Abstract
There are many reports on the frequency of non–sentinel lymph node involvement when isolated tumor cells are found in the sentinel node, but results and recommendations for the use of an axillary lymph node dissection differ among studies. This systematic review was conducted to give an overview of this issue and to provide recommendations for the use of an axillary lymph node dissection in these patients. We searched Medline, Embase, and Cochrane databases from January 1, 2002, through November 27, 2007, for articles on patients with invasive breast cancer who had isolated tumor cells in the sentinel lymph node (according to the sixth edition of the Cancer Staging Manual of the American Joint Committee on Cancer) and who also underwent axillary lymph node dissection. Of 411 selected articles, 29 (including 836 patients) were included in this review. These 29 studies were heterogeneous, reporting a wide range of non–sentinel lymph node involvement (defined as the presence of isolated tumor cells or micro- or macrometastases) associated with isolated tumor cells in the sentinel lymph node, with an overall pooled risk for such involvement of 12.3% (95% confidence interval = 9.5% to 15.7%). This pooled risk estimate was marginally higher than the risk of a false-negative sentinel lymph node biopsy examination (ie, 7%–8%) but marginally lower than the risk of non–sentinel lymph node metastases in patients with micrometastases (ie, approximately 20%) who are currently eligible for an axillary lymph node dissection. Because 36 (64%) of the 56 patients with isolated tumor cells in their sentinel lymph node also had non–sentinel lymph node macrometastases, those patients with isolated tumor cells in the sentinel lymph node without other indications for adjuvant systemic therapy might be candidates for axillary lymph node dissection.
Axillary lymph node status is one of the most important prognostic factors for breast cancer patients (1). A sentinel lymph node biopsy examination is a safe and accurate initial staging method for lymph nodes of patients with early-stage breast cancer and has largely replaced axillary lymph node dissection as the preferred staging method (2). Selective targeting of the sentinel lymph node with enhanced pathological analysis (ie, step sectioning and immunohistochemistry) has led to an increased rate of identifying positive lymph nodes (3,4). However, the clinical significance of small groups of metastatic cells in lymph nodes is unclear.
The sixth edition of the Cancer Staging Manual of the American Joint Committee on Cancer (AJCC), which was first published in 2002, distinguishes between isolated tumor cells (clusters with diameters of ≤0.2 mm) and micrometastases (tumor cell clusters of >0.2 but ≤2 mm) on the basis of cluster size, reflecting the increased use of the sentinel lymph node biopsy method for lymph node staging. According to the first publication (5), the category no regional lymph node metastases (pN0) was given an additional description “i” [pN0(i+)] for metastatic cells visible by immunohistochemistry only. That is, pN0(i+) indicated that lymph node metastases were not detected on hematoxylin-eosin–stained lymph node sections but were detected by immunohistochemistry and that each tumor cluster had a diameter of no more than 0.2 mm. These definitions were clarified in the 2003 revision (6), so that the i description referred to the presence (i+) or absence (i−) of isolated tumor cells (clusters with diameters of ≤0.2 mm) regardless of detection method. These patients have been assigned to the N0 group for staging and treatment purposes because “the unknown benefits of providing treatment for these small lesions would not overweigh the morbidity caused by the treatment itself” (7).
Although there is agreement that patients whose sentinel lymph node contain micro- and/or macrometastases (ie, those in the N1 category) should be subjected to axillary lymph node dissection, controversy exists as to whether there is a benefit associated with axillary lymph node dissection for patients whose sentinel lymph node contains isolated tumor cells. Several studies (8–10) report an association between the size of metastases in the sentinel lymph node and non–sentinel lymph node involvement. Before the introduction of the current definition of isolated tumor cells, such groups of metastatic cells were included in the micrometastases category (pN1a) and thus usually integrated into the group of metastases detected by immunohistochemistry only. A meta-analysis by Cserni et al. (11) reported an overall risk of non–sentinel lymph node involvement of 9%, when sentinel lymph node metastases were detectable by immunohistochemistry only. Since the introduction of the current definition of isolated tumor cells, a growing number of studies (12–14) have assessed the detection rate of non–sentinel lymph node metastases in patients who have isolated tumor cells in their sentinel lymph node, but the results and recommendations from these studies for the use of axillary lymph node dissection have varied. This systematic review was conducted to give an overview of these results and provide recommendations regarding the role of an axillary lymph node dissection in patients with isolated tumor cells in their sentinel lymph node.
