Abstract
Gene knockouts in mice have showed that Grb2 and GATA6 are essential for the formation of primitive endoderm in blastocysts. Here, we confirmed that implanted Grb2-null blastocysts lack primitive or extraembryonic endoderm cells either at E4.5 or E5.5 stages. We analyzed the relationship between Grb2 and GATA6 in the differentiation of embryonic stem (ES) cells to primitive endoderm in embryoid body models. Upon transfection with GATA6 expression vector, Grb2-null ES cells underwent endoderm differentiation as indicated by the expression of the extraembryonic endoderm markers Dab2 and GATA4. Transfection of GATA4 expression vector also had the same differentiation potency. When GATA6- or GATA4-transfected Grb2-null ES cells were allowed to aggregate, fragments of an endoderm layer formed on the surface of the spheroids. The results suggest that GATA6 is downstream of Grb2 in the inductive signaling pathway and the expression of GATA6 is sufficient to compensate for the defects caused by Grb2 deficiency in the development of the primitive and extraembryonic endoderm.
Keywords: primitive endoderm, blastocysts, ES cells, Ras/MAPK pathway, embryoid bodies, lineage differentiation
INTODUCTION
The primitive endoderm is one of the earliest cell lineages arising from the inner cell mass in early embryogenesis. Recent studies indicate that primitive endoderm cells originate within the interior of the pluripotent cells of the inner cell mass, and the differentiated cells sort and position on the surface to form an epithelial layer covering the inner cell mass that is referred to as the epiblast in the later stage of the blastocysts (Chazaud et al., 2006; Rula et al., 2007; Plusa et al., 2008; Meihac et al., 2009). Dab2 is required for sorting of the newly derived primitive endoderm cells to the surface to form an epithelial layer (Yang et al., 2002; 2007). The sorting and surface positioning of the primitive endoderm cells are thought to be determined by the ability of the primitive endoderm cells to generate apical polarity, rather than differential adhesive affinity (Yang et al., 2007; Gerbe et al., 2008; Moore et al., 2009).
Oct3/4 and Nanog are markers of pluripotency for the cells of the inner cell mass and epiblast (Pesce and Scholer, 2001; Cavaleri and Scholer, 2003; Mitsui et al., 2003; Chambers et al., 2007). Expression of Oct3/4 and Nanog is lost and several other genes are induced upon differentiation, and laminin, GATA4, GATA6, and Dab2 are common markers for the primitive endoderm lineage (Rossant and Tam, 2004; Cai et al., 2008). Primitive endoderm forms at the time of blastocyst implantation (Gardner, 1982). Parietal endoderm cells are subsequently derived from the primitive endoderm cells and migrate out to cover the surface of the blastocoels (Gardner, 1989). The parietal endoderm cells retain the expression of GATA4 and GATA6, and actively produce extracellular matrix components such as laminin to form a thick basement membrane, the Reichert’s membrane (Cai et al., 2008). The remaining primitive endoderm cells covering the epiblast mature into visceral endoderm cells, which retain GATA4 expression but lose GATA6 expression (Cai et al., 2008).
Characterization of mutant embryos from genetic knockout mice identified GATA6 as an essential gene for the development of extraembryonic endoderm (Koutsurakis et al., 1999; Morrisey et al., 1998), and GATA6 is required at the step of commitment to primitive endoderm fate (Cai et al., 2008). GATA family proteins consist of six transcription factors that bind “A/G GATA A/G” core sequence and regulate the development of various cell lineages and organs (Patient and McGhee, 2002). Among them, GATA4, in addition to GATA6, plays a role in the differentiation of primitive endoderm in embryoid bodies (Soudais et al., 1985). Unlike wildtype cells, GATA4-null embryonic stem (ES) cells do not spontaneously differentiate and form an endoderm layer upon aggregation as embryoid bodies (Soudais et al., 1985). However, the requirement of GATA4 is bypassed by addition of retinoic acid (Bielinska and Wilson, 1997; Capo-chichi et al., 2005). In contrast, GATA6 is essential for primitive endoderm differentiation both in vivo and in vitro (Cai et al., 2008).
Another essential gene for primitive endoderm development is Grb2 (Cheng et al., 1998; Chazaud et al., 2006). This conclusion was reached in the studies of dissected Grb2 null blastocysts in vitro, however the phenotype of Grb2-null blastocysts implanted inside uterine has not been reported (Cheng et al., 1998; Chazaud et al., 2006). Grb2, an adaptor protein that links receptor tyrosine kinase (RTK) to Sos, is a crucial component of the RTK-Grb2-Sos-Ras-MEK-Erk signaling cascades. Several studies support a pivotal role of this Ras/MAPK pathway in the differentiation of primitive endoderm (Cheng et al., 1998; Chazaud et al., 2006; Yamanaka et al., 2010; Nichols et al., 2009). Targeted disruption of fibroblast growth factor receptor 2 or its ligand fgf4, which activate the Ras/MAPK signal pathway in the cells of the inner cell mass, also abrogates primitive endoderm differentiation (Feldman et al., 1995; Arman et al., 1998). Suppression of Ras/MAPK pathway preserves pluripotency of the inner cell mass (Nichols et al., 2009). Furthermore, a constitutive active mutant of either Ras or MEK is sufficient to promote primitive endoderm differentiation of murine ES cells in culture (Verheijen et al., 1999). Activation of Ras/MAPK pathway is believed to suppress Nanog expression (Hamazaki et al., 2006), which subsequently releases its repression of GATA6 expression, since putative Nanog binding motif has been predicted in the promoter region of GATA6 (Hamazaki et al., 2006). Thus, it is thought that the differentiation of primitive endoderm is initiated through the repression of Nanog expression, which is expressed with GATA6 in a “salt and pepper” pattern in the inner cell mass of blastocysts as early as 3.5 days post coitum (Chazaud et al., 2006).
Additional crucial genes affecting primitive endoderm development are Nanog and Oct-3/4. These are transcription factors required for the maintenance of pluripotency of the epiblasts and their expression is expected to subside upon differentiation (Nichols et al., 1998; Cavaleri and Scholer, 2003; Chambers et al., 2007). In implanting blastocyst, the expression of Nanog is mutually exclusive with GATA4 and GATA6-positive cells that are markers for primitive endoderm (Chazaud et al., 2006). In vitro, Nanog deficient ES cells take up parietal endoderm fate (Mitsui et al., 2003; Chambers et al., 2003). In contrast, Oct3/4 is present initially in the primitive endoderm cells at the early stage of blastocyst development, and gradually subsides upon further differentiation (Nichols et al., 1998; Niwa et al., 2000; Pesce and Schöler, 2001). Nevertheless, suppression of Oct3/4 in ES cells in culture leads to a trophectoderm rather than primitive endoderm fate (Hough et al., 2006; Niwa et al., 2005).
