Abstract
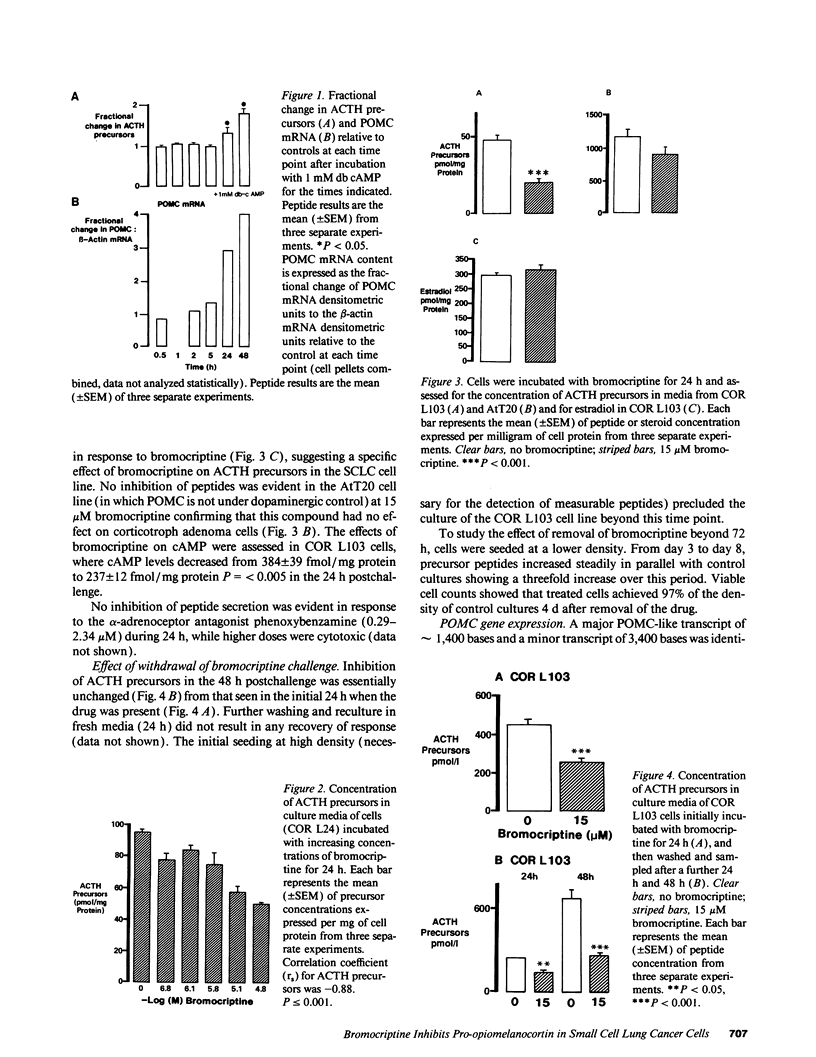
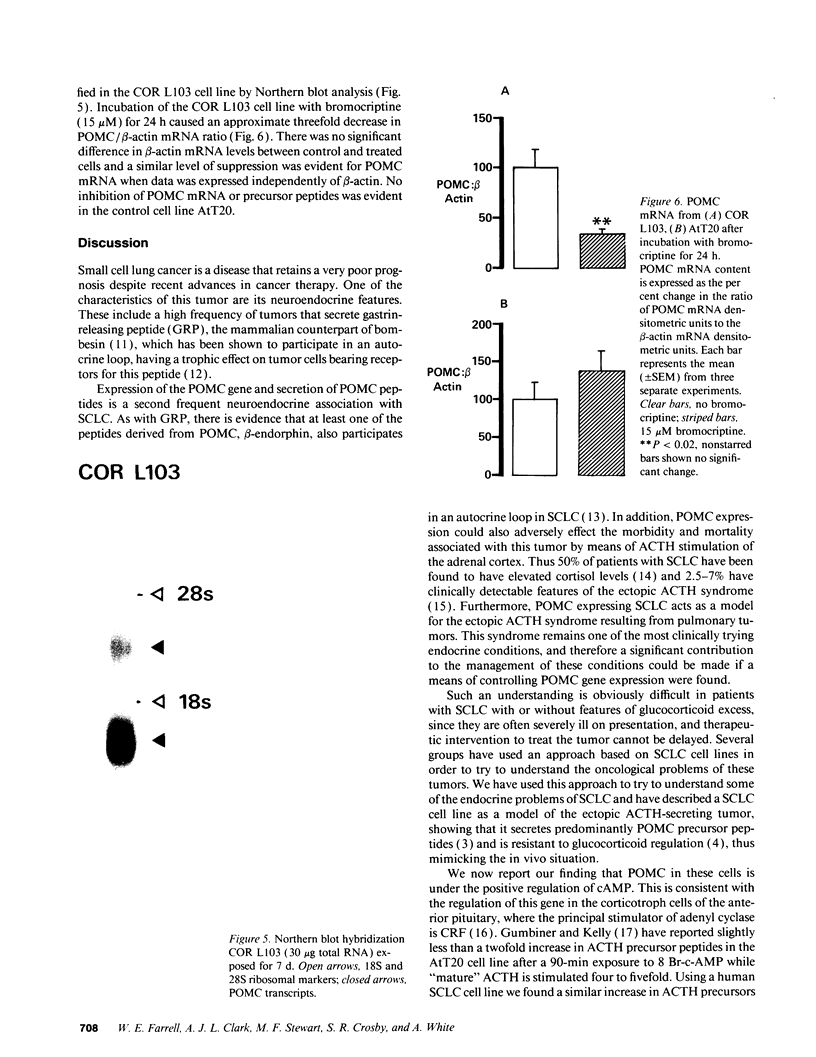
We have previously reported that a human small cell lung cancer (SCLC) cell line (COR L103) that expresses the proopiomelanocortin (POMC) gene and secretes ACTH precursor peptides is relatively resistant to glucocorticoid regulation. Using this model, we have now examined alternative regulatory mechanisms of the POMC gene and found that both the mRNA and ACTH precursor peptides were stimulated four- and two-fold, respectively, after 48 h incubation with db-cAMP. Next, we examined the dopamine agonist, bromocriptine, which acts predominantly through D2 receptors linked to adenyl cyclase to cause a reduction in intracellular cAMP. Bromocriptine suppressed cAMP levels and inhibited precursor peptide secretion within 24 h in a dose-dependent manner (0.15-15 microM). At the highest dose, peptide secretion was inhibited from 95 to 53 pmol/mg protein, and POMC mRNA was reduced by 50%, while beta-actin mRNA remained unchanged. This effect could not be mimicked by incubation of cells with the alpha-adrenergic antagonist, phenoxybenzamine, suggesting that the alpha-adrenergic effects of bromocriptine were not responsible for this observation. These cells also secrete estradiol, but the secretory rate was unaffected by bromocriptine, suggesting, with the beta-actin data, that the POMC inhibition was not a cytotoxic effect. No recovery in precursor peptide secretion was seen in a 48-h period after the removal of bromocriptine. However, when the postchallenge incubation was extended to 8 d, there was a recovery in secretory potential between day 3 and day 8 and normal growth kinetics in the 4 d after removal of the drug. In contrast to these findings, the mouse corticotroph cell line, AtT20, showed no response to bromocriptine, in keeping with reports that this agonist has no effect on anterior lobe corticotrophs. We conclude that bromocriptine effectively inhibits POMC expression in SCLC cells, and that this phenomenon might be of useful clinical application.