Patients and Methods
Literature Search Strategy
The electronic databases of Medline, Embase, and Cochrane were searched from January 1, 2002, through November 27, 2007, by use of variations of free text and controlled terms for breast cancer and sentinel lymph node and non–sentinel lymph node involvement. The year 2002 was selected because the sixth edition of the AJCC Cancer Staging Manual was published this year. Articles published in English, German, French, or Dutch were considered. Two reviewers (C. H. M. van Deurzen and M. de Boer) independently evaluated titles and abstracts of the identified papers. The full text of potentially relevant articles was then reviewed.
Study Inclusion Criteria
To be included in this review, studies had to meet three inclusion criteria. First, patients had to have invasive breast cancer. Second, patients must have had a sentinel lymph node biopsy procedure followed by an axillary lymph node dissection. Third, isolated tumor cells had to be classified according to the sixth edition of the AJCC Cancer Staging Manual (tumor cell clusters with a diameter of ≤0.2 mm). Studies were excluded that reported on a sentinel lymph node biopsy procedure after neoadjuvant chemotherapy, microinvasive breast cancer, or sentinel lymph node tumor deposits without size definition according to the sixth edition of the AJCC Cancer Staging Manual.
Data Extraction and Statistical Analysis
Data extraction was carried out independently by two reviewers (C. H. M. van Deurzen and M. de Boer). Any disagreement was resolved by consensus. Data were extracted by use of standardized data extraction forms. We documented sample size, inclusion and exclusion criteria, primary tumor characteristics, definition of isolated tumor cells, sentinel lymph node biopsy protocol (blue dye and/or colloid and the injection site), processing methods for sentinel lymph nodes and non–sentinel lymph nodes, level of axillary lymph node dissection, and number of patients with isolated tumor cells in their sentinel lymph node and with non–sentinel lymph node metastases. The AJCC classification of non–sentinel lymph node metastases, including isolated tumor cells (cell clusters with diameters of ≤0.2 mm), micrometastases (tumor cell clusters >0.2 but ≤ 2 mm), and macrometastases (tumor cell clusters >2 mm), was also recorded. In some instances, corresponding authors were contacted for additional information.
We calculated 95% confidence intervals (CIs) for the risks of non–sentinel lymph node involvement within the studies by use of the Rothman spreadsheet (15). A random-effects meta-analysis with an exact likelihood approach was used to calculate pooled risk estimates of non–sentinel lymph node involvement and 95% confidence intervals (16). The extent to which one or more study characteristics explained between-study heterogeneity was explored by use of metaregression analysis (17). The following variables were considered: the number of levels of the sentinel lymph node or non–sentinel lymph nodes that were stained with hematoxylin–eosin and/or by immunohistochemistry. Systematic differences between small and large studies were assessed by use of a funnel plot (18). Statistical analyses were performed with SAS version 9.1. All statistical tests were two-sided.
Results
The initial electronic search identified 411 potentially relevant articles of which we screened the title and abstract. After that screening, the full texts of 144 articles were obtained. After full text review and exclusion of overlapping series, 29 articles that met the inclusion and exclusion criteria for this study were identified and used for data extraction. The main characteristics and results of these studies are summarized in Table 1. These studies were heterogeneous regarding study design, size, inclusion criteria for sentinel lymph node biopsy examination, sentinel lymph node biopsy technique, and histopathological work-up. Overall, these 29 studies included 836 patients, of whom 108 had non–sentinel lymph node involvement.