In this study, we further compared the mutant phenotypes of Grb2 and GATA6 knockout embryos, and analyzed the relationship between Grb2 and GATA6 genes in ES cell differentiation and primitive endoderm development.
RESULTS
Failure of Primitive Endoderm Epithelial Layer Formation in Grb2-Null Embryos
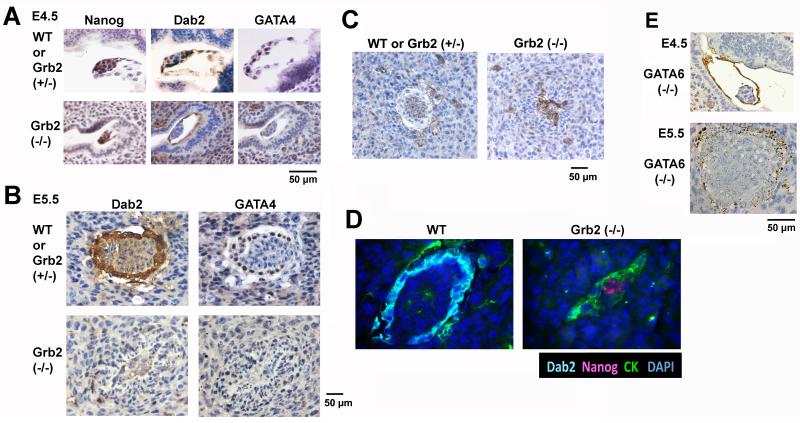
Previously, Grb2 homozygous knockout embryos were found to die at an early step of mouse embryonic development, at around E5-6 stages (Cheng et al., 1998). In vitro study of blastocysts suggested that Grb2 null blastocysts fail to undergo differentiation to form a primitive endoderm layer (Chazaud et al., 2006). We further examined the phenotype of Grb2 mutant embryos implanted on uterine wall. Following timed mating of Grb2 (+/−) parents, whole uterine horns were harvested at approximate 4.5 days post coitum and processed for histological analysis (Fig. 1A). In the sections, morphologically normal implanted blastocysts containing a Nanog-positive inner cell mass and Dab2- and GATA4-positive primitive endoderm layer were identified and assigned as wildtype or Grb2 (+/−). On the same sections, littermates containing Nanog-positive inner cell mass but lacking Dab2- and GATA4-positive primitive endoderm cells were observed, and these abnormal blastocysts were designated as the presumptive Grb2-null embryos (Fig. 1A). The Grb2-null embryos were found to constitute approximately the expected ratio of one-fourth of the total embryos: 4 mutant E4.5 embryos were found among a total of 13 E4.5 embryos analyzed. Thus, we confirmed that Grb2 is required for primitive endoderm formation in implanted blastocysts.
Fig. 1.
Failure of primitive endoderm epithelial layer formation in Grb2-null embryos. Embryos enclosed in uterine horns from timed matings of Grb2 (+/−) parents were harvested, fixed, and embedded in paraffin. Adjacent sections were immunostained for Nanog, Dab2, and GATA4. Embryos containing Dab2 and GATA4-positive endoderm cells are assigned as either wildtype or Grb2 heterozygous. Abnormal embryos lacking endoderm markers were presumptive Grb2 (−/−). A: Representative E4.5 implanted embryos from timed matings between Grb2 (+/−) parents are shown. Littermates are compared between a wildtype or Grb2 heterozygous and a presumptive Grb2 (−/−) embryo. B: Representative E5.5 embryos from timed matings between Grb2 (+/−) parents are shown, comparing Dab2 and GATA4 immunostaining of a wildtype or Grb2 heterozygous with a presumptive Grb2 (−/−) embryo. C: Representative cytokeratin 8 immunostaining of E5.5 embryos from timed matings between Grb2 (+/−) parents are shown, comparing a wildtype or Grb2 heterozygous with a presumptive Grb2 (−/−) embryo. D: Representative wildtype or Grb2 heterozygous and a presumptive Grb2 (−/−) E5.5 embryos are shown, of immunofluorescence staining of Dab2, cytokeratin 8 (CK), Nanog, and DAPI. E: Representative Dab2 immunostaining of E4.5 and E5.5 implanted, presumptive GATA6-null embryos from timed matings between GATA6 (+/−) parents are shown for comparison. Scale bars are provided next to the images.
