age in the United States. Neuropsychologia. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Guerguerian R, Lewine RR. Brain torque and sex differences in schizophrenia. Schizophr Res. 1998;30:175–181. doi: 10.1016/s0920-9964(97)00144-8. [DOI] [PubMed] [Google Scholar]
- Gur RE. Is schizophrenia a lateralized brain disorder? Editor’s introduction. Schizophr Bull. 1999;25:7–9. doi: 10.1093/oxfordjournals.schbul.a033368. [DOI] [PubMed] [Google Scholar]
- Gur RE, Chin S. Laterality in functional brain imaging studies of schizophrenia. Schizophr Bull. 1999;25:141–156. doi: 10.1093/oxfordjournals.schbul.a033361. [DOI] [PubMed] [Google Scholar]
- Habib M, Robichon F, Lévrier O, Khalil R, Salamon G. Diverging asymmetries of temporo-parietal cortical areas: a reappraisal of Geschwind/Galaburda theory. Brain Lang. 1995;48:238–258. doi: 10.1006/brln.1995.1011. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Herve PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. NeuroImage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, O’Donnell DW, Stritzke P. Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull. 1992;18:257–272. doi: 10.1093/schbul/18.2.257. [DOI] [PubMed] [Google Scholar]
- Hustler JJ, Loftus WC, Gazzaniga MS. Individual variation of cortical surface area asymmetries. Cereb Cortex. 1998;8:11–17. doi: 10.1093/cercor/8.1.11. [DOI] [PubMed] [Google Scholar]
- Ide A, Rodríguez E, Zaidel E, Aboitiz F. Bifurcation patterns in the human sylvian fissure: hemispheric and sex differences. Cereb Cortex. 1996;6:717–725. doi: 10.1093/cercor/6.5.717. [DOI] [PubMed] [Google Scholar]
- Jancke L, Schlaug G, Huang Y, Steinmetz H. Asymmetry of the planum parietale. NeuroReport. 1994;5:1161–1163. doi: 10.1097/00001756-199405000-00035. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zatz LM, Moses JA, Jr, Cardellino JP. Computed tomography in schizophrenics and normal volunteers: II. Cranial asymmetry. Arch Gen Psychiatry. 1982;39:771–773. doi: 10.1001/archpsyc.1982.04290070007002. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, O’Craven KM, Ticho BS, Goldstein AM, Makris N, Henson JW. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53:1260–1265. doi: 10.1212/wnl.53.6.1260. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Polk M, Black SE, Howell J. Sex, handedness, and the morphometry of cerebral asymmetries on magnetic resonance imaging. Brain Res. 1990;530:40–48. doi: 10.1016/0006-8993(90)90655-u. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Sex-linked differences in the anatomy of the perisylvian language cortex: a volumetric MRI study of gray matter volumes. Neuropsychology. 2004;18:738–747. doi: 10.1037/0894-4105.18.4.738. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Koff E, Naeser MA, Pieniadz JM, Foundas AL, Levine HL. Computed tomographic scan hemispheric asymmetries in right- and left-handed male and female subjects. Arch Neurol. 1986;43:487–491. doi: 10.1001/archneur.1986.00520050059023. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Gender differences in the normal lateralization of the supratemporal cortex: MRI surface-rendering morphometry of Heschl’s gyrus and the planum temporale. Cereb Cortex. 1994;4:107–118. doi: 10.1093/cercor/4.2.107. [DOI] [PubMed] [Google Scholar]
- Kulynych JJ, Luevano LF, Jones DW, Weinberger DR. Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry. 1997;41:995–999. doi: 10.1016/S0006-3223(96)00292-2. [DOI] [PubMed] [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann N Y Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- LeMay M. Asymmetries of the skull and handedness. Phrenology revisited. J Neurol Sci. 1977;32:243–253. doi: 10.1016/0022-510x(77)90239-8. [DOI] [PubMed] [Google Scholar]
- Luchins DJ, Meltzer HY. A blind, controlled study of occipital cerebral asymmetry in schizophrenia. Psychiatry Res. 1983;10:87–95. doi: 10.1016/0165-1781(83)90107-5. [DOI] [PubMed] [Google Scholar]
- Luchins DJ, Weinberger DR, Wyatt RJ. Schizophrenia: evidence of a subgroup with reversed cerebral asymmetry. Arch Gen Psychiatry. 1979;36:1309–1311. doi: 10.1001/archpsyc.1979.01780120039005. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 2006;16:1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Avis D, Evans AC. Multiple surface identification and matching in magnetic resonance imaging. Proc SPIE. 1994;2359:160–169. [Google Scholar]
- Mackay CE, Barrick TR, Roberts N, DeLisi LE, Maes F, Vandermeulen D, Crow TJ. Application of a new image analysis technique to study brain asymmetry in schizophrenia. Psychiatry Res. 2003;124:25–35. doi: 10.1016/s0925-4927(03)00088-x. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development (The International Consortium for Brain Mapping; ICBM) NeuroImage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- McManus IC, Bryden MP. Geschwind’s theory of cerebral laterization: developing a formal causal model. Psychol Bull. 1992;110:237–253. doi: 10.1037/0033-2909.110.2.237. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Moussai J, Zoumalan C, Rayman J, Toga AW. 3D mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry. 2001;158:244–255. doi: 10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Kim S, Thompson PM, Szeszko P, Robinson D, Luders E, Toga AW. Abnormal gyral complexity in first-episode schizophrenia. Biol Psychiatry. 