Table 1.
Overview of breast cancer studies reporting on the detection rate of non–sentinel lymph node involvement after axillary lymph node dissection in patients with isolated tumor cells in their sentinel lymph node*
| Author (reference) | No. of patients | Inclusion criteria for SLN procedure | No. of levels assessed† |
Positive non-SLNs‡ |
No. of positive non-SLNs§ |
||||||
| SLN |
Non-SLN |
||||||||||
| H&E | IHC | H&E | IHC | Total No. | % (95% CI) | ITC | Micro | Macro | |||
| Delaloye et al. (19) | 2 | cT <3 cm | 10–12 | NR | M | M | 0 | 0 (0 to 66) | NA | NA | NA |
| Barranger et al. (20) | 1 | cN0 | 4 | NR | M | 0 | 0 | 0 (0 to 79) | NA | NA | NA |
| McCready et al. (21) | 17 | cN0 | 3 | 2 | 1 | 0 | 2 | 12 (3 to 34) | 0 | 0 | 2 |
| Dabbs et al. (22) | 63 | NA | 3 | NR | 1 | M | 7 | 11 (5 to 21) | 0 | 0 | 7 |
| Menes et al. (13) | 31 | cN0 | 5 | NR | 1 | M | 6 | 19 (9 to 36) | M | M | M |
| Zgajnar et al. (23) | 5 | cN0, cT <3 cm | Ser Sec | Ser Sec | 1 | M | 0 | 0 (0 to 43) | NA | NA | NA |
| Changsri et al. (24) | 22 | NA | 3 | NR | 1 | M | 6 | 27 (13 to 48) | 6 | 0 | 0 |
| Leidenius et al. (25) | 19 | NA | 4 | NR | 2 | 2 | 5 | 26 (12 to 49) | M | M | M |
| Viale et al. (26) | 116 | cN0, cT ≤3 cm | ≥15 | NR | 3–6 | M | 17 | 15 (9 to 22) | 1 | 3 | 13 |
| Schrenk et al. (27) | 44 | cN0 | ≥4 | NR | M | 0 | 4 | 9 (4 to 21) | 0 | 1 | 3 |
| de Widt-Levert et al. (28) | 13 | cN0, cT1 | ≥3 | ≥3 | 2 | M | 1 | 8 (1 to 33) | M | M | M |
| Klevesath et al. (29) | 4 | cN0, cT ≤4 cm | 3 | NR | 1 | 0 | 0 | 0 (0 to 49) | NA | NA | NA |
| Calhoun et al. (30) | 61 | NA | M | NR | 1 | M | 3 | 5 (2 to 13) | 0 | 2 | 1 |
| Krauth et al. (31) | 19 | cN0, cT1-2 | Ser Sec | NR | 1 | NR | 5 | 26 (12 to 49) | M | M | M |
| Choi et al. (32) | 2 | cN0 | 21 | 2 | M | M | 0 | 0 (0 to 66) | NA | NA | NA |
| Houvenaeghel et al. (12) | 187 | cT0-2 | 2–6‖ | NR | M | M | 30 | 16 (11 to 22) | M | M | M |
| van Rijk et al. (33) | 54 | NA | 3 | NR | 1 | 0 | 4 | 7 (3 to 18) | 2 | 0 | 2 |
| Amin and Hoda (34) | 21 | NA | 3 | 1 | M | M | 4 | 19 (8 to 40) | M | M | M |
| Falconieri et al. (35) | 19 | pT1-2 | >15 | NR | M | M | 0 | 0 (0 to 17) | NA | NA | NA |
| Ryden et al. (36) | 6 | cN0, cT <3 cm | 5 | 3 | 1 | M | 0 | 0 (0 to 39) | NA | NA | NA |
| Bolster et al. (37) | 48 | cN0, cT1-2 | ≥3 | NR | 1–2 | M | 7 | 15 (7 to 27) | 0 | 2 | 5 |
| Groen et al. (38) | 18 | cN0, cT1-2 | 3 | 3 | 1 | M | 3 | 17 (6 to 39) | 0 | 1 | 2 |
| Langer et al. (39) | 11 | cN0, pT ≤3 cm | >3 | NR | M | M | 1 | 9 (2 to 38) | M | M | M |
| Fait and Chrenko (40) | 4 | cN0, cT <4 cm | M | M | M | M | 0 | 0 (0 to 49) | NA | NA | NA |
| Herbert et al. (41) | 4 | NA | 5 | NR | M | M | 0 | 0 (0 to 49) | NA | NA | NA |