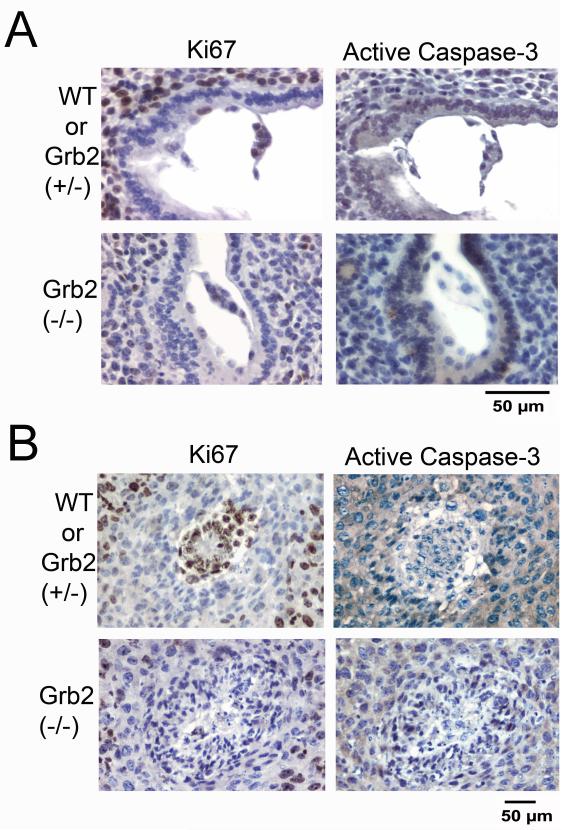
Grb2-null embryos persist to E5.5 stage, showing deformed embryos lacking a Dab2- and GATA4-positive extraembryonic endoderm observed in wildtype or Grb2 (+/−) littermates (Fig. 1B). Similar to the normal embryos, trophectoderm cells (indicated by cytokeratin 8 staining) are present, scattered around the implanted site of the presumptive Grb2-null E5.5 embryos (Fig. 1C). Noticeably, large numbers of the Grb2-null embryonic cells are also positive for cytokeratin, suggesting possible differentiation of the Grb2-null inner cell mass to trophectoderm lineage (Fig. 1C). In the mutant embryos, Nanog-positive embryonic cells are still observable at E5.5, at which stage normal embryos no longer contain Nanog-expressing cells (Fig. 1D). In comparison, the Grb2-null embryos at E4.5 stage are indistinguishable from those of GATA6-null embryos (Fig. 1E), which also lack a primitive endoderm layer (Cai et al., 2008). Although both Grb2- and GATA6-null embryos lack cell organization and structure at E5.5 stage, the Grb2-null embryos (Fig. 1B) are significantly smaller than the GATA6 null embryos (Fig. 1E). We used staining of ki67 and activated caspase-3 to monitor proliferation and apoptotic cell death respectively in the embryos (Fig. 2). At E4.5, cells within the normal/wildtype embryos and the deciduas are highly proliferative, but the lack of cell proliferation in the Grb2 (−/−) embryo is apparent, though deciduas carrying the defective embryo are positive for ki67 staining (Fig. 2A). By E5.5, the wildtype embryos remain highly proliferative and expanded, while the Grb2-deficient embryos are not expended (Fig. 2B). Little caspase-3 activation was observed in either wildtype or Grb2-null embryos (Fig. 2A, B). Cell stained for activated caspase-3 were observed in other location in the same slides (ovaries and uteri), serving as a positive control (not shown).
Fig. 2.
Reduced proliferation of Grb2-null embryos. E4.5 and E5.5 embryos enclosed in uterine horns from timed matings of Grb2 (+/−) parents were harvested, fixed, and embedded in paraffin. Adjacent sections were stained with markers for Nanog, Dab2, and GATA4 to assign genotypes to be either wildtype or Grb2 heterozygous, or presumptive Grb2 (−/−). Examples of adjacent sections of a wildtype and a Grb2-null embryo stained with either ki67 or activated/cleaved caspase-3 are shown: A: E4.5; B: E5.5.
Thus, like GATA6, Grb2 is essential for primitive endoderm differentiation; however, unlike GATA6, Grb2 is also required for cell proliferation and the expansion of the inner cell mass. GATA6 mutant embryos have relatively large inner cell mass at E5.5 (Cai et al., 2008), suggesting the GATA6 is not required for cell proliferation. Grb2 appears to be not required for the differentiation and development of trophectoderm.
Grb2-Null ES Cells Are Incompetent in Differentiation into Primitive Endoderm Lineage
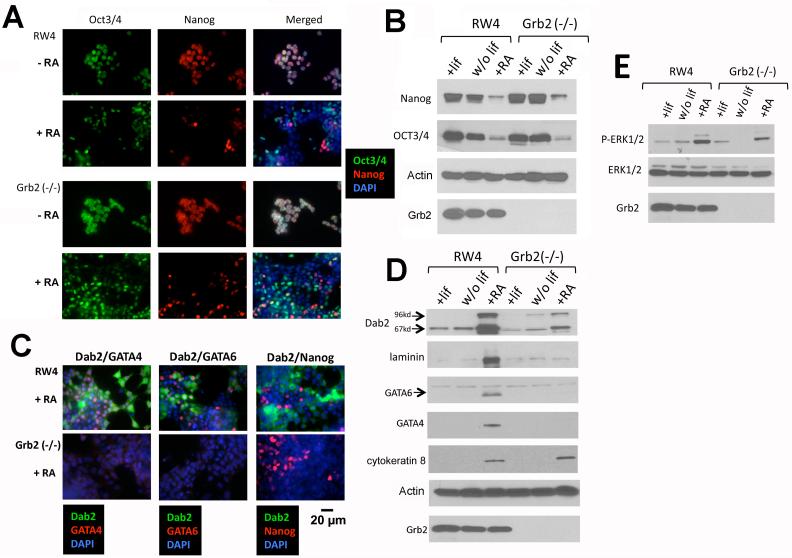
ES cells differentiate towards extraembryonic lineages following retinoic acid treatment or cell aggregation to form embryoid bodies (Capo-chichi et al., 2005). We further studied the propensity of Grb2 (−/−) ES cells to undergo differentiation in culture. Like wildtype RW4 ES cells, prior to treatment, Grb2-null ES cells ubiquitously express pluripotent markers Oct3/4 and Nanog (Fig. 3A). Upon treatment with retinoic acid, Oct3/4 and Nanog expression were reduced in a large fraction of Grb2 null ES cells as judged by immunofluorescence microscopy (Fig. 3A). Both Nanog and Oct3/4 were reduced to a comparable extent in both wildtype and Grb2-null ES cells assayed by Western blot (Fig. 3B). However, unlike RW4 cells, Grb2-null ES cells did not undergo endoderm differentiation since the markers, GATA4, GATA6, and Dab2, were not induced (or greatly reduced) as assayed by both immunofluorescence staining and Western blot (Fig. 3C,D). Nevertheless, cytokeratin 8 (a trophectoderm marker) expression was equally induced in Grb2-null ES cells upon retinoic acid treatment (Fig. 3D), indicating Grb2 is not essential for trophectoderm differentiation. Retinoic acid was much stronger than the withdrawal of LIF in inducing differentiation of RW4 ES cells, but retinoic acid still did not stimulate a significant induction of primitive endoderm markers in Grb2-null ES cells. We observed that retinoic acid induced an increased MAPK activation in both RW4 and Grb2 (−/−) ES cells (Fig. 3E), suggesting activation of MAPK pathway may play a role in ES cell differentiation, but MAPK activation is not sufficient to induce the primitive endoderm lineage.
Fig. 3.