Full text
PDF



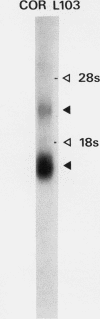
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Wynn P. C., Harwood J. P., Hauger R. L., Millan M. A., Grewe C., Catt K. J. Receptor-mediated actions of corticotropin-releasing factor in pituitary gland and nervous system. Neuroendocrinology. 1986;43(1):79–88. doi: 10.1159/000124513. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Stewart M. F., Lavender P. M., Farrell W., Crosby S. R., Rees L. H., White A. Defective glucocorticoid regulation of proopiomelanocortin gene expression and peptide secretion in a small cell lung cancer cell line. J Clin Endocrinol Metab. 1990 Feb;70(2):485–490. doi: 10.1210/jcem-70-2-485. [DOI] [PubMed] [Google Scholar]
- Cochet M., Chang A. C., Cohen S. N. Characterization of the structural gene and putative 5'-regulatory sequences for human proopiomelanocortin. Nature. 1982 May 27;297(5864):335–339. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- Crosby S. R., Stewart M. F., Ratcliffe J. G., White A. Direct measurement of the precursors of adrenocorticotropin in human plasma by two-site immunoradiometric assay. J Clin Endocrinol Metab. 1988 Dec;67(6):1272–1277. doi: 10.1210/jcem-67-6-1272. [DOI] [PubMed] [Google Scholar]
- Croughs R. J., Koppeschaar H. P., van't Verlaat J. W., McNicol A. M. Bromocriptine-responsive Cushing's disease associated with anterior pituitary corticotroph hyperplasia or normal pituitary gland. J Clin Endocrinol Metab. 1989 Feb;68(2):495–498. doi: 10.1210/jcem-68-2-495. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Davis T. P., Burgess H. S., Crowell S., Moody T. W., Culling-Berglund A., Liu R. H. Beta-endorphin and neurotensin stimulate in vitro clonal growth of human SCLC cells. Eur J Pharmacol. 1989 Feb 28;161(2-3):283–285. doi: 10.1016/0014-2999(89)90862-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gibson A., Samini M. Bromocriptine is a potent alpha-adrenoceptor antagonist in the perfused mesenteric blood vessels of the rat. J Pharm Pharmacol. 1978 May;30(5):314–315. doi: 10.1111/j.2042-7158.1978.tb13238.x. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Hale A. C., Besser G. M., Rees L. H. Characterization of pro-opiomelanocortin-derived peptides in pituitary and ectopic adrenocorticotrophin-secreting tumours. J Endocrinol. 1986 Jan;108(1):49–56. doi: 10.1677/joe.0.1080049. [DOI] [PubMed] [Google Scholar]
- Kohler P. C., Trump D. L. Ectopic hormone syndromes. Cancer Invest. 1986;4(6):543–554. doi: 10.3109/07357908609039834. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Klijn J. G., de Quijada M., Timmermans H. A., Uitterlinden P., de Jong F. H., Birkenhäger J. C. The mechanism of the suppressive action of bromocriptine on adrenocorticotropin secretion in patients with Cushing's disease and Nelson's syndrome. J Clin Endocrinol Metab. 1980 Aug;51(2):307–311. doi: 10.1210/jcem-51-2-307. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., de Lange S. A., Stefanko S. Z. Adrenocorticotropin-secreting pituitary adenomas originate from the anterior or the intermediate lobe in Cushing's disease: differences in the regulation of hormone secretion. J Clin Endocrinol Metab. 1982 Feb;54(2):286–291. doi: 10.1210/jcem-54-2-286. [DOI] [PubMed] [Google Scholar]
- Liddle G. W., Nicholson W. E., Island D. P., Orth D. N., Abe K., Lowder S. C. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283–314. doi: 10.1016/b978-0-12-571125-8.50009-0. [DOI] [PubMed] [Google Scholar]
- McNicol A. M., Teasdale G. M., Beastall G. H. A study of corticotroph adenomas in Cushing's disease: no evidence of intermediate lobe origin. Clin Endocrinol (Oxf) 1986 Jun;24(6):715–722. doi: 10.1111/j.1365-2265.1986.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E., Mitchell W. M., Island D. P., Shapiro M., Byyny R. L. ACTH and MSH production by a single cloned mouse pituitary tumor cell line. Endocrinology. 1973 Feb;92(2):385–393. doi: 10.1210/endo-92-2-385. [DOI] [PubMed] [Google Scholar]
- Pardy K., Carter D., Murphy D. Dopaminergic mediation of physiological changes in proopiomelanocortin messenger ribonucleic acid expression in the neurointermediate lobe of the rat pituitary. Endocrinology. 1990 Jun;126(6):2960–2964. doi: 10.1210/endo-126-6-2960. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Schumacher U. K. Plasma bombesin concentrations in patients with extensive small cell carcinoma of the lung. Lancet. 1982 Feb 27;1(8270):509–509. doi: 10.1016/s0140-6736(82)91478-7. [DOI] [PubMed] [Google Scholar]
- Sorenson G. D., Pettengill O. S., Brinck-Johnsen T., Cate C. C., Maurer L. H. Hormone production by cultures of small-cell carcinoma of the lung. Cancer. 1981 Mar 15;47(6):1289–1296. doi: 10.1002/1097-0142(19810315)47:6<1289::aid-cncr2820470610>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A., Stewart M. F., Farrell W. E., Crosby S. R., Lavender P. M., Twentyman P. R., Rees L. H., Clark A. J. Pro-opiomelanocortin gene expression and peptide secretion in human small-cell lung cancer cell lines. J Mol Endocrinol. 1989 Jul;3(1):65–70. doi: 10.1677/jme.0.0030065. [DOI] [PubMed] [Google Scholar]