| Cserni et al. (42–44)¶ | 22 | ILC (43), pT ≤ 1.5 cm (44) | Ser Sec | NR | M | M | 0 | 0 (0 to 15) | NA | NA | NA |
| van Deurzen et al. (45) | 23 | cN0 | ≥5 | 5 | 1–2 | 0 | 3 | 13 (5 to 32) | 0 | 2 | 1 |
| Total | 836 | 108 | 12 (10 to 16) | 9 | 11 | 36 | |||||
SLN = sentinel lymph node; H&E = hematoxylin–eosin; IHC = immunohistochemistry; ITC = isolated tumor cells; NR = not routinely; M = missing data; NA = not applicable; Ser Sec = serial section; Micro = tumor cell cluster of >0.2 but ≤2 mm; Macro = tumor cell cluster with diameter of >2 mm; cT = clinical tumor stage; cN0 = clinically no regional lymph node metastasis; cT1 = clinical tumor diameter ≤2 cm; cT1-2 = clinical tumor diameter ≤5 cm; cT0-2 = clinically no evidence of primary tumor through a clinical tumor diameter ≤5 cm; pT1-2 = pathological tumor diameter ≤ 5 cm; pT = pathological tumor diameter; cT = clinical tumor diameter; ILC = infiltrative lobular carcinoma; pT <1.5 cm = pathological tumor size ≤1.5 cm.
Number of lymph node sections assessed by pathological analysis.
Total number of patients with isolated tumor cells in the sentinel lymph node and non–sentinel lymph node metastases (including isolated tumor cells and mirco- and macrometastases).
American Joint Committee on Cancer classification of non–sentinel lymph node metastases.
Different protocols were used.
Three overlapping reports from the same author, results after excluding overlapping patients.
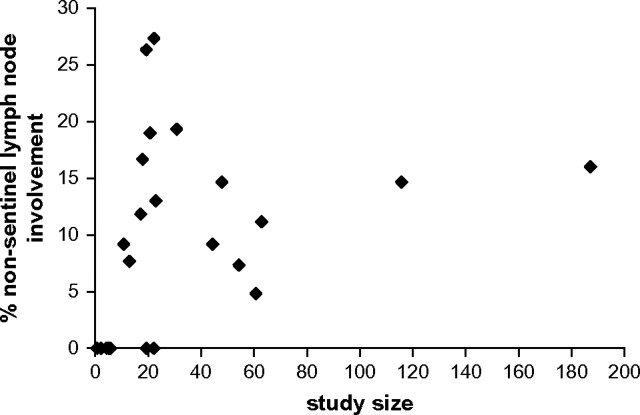
Sentinel lymph nodes were usually identified by use of blue dye in combination with radioactive colloid that was injected peritumorally. The detection rate of non–sentinel lymph node metastases ranged from 0% through 27%, with a pooled risk for non–sentinel lymph node metastases of 12.3% (95% CI = 9.5% to 15.7%). Data on the size of non–sentinel lymph node metastases and whether such metastases were considered in the treatment of the patient were scarce. In 10 of the 17 studies with non–sentinel lymph node involvement, information was available on the type of metastases that was classified according to the sixth edition of the AJCC Cancer Staging Manual. The pooled risk of non–sentinel lymph node macrometastases, after a positive sentinel lymph node was found, in these studies was 63.5% (95% CI = 38.1% to 83.1%). The funnel plot did not indicate systematic differences in the frequency of non–sentinel lymph node metastases between large studies and smaller studies (Figure 1). However, the range of non–sentinel lymph node metastases (5%–19%) in larger studies (ie, those with >30 patients) was smaller than the range (0%–27%) in smaller studies (ie, those with <30 patients).