Retinoic acid induces differentiation of ES cells in cultures. Following withdrawal of LIF from medium or addition of retinoic acid (1 μM) for 4 days in culture, the differentiation of RW4 wildtype and Grb2 (−/−) ES cells was monitored by the loss of pluripotent markers Oct3/4 and Nanog, and the induction of endoderm lineage markers Dab2, GATA4, and GATA6, by both immunofluorescence microscopy and Western blots. A: Double immunostaining of Oct3/4 and Nanog was performed in RW4 wildtype and Grb2 (−/−) Es cells with or without treatment with retinoic acid. DAPI staining reveals the nuclei. B: The loss of Oct3/4 and Nanog expression following retinoic acid treatment was assayed by Western blots. C: Double immunostaining of Dab2 and GATA4, Dab2 and GATA6, or Dab2 and Nanog, was performed in RW4 wildtype and Grb2 (−/−) ES cells treated with retinoic acid. DAPI staining reveals the nuclei. D: The induction of extra-embryonic endoderm markers Dab2, laminin, GATA6, and GATA4, and trophectoderm marker cytokeratin 8, was assayed by Western blots. E: The activation of MAPK was assayed by Western using phospho-specific antibodies to phosphorylated ERK1/2.
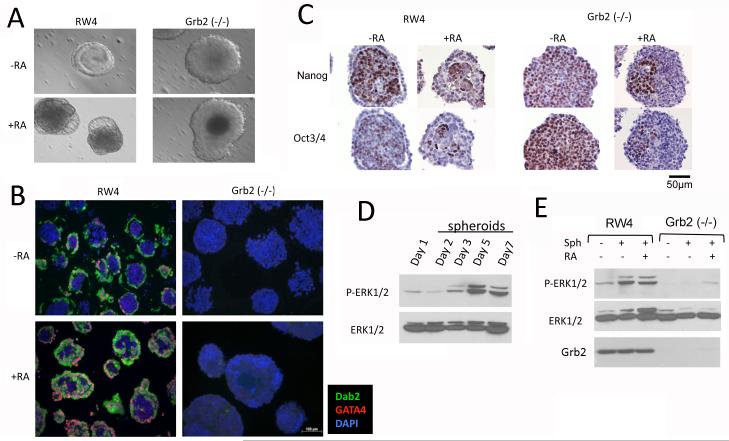
Unlike RW4 ES cells, Grb2 null ES cells did not undergo endoderm differentiation upon aggregation to form embryoid bodies either with or without retinoic acid (Fig. 4). Consistent with previous results (Capo-chichi et al., 2005), spontaneous differentiation of RW4 embryoid bodies to form an outer shell was readily observed after 5 days in suspension culture, while an outer shell was absent in all embryoid bodies formed from aggregation of Grb2-null ES cells (Fig. 4A). Immunofluorescence staining with primitive endoderm markers GATA4 and Dab2 confirmed that the aggregates of Grb2 null ES cells lack primitive endoderm cells or layers, compared to wildtype RW4 embryoid bodies (Fig. 4B). Addition of retinoic acid to the cell aggregates exaggerated the endoderm differentiation of RW4 embryoid bodies, but failed to stimulate endoderm differentiation in the aggregates of Grb2-null ES cells (Fig. 4B). While RW4 embryoid bodies consist of an endoderm shell enclosing the interior cells positive for both Oct3/4 and Nanog, the Grb2-null cells in the aggregates are uniformly positive for both Oct3/4 and Nanog (Fig. 4C). Retinoic acid treatment decreased the fraction of Oct3/4 and Nanog-positive cells in the aggregates from either RW4 or Grb2-null ES cells (Fig. 4C).
Fig. 4.
Grb2-null ES cells are unable to form primitive endoderm in embryoid bodies. RW4 wildtype and Grb2 (−/−) ES cells were allowed to aggregate in non-adherent culture flasks to form embryoid bodies both in the presence and absence of 1 μM retinoic acid. A: Representative day 5 embryoid bodies viewed in bright field under a microscope show an outer layer for wildtype embryoid bodies and absence of an outer layer in Grb2 (−/−) cell aggregates. B: Embryoid bodies were harvested on day 5 and processed for histology. Immunofluorescence microscopy shows the presence of an outer endoderm layer stained with endoderm markers Dab2 (green) and GATA4 (red) in RW4 but not in Grb2 (−/−) embryoid bodies. Nuclei were stained with DAPI. C: The embryoid bodies were stained with Nanog and Oct3/4. D: Western blot was used to determine the time course of MAPK activation during the formation of embryoid bodies. E: Embryoid bodies were harvested on day 5 of the cultures and were assayed for MAPK activation by Western blots.
We observed an increased MAPK activation in the course of aggregation of RW4 ES cells, with a peak on day 5 (Fig. 4D). However, MAPK activation was much weaker in the aggregations of Grb2-null ES cells (Fig. 4E).
Collectively, these results suggest that retinoic acid can induce Grb2-null ES cell differentiation by suppressing pluripotent markers Oct3/4 and Nanog; however, the cells differentiated into lineage(s) other than the primitive endoderm.
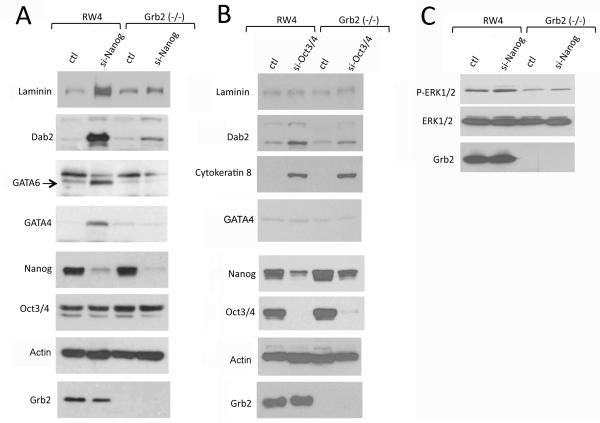
Suppression of Nanog Is Insufficient for Endoderm Differentiation of Grb2-Null ES Cells
It has been suggested that ES cells undergo endoderm differentiation following transcriptional suppression of Nanog through Grb2-mediated MAPK activation (Hamazaki et al., 2006). We used RNA interference to suppress Nanog expression in Grb2-null ES cells and investigated endoderm differentiation (Fig. 5). In RW4 ES cells, suppression of Nanog induced endoderm differentiation, indicated by the induction of markers such as laminin, Dab2, GATA4, and GATA6, consistent with previous reports (Hamazaki et al., 2006). However, similar suppression of Nanog in Grb2-null ES cells failed to induce endoderm differentiation (Fig. 5A). Suppression of Nanog did not appear to influence Oct3/4 level (Fig. 5A); however, suppression of Oct3/4 reduced Nanog in both wildtype and Grb2 null ES cells (Fig. 5B). Oct3/4 suppression did not significantly induce endoderm differentiation, but did cause trophectoderm differentiation, indicated by the induced expression of cytokeratin 8 in both RW4 and Grb2-null ES cells. We found that Nanog suppression did not significantly alter MAPK activation either in RW4 or Grb2 (−/−) ES cells (Fig. 5C). Thus, these data further confirm that Grb2 deficiency blocks the formation of primitive endoderm lineage, but does not interfere with differentiation into trophectoderm lineage.