2004a;55:859–867. doi: 10.1016/j.biopsych.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. NeuroImage. 2004b;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, DeLuca H, Thompson PM. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005a;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005b;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Torres I, Flaum M, Andreasen NC. Brain morphology in first-episode schizophrenia. Am J Psychiatry. 1995;152:1721–1723. doi: 10.1176/ajp.152.12.1721. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Tomaiuolo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC. Human cingulate and paracingulate sulci: pattern, variability, asymmetry, and probabilistic map. Cereb Cortex. 1996;6:207–214. doi: 10.1093/cercor/6.2.207. [DOI] [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. On the other hand. Behav Genet. 2005;35:343–350. doi: 10.1007/s10519-005-3226-z. [DOI] [PubMed] [Google Scholar]
- Peters M, Murphy K. Cluster analysis reveals at least three, and possibly five distinct handedness groups. Neuropsychologia. 1992;30:373–380. doi: 10.1016/0028-3932(92)90110-8. [DOI] [PubMed] [Google Scholar]
- Petty RG. Structural asymmetries of the human brain and their disturbance in schizophrenia. Schizophr Bull. 1999;25:121–139. doi: 10.1093/oxfordjournals.schbul.a033360. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Burgel U, Geyer S, Schormann T, Schleicher A, Freund HJ, Zilles K. Variability and asymmetry in the human precentral motor system. A cytoarchitectonic and myeloarchitectonic brain mapping study. Brain. 2001;124:2232–2258. doi: 10.1093/brain/124.11.2232. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sc. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Satz P, Green MF. Atypical handedness in schizophrenia: some methodological and theoretical issues. Schizophr Bull. 1999;25:63–78. doi: 10.1093/oxfordjournals.schbul.a033367. [DOI] [PubMed] [Google Scholar]
- Schachter SC, Ransil BJ, Geschwind N. Associations of handedness with hair color and learning disabilities. Neuropsychologia. 1987;25:269–276. doi: 10.1016/0028-3932(87)90137-0. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H, Barta P, Pearlson G, Murry R. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives—The Maudsley Family Study. Schizophr Res. 1999;40:111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med. 1992;327(c) doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Trans Med Imag. 1998;17:653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Steinmetz H. Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci Biobehav Rev. 1996;20:587–591. doi: 10.1016/0149-7634(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Volkmann J, Jancke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 1991;29:315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Gunning-Dixon F, Ashtari M, Snyder PJ, Lieberman JA, Bilder RM. Reversed cerebellar asymmetry in men with first-episode schizophrenia. Biol Psychiatry. 2003;53:450–459. doi: 10.1016/s0006-3223(02)01529-9. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Moussai J, Zohoori S, Goldkorn A, Khan AA, Mega MS, Small GW, Cummings JL, Toga AW. Cortical variability and asymmetry in normal aging and Alzheimer’s disease. Cereb Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Woods RP, Mega MS, Toga AW. Mathematical/computational challenges in creating deformable and probabilistic atlases of the human brain. Hum Brain Mapp. 2000;9:81–92. doi: 10.1002/(SICI)1097-0193(200002)9:2<81::AID-HBM3>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. NeuroImage. 2004;23(Suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Toga AW, Narr KL, Thompson PM, Luders E. Brain Asymmetry. Encyclopedia of Neuroscience. (2) in press. [Google Scholar]
- Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, Honer WG, Falkai P. Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J of Psychiatry. 2000;157:34–39. doi: 10.1176/ajp.157.1.34. [DOI] [PubMed] [Google Scholar]
- Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB. Sexually-dimorphic patterns of cortical asymmetry, and the role for sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology. 1998;23:519–547. doi: 10.1016/s0306-4530(98)00019-5. [DOI] [PubMed] [Google Scholar]
- Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229:665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. J Comp Neurology. 1992;323:326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- Woods RP, Dodrill CB, Ojemann GA. Brain injury, handedness, and speech lateralization in a series of amobarbital studies. Ann Neurol. 1988;23:510–518. doi: 10.1002/ana.410230514. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comp Ass Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Dawant BM. Brain segmentation and white matter lesion detection in MR images. Crit Rev Biomed Eng. 1994;22:401–465. [PubMed] [Google Scholar]
- Zilles K, Dabringhaus A, Geyer S, Amunts K, Qu M, Schleicher A, Gilissen E, Schlaug G, Steinmetz H. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neurosci Biobehav Rev. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]