Figure 1.
Funnel plot. The 29 studies on non–sentinel lymph node involvement associated with isolated breast cancer cells in the sentinel node were analyzed (see Table 1 for these studies). The study size (defined as the number of patients included with isolated tumor cells in the sentinel node and who also underwent axillary lymph node dissection) is plotted on the horizontal axis, and the percentage of these patients with non–sentinel lymph node metastases is plotted on the vertical axis. Each point represents a published study.
Metaregression analysis revealed that the number of assessed levels of the sentinel lymph node or non–sentinel lymph nodes stained with hematoxylin–eosin and/or immunohistochemistry (ie, defined as the number of lymph node sections assessed by pathological analysis) did not contribute substantially to between-study heterogeneity in non–sentinel lymph node involvement. Because the included articles did not further specify clinicopathologic features of patients with a sentinel lymph node containing isolated tumor cells, we could not formulate recommendations for the use of axillary lymph node dissection for patients with sentinel lymph node involvement.
Discussion
In this systematic literature review, we have summarized the available studies reporting on the risk of non–sentinel lymph node involvement after isolated tumor cells were found in the sentinel lymph node. The overall incidence of non–sentinel lymph node involvement ranged from 0% to 27%. The pooled overall risk of non–sentinel lymph node involvement was 12.3% (95% CI = 9.5% to 15.7%), and 63.5% (95% CI = 38.1% to 83.1%) of non–sentinel lymph node metastases were macrometastases.
This study had several limitations. First, the studies included in the analysis did not specify the clinicopathologic details of those patients with isolated tumor cells and non–sentinel lymph node involvement. Therefore, with the currently available data, we cannot generate recommendations for the use of an axillary lymph node dissection in subgroups of patients with isolated tumor cells in the sentinel lymph node. Second, many of the included studies in this analysis did not further classify non–sentinel lymph node metastases (ie, isolated tumor cells or micro- or macrometastases). Therefore, the risk calculation for macrometastases in the non–sentinel lymph node was based on a relatively small number of studies.
The large range of non–sentinel lymph node involvement may stem partially from differences in study design, inclusion criteria for a sentinel lymph node biopsy procedure in this study, sentinel lymph node biopsy technique, and/or the processing and interpretation of metastatic deposits. First, included studies may be limited, in part, by their retrospective design because not all patients with a sentinel lymph node containing isolated tumor cells may have undergone an axillary lymph node dissection. The subgroup of patients with a sentinel lymph node containing isolated tumor cells who underwent an axillary lymph node dissection may have been selected because they appeared to have more aggressive primary disease. Second, exclusion criteria for sentinel lymph node biopsy procedure differed by the largest included clinical tumor diameter (ie, 3 or 5 cm). Third, the sentinel lymph node biopsy protocol differed by the type of substance injected (colloid and/or blue dye) and injection site (subdermal, peritumoral, or periareolar), although the influence of these variables was probably minimal. Fourth, the included studies used different protocols for sentinel lymph node and non–sentinel lymph node analyses, with a wide range in the number of slides, depth of section intervals, and use of immunohistochemistry. The detection rate of isolated tumor cells in the sentinel lymph node decreased with less extensive pathological work-up (ie, analysis of a low number of lymph node sections or a lack of routine use of immunohistochemistry) and some sentinel lymph nodes classified as having isolated tumor cells may, in fact, have had micrometastases at deeper levels; these variations would be expected to affect the risk and type of non–sentinel lymph node involvement observed. The risk of non–sentinel lymph node involvement was affected by the size of sentinel lymph node metastases; the larger the metastasis in the sentinel lymph node, the higher the risk of non–sentinel lymph node involvement. Because pathological sampling involves assessment of a fraction of the lymph node volume only, there may be discordance between the detected sentinel lymph node metastatic size and its true size. In this systematic review, however, the