Fig. 5.
Suppression of Oct3/4 or Nanog in Grb2-null ES cells does not induce endoderm differentiation. RW4 wildtype and Grb2 (−/−) ES cells were treated with siRNA to Nanog, Oct3/4, or scrambled controls for 3 days in LIF-containing ES cell culture medium. The cells were then collected and subjected to Western blot analysis to determine cell differentiation and lineages. A: The ES cells were treated with control or siRNA to Nanog. Western blot was performed for endoderm markers, laminin, Dab2, GATA6, GATA4; and pluripotent markers Nanog and Oct3/4. B: The cells were treated with control or siRNA to Oct3/4. Western blots were performed for the endoderm markers: laminin, Dab2, GATA6, GATA4; the trophectoderm marker cytokeratin 8; and the pluripotent markers Nanog and Oct3/4. C: MAPK activation was assayed in Nanog siRNA treated and control cells by Western blot using phospho-specific antibodies.
Ectopic Expression of GATA6 or GATA4 Is Sufficient to Restore Endoderm Differentiation in Grb2-Null ES Cells in Culture
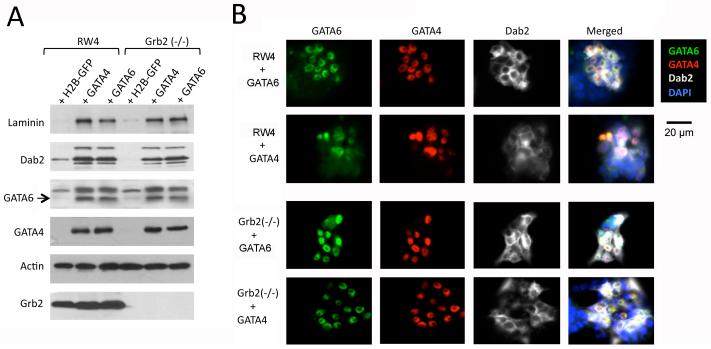
Both Grb2 and GATA6 are required for primitive endoderm differentiation and the mutant embryos exhibit similar phenotypes, suggesting that the two genes act in the same differentiation pathway. To analyze the relationship between GATA6 with Grb2, we next tested whether ectopic expression of GATA6 is sufficient to rescue the defective endoderm differentiation of Grb2-null ES cells. In RW4 wildtype ES cells, transfection and expression of GATA4 or GATA6 induced each other and endoderm differentiation (Fig. 6), consistent with previous reports (Fujikura et al., 2002; Capo-chichi et al., 2005). Ectopic expression of GATA4 or GATA6 in Grb2-null ES cells induced endoderm markers laminin, Dab2, GATA4 or GATA6 in Grb2-null ES cells to a similar degree as in RW4 ES cells (Fig. 6). Thus, forced expression of GATA4 or GATA6 is sufficient to override the requirement of Grb2 in endoderm differentiation.
Fig. 6.
Ectopic expression of GATA6 or GATA4 is sufficient to restore the endoderm differentiation in Grb2 (−/−) ES cells in monolayer cultures. RW4 or Grb2 (−/−) ES cells were transfected with expression vectors for GATA4, GATA6, H2B-GFP, or vector control. Following transfection for 3 days, the cells were harvested for analysis by Western blots and immunofluorescence microscopy. A: Western blot analysis was performed for laminin, Dab2, GATA6, and GATA4 in the transfected ES cells. B: Immunostaining for GATA6 (green), GATA4 (red), and Dab2 (white) was performed simultaneously in the transfected cells. DAPI (blue) was used to mark the nuclei.
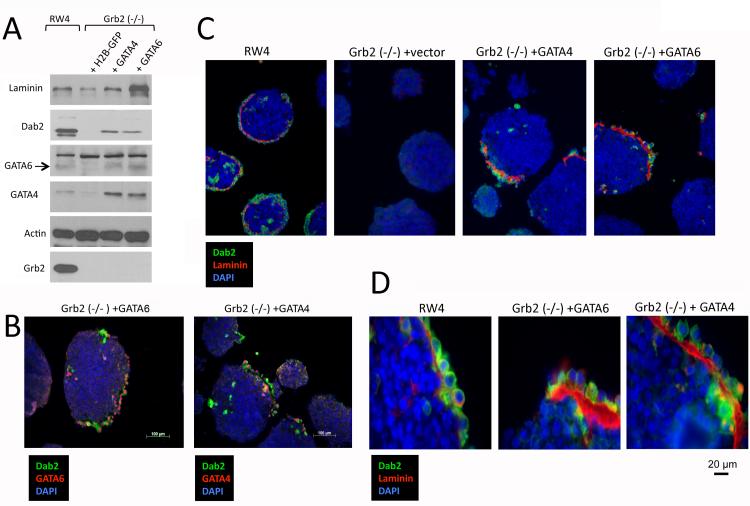
Ectopic GATA6 Expression Enables Grb2-Null ES Cells to Form Primitive Endoderm in Embryoid Bodies
The transfection efficiency in both RW4 and Grb2-null ES cells was estimated to be about 40% based on transfection controls using histone H2B expression plasmids, and a similar degree of GATA4 and GATA6 expression was observed in the mixture of differentiated and undifferentiated ES cells (Fig. 6). When we allowed the GATA4 or GATA6-transfected and non-transfected mixture of ES cells to aggregate and form embryoid bodies, the differentiated extraembryonic endoderm cells sorted to the surface to form endoderm layer, as established earlier (Rula et al., 2007; Moore et al., 2009). Similar to the aggregates from RW4, endoderm markers were assayed positive by Western blots in the aggregates from the mixture of transfected Grb2-null ES cells (Fig. 7A). Dab2-positive extraembryonic endoderm outer layers were observed by immunofluorescence microscopy in aggregates from either GATA4- or GATA6-transfected Grb2-null ES cells (Fig. 7B). No significant difference was observed between embryoid bodies formed from either GATA4- or GATA6-transfected Grb2-null ES cells. The transfected Grb2-null ES cells marked by Dab2 expression (green) formed a partial endoderm layer with a distinctive laminin (red) basement membrane (Fig. 7C), as shown in a higher magnification (Fig. 7D). Comparing to embryoid bodies from RW4 cells, embryoid bodies formed by GATA4 or GATA6-transfected Grb2-null ES cells had a much thicker basement membrane. The endoderm layer from the GATA4- or GATA6-transfected Grb2-null ES cells resembles the Reichert’s membrane forming parietal endoderm cells. Thus, expression of either GATA4 or GATA6 can bypass the requirement for Grb2 in the formation of extraembryonic endoderm. We conclude that Grb2 is upstream of GATA6 in the signaling cascade to induce extraembryonic endoderm differentiation in early mouse embryos.