number of assessed levels (defined as the number of lymph node sections assessed by pathological analysis) of the sentinel lymph node and non–sentinel lymph nodes stained with hematoxylin–eosin and by use of immunohistochemistry could not explain the between-study heterogeneity. Non–sentinel lymph nodes are generally examined less extensively without the routine use of immunohistochemistry, and this practice may underestimate the actual risk of small non–sentinel lymph node metastases after isolated tumor cells are found in the sentinel lymph node. The use of immunohistochemistry in the work-up of non–sentinel lymph nodes increases the detection rate of (small) non–sentinel lymph node metastases (46), so omitting immunohistochemistry for non–sentinel lymph nodes is likely to result in a relatively high percentage of non–sentinel lymph node macrometastases. However, Changsri et al. (24) reported only on isolated tumor cells in non–sentinel lymph nodes, by examining a single section of the non–sentinel lymph node by hematoxylin–eosin only. In their study, all patients with isolated tumor cells in their sentinel lymph node also had isolated tumor cells in their non–sentinel lymph nodes. They concluded that additional metastases in non–sentinel lymph nodes of patients with isolated tumor cells in the sentinel lymph node should also be of a small size, which is in contrast with the results of this systematic review. Finally, the heterogeneity of the results of these studies could be due to various interpretations of the current definition of isolated tumor cells, which do not permit a reproducible distinction between micrometastases and isolated tumor cell (47–49).
In, to our knowledge, the largest study of isolated tumor cells in the sentinel lymph node followed by axillary lymph node dissection (n = 187), Houvenaeghel et al. (12) reported a 16% rate of non–sentinel lymph nodes involvement. This retrospective multicenter study reported no statistically significant difference in the risk of non–sentinel lymph node involvement between sentinel lymph nodes that contained isolated tumor cells (16%) and those that contained micrometastases (14.3%). However, they reported a 9.9% detection rate of non–sentinel lymph node involvement in a large group (n = 212) of sentinel lymph nodes containing micrometastases that had an unknown diameter (which also included isolated tumor cells). No mention was made as to whether additional non–sentinel lymph node metastases were associated with therapeutic decision making. They found that the only proposed subgroups of patients for whom axillary lymph node dissection could be avoided were patients with a small primary tumor (pT1a or pT1b) or with pT1 tubular, colloidal, or medullary tumors, with a risk for non–sentinel lymph node involvement of 5% or less.
In the second largest study (n = 116), Viale et al. (14) reported non–sentinel lymph node involvement in 15% of patients whose sentinel lymph node contained isolated tumor cells; this non–sentinel lymph node involvement was further classified as isolated tumor cells (6%) or micrometastasis (18%) or macrometastasis (76%). They included clinically lymph node–negative patients with early breast cancer (breast tumor diameter of ≤3 cm). The 6.7% difference in the detection rate of non–sentinel lymph node involvement between sentinel lymph nodes containing isolated tumor cells (14.7%) and micrometastases (21.4%) was not statistically significant. The identification of these non–sentinel lymph node metastases, which were mostly macrometastases, affected further systemic therapy in most patients whose sentinel lymph node contained isolated tumor cells or micrometastases. They concluded that the current categories, which were introduced by the sixth edition of the AJCC Cancer Staging Manual, cannot safely be adopted to tailor axillary surgical treatment for patients undergoing a sentinel lymph node biopsy procedure. A predictive model that was based on features associated with non–sentinel lymph node involvement could not identify a subgroup of patients with less than 13% non–sentinel lymph node involvement. From these results, Viale et al. recommended axillary lymph node dissection if there was any evidence of sentinel lymph node involvement in all settings except clinical trials. However, it must be emphasized that their assessment of the sentinel lymph node (by frozen sections and without routine immunohistochemistry) was likely to have decreased the detection rate of isolated tumor cells in the sentinel lymph node, which would, therefore, affect the detectable rate of non–sentinel lymph node involvement.
The decision of whether or not to proceed with an axillary lymph node dissection after finding isolated tumor cells in the sentinel lymph node was based on the risk of the procedure itself and on the risk and clinical significance of leaving residual disease in the axilla. The size of metastatic tumor material in the sentinel lymph node was one of the strongest predictors of non–sentinel lymph node involvement, although other characteristics (increased tumor size, more than one positive sentinel lymph node, and lymphovascular invasion in the primary tumor) also have statistically significant predictive value (50). From the studies included in this systematic review, the pooled risk of non–sentinel lymph node involvement among patients whose sentinel lymph node contained isolated tumor cells was approximately 12.3%, which is only marginally lower than the risk of non–sentinel lymph node involvement in patients with sentinel lymph node micrometastases (ie, approximately 20%) (11,14,51) who are currently eligible for axillary lymph node dissection. It could therefore be argued that patients whose sentinel lymph node contained isolated tumor cells should also undergo an axillary lymph node dissection. However, this risk of non–sentinel lymph node involvement in patients with isolated tumor cells in the sentinel node is only marginally higher than the risk of a false-negative sentinel lymph node biopsy examination (7%–8%) (52,53), and we do not advocate axillary lymph node dissection for patients with sentinel lymph node–negative breast cancer. In addition, the clinical significance of residual axillary disease when axillary lymph node dissection is not performed is unclear. The risk of axillary recurrence if residual disease is present is probably low, and whether axillary lymph node dissection provides a survival benefit for sentinel lymph node–positive patients is not clear. Several studies (54–56), although limited by size and follow-up time, reported a very low rate of axillary recurrence in patients with a positive sentinel lymph node who did not undergo axillary lymph node dissection. This result may be due to selection of low-risk patients, limited outgrowth potential of residual tumor, or the effects of local radiotherapy. Furthermore, most breast cancer patients receive adjuvant systemic therapy when their primary tumor has unfavorable characteristics, and such therapy may also eradicate residual lymph node metastases. With regard to local control, a wait-and-see policy may therefore be acceptable when the sentinel lymph node contains isolated tumor cells. However, a substantial proportion (approximately 64%) of non–sentinel lymph node metastases identified in patients with sentinel lymph nodes containing isolated tumor cells are macrometastases, which require adjuvant systemic therapy. Failure to detect these non–sentinel lymph node macrometastases by omitting axillary lymph node dissection could thus result in undertreatment of those patients whose sentinel lymph node contains isolated tumor cells but whose primary tumor characteristics do not indicate the need for adjuvant therapy.
In conclusion, results on isolated tumor cells in the sentinel lymph node are somewhat controversial, and there is still doubt about the need for axillary lymph node dissection after finding isolated tumor cells in the sentinel lymph node. A wait-and-see policy is probably acceptable for most patients whose sentinel lymph node contains isolated tumor cells. However, for patients with a sentinel lymph node that contains isolated tumor cells, axillary lymph node dissection could be used if the indication for adjuvant systemic therapy is influenced by finding macrometastases on full lymph node staging. In this respect, the risk of non–sentinel lymph node involvement also depends on other risk factors (ie, primary tumor size and the presence of lymphovascular invasion). Molecular profiling of metastatic deposits, which could be distinct from the primary tumor (57), could be used to further select patients who might benefit from axillary lymph node dissection. Findings from large ongoing clinical trials, such as the International Breast Cancer Study Group 2301 trial, which has randomly assigned sentinel lymph node–positive patients to axillary lymph node dissection or surveillance, may provide greater clinical evidence regarding policies on axillary lymph node dissection after the identification of a positive sentinel lymph node.
Funding
This work was partly supported by a grant from the AntoniusMesosGroup, Oncology Center, Nieuwegein, The Netherlands.