Fig. 7.
Ectopic expression of GATA6 or GATA4 enables Grb2 (−/−) ES cells to form endoderm layer in embryoid bodies. Embryoid bodies were produced from aggregation of RW4 or Grb2 (−/−) ES cells transfected with expression vectors for GATA4, GATA6, and histone H2B-GFP (control). The 3-day embryoid bodies were processed for analysis by Western blot and immunofluorescence microscopy. A: Western blot was performed to compare markers of the cell aggregates. B: Representative embryoid bodies show the presence of partial endoderm layer from Grb2-null ES cells transfected with GATA4 or GATA6. The endoderm cells were stained with Dab2 (green), and GATA4 (red) in GATA4-transfected cells, and GATA6 (red) in GATA6-transfected cells. DAPI marks the nuclei. C: Representative embryoid bodies show the presence of partial endoderm layer from Grb2-null ES cells transfected with GATA4 or GATA6, compared to embryoid bodies from RW4 ES cells. The endoderm cells are stained with Dab2 (green), and laminin (red) for basement membrane. DAPI marks the nuclei. D: The stainings for Dab2 (green) and laminin (red) are shown in a higher magnification for the presence of partial endoderm layer from Grb2-null ES cells transfected with GATA4 or GATA6, comparing to embryoid bodies from RW4 ES cells.
DISCUSSION
Gene knockout studies showed that Grb2 (Cheng et al., 1998; Chazaud et al., 2006) and GATA6 (Cai et al., 2008) are essential for the differentiation and formation of the primitive endoderm in early embryos. Here, we found that implanted Grb2-null blastocysts contained no primitive endoderm cells and resembled the mutant GATA6-null embryos at E4.5 stage. We showed that ectopic expression of GATA6 is able to restore the ability of Grb2-null ES cells to undergo endoderm differentiation and form endoderm layer in embryoid bodies. Thus, we establish that GATA6 is downstream of Grb2 in a signaling pathway to induce primitive endoderm differentiation in the early embryos.
Previously, the general model is that Grb2-mediated Ras/MAPK activation suppresses Nanog transcription, and a reduction of nanog level will release its suppression of GATA4 and GATA6 expression, leading to primitive endoderm differentiation from the cells of the inner cell mass (Hamazaki et al., 2006). However, our experiments of suppressing Nanog in Grb2-null ES cells indicate that suppression of Nanog is insufficient to induce endoderm lineage, and thus Grb2 is involved in a pathway independent of Nanog to induce primitive endoderm differentiation. Likewise, suppression of Oct3/4 also leads to a reduction of Nanog, but the ES cells differentiated into trophectoderm instead of endoderm, and Grb2 is not required for trophectoderm differentiation of ES cells. Although Ras/MAPK pathway also promotes trophectoderm lineage differentiation (Lu et al., 2008), Grb2 is not essential for the formation of trophectoderm in early mouse embryos as well as in ES cell differentiation in to trophectoderm-like cells. Consistently, the appearance of trophectoderm was also observed in blastocysts cultured in the presence of inhibitors for Ras/MAPK pathway (Nichols et al. 2009).
The rescue experiments suggest that the critical role of Grb2 is to induce GATA4 or GATA6 in extraembryonic endoderm differentiation. The observed difference between endoderm from the wildtype and the transfected Grb2-null ES cells is the thickened basement membrane. Endoderm of the wildtype embryoid bodies has a thin basement membrane, which resembles that of the visceral endoderm; however the endoderm basement membrane of embryoid bodies formed by GATA4- or GATA6-transfected Grb2 null ES cells is much thicker, and resembles the multilayered Reichert’s membrane produced by parietal endoderm cells. It is known that GATA6 expression declines after primitive endoderm differentiation and further maturation into visceral endoderm cells, while GATA6 expression persists in parietal endoderm cells (Cai et al., 2008). This suggests that persistent expression of GATA6 either due to forced ectopic expression, or induced by expression of the transfected GATA4 may stimulate parietal endoderm lineage of the transfected Grb2-null ES cells.
In sum, in the current study we have established that Grb2-mediated pathway mainly stimulates GATA6 expression in the primitive endoderm differentiation of the early embryos. In addition, Grb2 is also required for proliferation and expansion of the inner cell mass. These data also suggests additional complexity in the cellular pathway in primitive endoderm differentiation.
EXPERIMENTAL PROCEDURES
Mutant Mice and Embryos
Two mating pairs of Grb2 (+/−) mice (129 Grb2<tm1Paw>) (Cheng et al., 1998) were given by the Toronto Centre for Phenogenomics (Toronto, Ontario) with agreement by Dr. Anthony Pawson (Samuel Lunenfeld Research Institute, Toronto, Canada) to establish a breeding colony at the University of Miami. The Grb2 (+/−) mice were used for timed matings to obtain embryos of the desired age. For the analysis of E4.5 and E5.5 embryos, the entire uterine horns were harvested for histology. The blocks were sectioned and one in every 5 adjacent slides were examined by H&E staining to identify the implanted embryos.
Since the early embryonic lethality of Grb2-null pre- and post-implanted blastocysts has been well established (Cheng et al., 1998; Chazaud et al., 2006), we used the abnormal morphology and the lack of staining for the primitive endoderm markers Dab2 and GATA4 to distinguish Grb2-null embryos. Littermates containing an endoderm layer that are positive for Dab2 and GATA4 staining were identified as wildtype or Grb2 (+/−). GATA6-null embryos were used for comparison (Cai et al., 2008).
Culture and Maintenance of Mouse ES Cells and Embryoid Bodies
RW4 (Capo-chichi et al., 2005) and Grb2 (−/−) (Cheng et al., 1998) mouse embryonic stem (ES) cells were used in experiments. A frozen vial of the original Grb2 (−/−) ES cells was given by Dr. Pawson (Samuel Lunenfeld Research Institute, Toronto, Canada). These mouse ES cells were maintained on a layer of irradiated mouse embryonic fibroblasts in ES medium consisting of DMEM with 15% FBS, 1 mM glutamine, 1% non-essential amino acids, 0.1 mM β-mercaptoethanol, and 1,000 U/ml leukemia inhibitory factor (LIF). Three days prior to the experiment, the ES cells were trypsinized and seeded in ES culture medium containing LIF on plates coated with 0.1% of gelatin. All-trans-retinoic acid (RA) dissolved in DMSO was added 24 hours after plating at a final concentration of 1 μM to induce differentiation. An equal volume of DMSO vehicle was added to the control cells.
To form embryoid bodies, dispersed ES cells were allowed to aggregate in ES culture medium without LIF in non-adhesive poly-hema (Sigma) coated petri dishes. In some cases, RA was added 24 hours after the initiation of ES cell aggregation. After 4-7 days in suspension culture, the ES cell aggregates/spheroids were collected by brief centrifugation and then were used for analysis.
Cell Transfection
Wild type mouse ES cells RW-4 and Grb2 (−/−) cells were seeded at 2.5×105 on gelatin coated 6-well plates. Plasmid DNA was purified using Qiagen Maxiprep columns. GATA4 and GATA6 expression constructs were described previously (Capo-chichi et al., 2005). Lipofectamine LTX (Invitrogen) reagent was used for transfection according to the manufacturer’s protocols. Empty vector or H2BGFP expression plasmid was used as control and as an indicator of transfection efficiency. ES cells were collected for analysis by Western blot or immunofluorescence microscopy 72 hours after transfection.
Small Interfering RNA (siRNA)
Pre-designed Mission siRNA against mouse Nanog (SASI_Mm02_00333133) and Oct3/4 (SASI_Mm01_00091228) were purchased from Sigma. The siRNA oligonucleotides were transfected into cells using Lipofectamine RNAiMAX (Invitrogen) in serum-reduced Opti-MEM medium in accordance to the manufacturer’s recommendation. Transfection efficiency was determined by the cellular uptake of fluorescein-labeled dsRNA oligomer from Invitrogen. The typically efficiency was 90%. The cells were maintained in full ES cell culture media and analyzed 2-3 days following siRNA transfection.
Western Blot Analysis and Antibodies
Following treatment, ES cells were washed twice with cold PBS and collected in RIPA buffer (20 mM Tris pH 7.5, 50 mM NaCl, 0.1% SDS, 0.5% Sodium deoxycholate). The samples were then lyzed in SDS gel loading buffer and boiled for 10 minutes. The proteins were separated on 8% SDS-polyacrylamide gels, and immunoblotting was performed according to standard procedures and the signals were detected using the GE-Amersham ECL Western blotting kits.
Monoclonal anti-Dab2 (Catalog# 610465), Grb2 (Catalog# 610111), and ERK1/2 (Catalog# 610031) antibodies were purchased from BD Sciences. Polyclonal pan laminin (Catalog# L9393) and monoclonal β-actin (Catalog# A2228) antibodies were from Sigma. Polyclonal anti-phospho-Erk (Catalog# 4370) antibodies were purchased from Cell Signaling Technology. Polyclonal anti-Nanog (Catalog# sc-1000) antibodies were obtained from EMD Bioscience. Affinity purified rabbit polyclonal Anti-Activated caspase-3 (Catalog# 9661) antibodies were from Cell Signal, Inc. The antibodies recognize only the cleaved 17/19 KDa activated capspase-3 enzyme but not the full-length or other cleaved fragments. Anti-ki67 (Catalog# TEC-3) antibodies were from Dako. Monoclonal anti-GATA4 (Catalog# sc-9053) and Oct3/4 (Catalog# 6sc-5279) antibodies were from Santa Cruz Biotechnology. Anti-GATA6 rabbit polyclonal antibodies were produced and characterized as detailed previously (Cai et al., 2008). These antibodies were used for Western blot, immunofluorescence microscopy, and immunohistochemistry.
Immunofluorescence Microscopy
Wildtype RW4 and Grb2 (−/−) mouse ES cells were first seeded onto gelatin-coated cover slips at 0.5×105 cells per well in 12-well plates. These cells were differentiated with 1 μM RA for 3 days or transfected with expression plasmids. For immunofuorescence microscopy, the cells were fixed with 4% paraformaldehyde at room temperature for 10 minutes, permeabilized with 0.5% Triton X-100 for 5 minutes, blocked with 5% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. The primary antibodies were diluted into 3% BSA in PBS and incubated with the slides for 1 hour. The dilutions were: anti-GATA6 and anti-Nanog (Calbiochem) at 1:1,000; anti-Dab2 at 1:2,000; anti-GATA4 and anti-Oct3/4 at 1:400. The cover slips then were washed three times with PBS and incubated with either Alexa Fluor 488- or 596-conjugated secondary antibodies (Molecular Probes) in 3% BSA at room temperature for 1 hour. The unbound secondary antibodies were washed away with PBS for four times, and the nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 minutes. The cover slips were mounted in anti-fade media Prolong Gold (Invitrogen) according to the manufacturer’s directions. Immunofluorescence staining was viewed with 20X or 63X (oil) objective lens on a Zeiss Axio observer Z1 microscope linked to Zeiss AxioCam MR R3 camera. Images were acquired and processed using AxioVision software.
Histology and Immunohistochemistry
Following timed matings of Grb2 (+/−) parents, uteri containing embryos were harvested at E4.5 or E5.5. The entire uterine horns were fixed in 10% formalin, paraffin-embedded, sectioned into 6 μm slices, and adhered to positively charged slides. The sections were dewaxed in Xylene and hydrated through graded ethanol. Antigen retrieval was performed by boiling the sections submerged in antigen retrieval buffer (Dako) in a glass slide holder for 20 minutes in a kitchen steamer. The endogenous peroxidase activity was quenched by incubation with 3% H2O2 for 15 minutes. The slides were then blocked with 5% BSA at room temperature for 30 minutes, and subsequently incubated with antibodies: anti-GATA4 (1:2,000), anti-Dab2 (1:1,000), anti-Nanog (1:1,000), rat monoclonal cytokeratin 8/Troma-1 antibodies (Developmental Studies Hybridoma Bank, Ames, IA) (1:600), in 5% BSA at 4°C overnight. The slides were then washed and incubated with appropriate secondary antibodies conjugated with polymer horseradish peroxidase for 1 hour at room temperature. For Troma-1 staining, polyclonal rabbit anti-rat “bridging” antibodies (DAKO) at 1:500 dilution were used. Diaminobenzidine (DAB) was used as chromogen for the immunoperoxidase reaction. The slides were counterstained with hematoxylin, dehydrated in xylene and mounted in Permount.
ACKNOWLEDGMENTS
We thank our colleagues, Drs. Elizabeth Smith and Robert Moore, for comments, critical review, and editing of this manuscript. We appreciate the gift of the Grb2 null ES cells and mice from Dr. Tony Pawson (Samuel Lunenfeld Research Institute, Toronto, Canada). These studies were supported by R01 CA095071 and R01 CA79716 to X.X. Xu from NCI, NIH.
REFERENCES
- Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci U S A. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Wilson DB. Induction of yolk sac endoderm in GATA-4-deficient embryoid bodies by retinoic acid. Mech Dev. 1997;65:43–54. doi: 10.1016/s0925-4773(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Cai KQ, Capo-Chichi CD, Rula ME, Yang DH, Xu XX. Dynamic GATA6 expression in primitive endoderm formation and maturation in early mouse embryogenesis. Dev Dyn. 2008;237:2820–2829. doi: 10.1002/dvdy.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-Chichi CD, Rula ME, Smedberg JL, Vanderveer L, Parmacek MS, Morrisey EE, Godwin AK, Xu XX. Perception of differentiation cues by GATA factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev Biol. 2005;286:574–586. doi: 10.1016/j.ydbio.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Cavaleri F, Schöler HR. Nanog: a new recruit to the embryonic stem cell orchestra. Cell. 2003;113:551–552. doi: 10.1016/s0092-8674(03)00394-5. [DOI] [PubMed] [Google Scholar]
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL. Investigation of cell lineage and differentiation in the extraembryonic endoderm of the mouse embryo. J Embryol Exp Morphol. 1982;68:175–198. [PubMed] [Google Scholar]
- Gardner RL. Origin and differentiation of extraembryonic tissues in the mouse. Int Rev Exp Pathol. 1983;24:63–133. [PubMed] [Google Scholar]
- Gardner RL. Cell allocation and lineage in the early mouse embryo. Ciba Found Symp. 1989;144:172–181. [PubMed] [Google Scholar]
- Gerbe F, Cox B, Rossant J, Chazaud C. Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Dev Biol. 2008;313:594–602. doi: 10.1016/j.ydbio.2007.10.048. [DOI] [PubMed] [Google Scholar]
- Goldin SN, Papaioannou VE. Paracrine action of FGF4 during periimplantation development maintains trophectoderm and primitive endoderm. Genesis. 2003;36:40–47. doi: 10.1002/gene.10192. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Kehoe SM, Nakano T, Terada N. The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol Cell Biol. 2006;26:7539–7549. doi: 10.1128/MCB.00508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough SR, Clements I, Welch PJ, Wiederholt KA. Differentiation of mouse embryonic stem cells after RNA interference-mediated silencing of OCT4 and Nanog. Stem Cells. 2006;24:1467–1475. doi: 10.1634/stemcells.2005-0475. [DOI] [PubMed] [Google Scholar]
- Lu CW, Yabuuchi A, Chen L, Viswanathan S, Kim K, Daley GQ. Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet. 2008;40:921–926. doi: 10.1038/ng.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]; Development. 126:723–732. Corrected and republished in: [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Adams RJ, Morris SA, Danckaert A, Le Garrec JF, Zernicka-Goetz M. Active cell movements coupled to positional induction are involved in lineage segregation in the mouse blastocyst. Dev Biol. 2009;331:210–221. doi: 10.1016/j.ydbio.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Moore R, Cai KQ, Escudero DO, Xu XX. Cell adhesive affinity does not dictate primitive endoderm segregation and positioning during murine embryoid body formation. Genesis. 2009;47:579–589. doi: 10.1002/dvg.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, Rossant J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Pesce M, Schöler HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- Pfister S, Steiner KA, Tam PP. Gene expression pattern and progression of embryogenesis in the immediate post -implantation period of mouse development. Gene Expr Patterns. 2007;7:558–573. doi: 10.1016/j.modgep.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol. 2004;15:573–581. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rossant J, Chazaud C, Yamanaka Y. Lineage allocation and asymmetries in the early mouse embryo. Philos Trans R Soc Lond B Biol Sci. 2003;358:1341–8134. doi: 10.1098/rstb.2003.1329. discussion 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Tam PP. Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell. 2004;7:155–164. doi: 10.1016/j.devcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Rula ME, Cai KQ, Moore R, Yang DH, Staub CM, Capo-chichi CD, Howe PH, Smith ER, Xu XX. Cell autonomous sorting and surface positioning in the formation of primitive endoderm in embryoid bodies. Genesis. 2007;45:327–338. doi: 10.1002/dvg.20298. [DOI] [PubMed] [Google Scholar]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- Verheijen MH, Wolthuis RM, Bos JL, Defize LH. The Ras/Erk pathway induces primitive endoderm but prevents parietal endoderm differentiation of F9 embryonal carcinoma cells. J Biol Chem. 1999;274:1487–1494. doi: 10.1074/jbc.274.3.1487. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD, Xu XX. Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol. 2002;251:27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
- Yang DH, Cai KQ, Roland IH, Smith ER, Xu XX. Disabled-2 is an epithelial surface positioning gene. J Biol Chem. 2007;282:13114–13122. doi: 10.1074/jbc.M611356200. [DOI] [PubMed] [Google Scholar]