References
- 1.Fisher ER, Anderson S, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06: 10-year pathologic and clinical prognostic discriminants. Cancer. 1993;71(8):2507–2514. doi: 10.1002/1097-0142(19930415)71:8<2507::aid-cncr2820710813>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 3.van der Heiden van der Loo, Bezemer PD, Hennipman A. et al. Introduction of sentinel node biopsy and stage migration of breast cancer. Eur J Surg Oncol. 2006;32(7):710–714. doi: 10.1016/j.ejso.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Cserni G, Amendoeira I, Apostolikas N, et al. Pathological work-up of sentinel lymph nodes in breast cancer. Review of current data to be considered for the formulation of guidelines. Eur J Cancer. 2003;39(12):1654–1667. doi: 10.1016/s0959-8049(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 5.Singletary SE, Greene FL. Revision of breast cancer staging: the 6th edition of the TNM Classification. Semin Surg Oncol. 2003;21(1):53–59. doi: 10.1002/ssu.10021. [DOI] [PubMed] [Google Scholar]
- 6.Singletary SE, Greene FL, Sobin LH. Classification of isolated tumor cells: clarification of the 6th edition of the American Joint Committee on Cancer Staging Manual. Cancer. 2003;98(12):2740–2741. doi: 10.1002/cncr.11865. [DOI] [PubMed] [Google Scholar]
- 7.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 8.van-Iterson V, Leidenius M, Krogerus L, von-Smitten K. Predictive factors for the status of non-sentinel nodes in breast cancer patients with tumor positive sentinel nodes. Breast Cancer Res Treat. 2003;82(1):39–45. doi: 10.1023/B:BREA.0000003918.59396.e4. [DOI] [PubMed] [Google Scholar]
- 9.Nos C, Harding-MacKean C, Freneaux P, et al. Prediction of tumour involvement in remaining axillary lymph nodes when the sentinel node in a woman with breast cancer contains metastases. Br J Surg. 2003;90(11):1354–1360. doi: 10.1002/bjs.4325. [DOI] [PubMed] [Google Scholar]
- 10.Yu JC, Hsu GC, Hsieh CB, Sheu LF, Chao TY. Prediction of metastasis to non-sentinel nodes by sentinel node status and primary tumor characteristics in primary breast cancer in Taiwan. World J Surg. 2005;29(7):813–818. doi: 10.1007/s00268-005-7744-x. [DOI] [PubMed] [Google Scholar]
- 11.Cserni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91(10):1245–1252. doi: 10.1002/bjs.4725. [DOI] [PubMed] [Google Scholar]
- 12.Houvenaeghel G, Nos C, Mignotte H, et al. Micrometastases in sentinel lymph node in a multicentric study: predictive factors of nonsentinel lymph node involvement—Groupe des Chirurgiens de la Federation des Centres de Lutte Contre le Cancer. J Clin Oncol. 2006;24(12):1814–1822. doi: 10.1200/JCO.2005.03.3225. [DOI] [PubMed] [Google Scholar]
- 13.Menes TS, Tartter PI, Mizrachi H, Constantino J, Estabrook A, Smith SR. Breast cancer patients with pN0(i+) and pN1(mi) sentinel nodes have high rate of nonsentinel node metastases. J Am Coll Surg. 2005;200(3):323–327. doi: 10.1016/j.jamcollsurg.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241(2):319–325. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman KJ. Epidemiology. An Introduction. New York: Oxford University Press; 2002. [Google Scholar]
- 16.Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol. 2008;61(1):41–51. doi: 10.1016/j.jclinepi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9(1):1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care. Meta-analysis in Context. London: BMJ Publishing Group; 2001. pp. 189–208. [Google Scholar]
- 19.Delaloye JF, Monod JF, Friedli A, et al. Un reseau pour la recherche du ganglion sentinelle du cancer du sein. [Network for sentinel lymph node research in breast cancer] Rev Med Suisse Romande. 2003;123(5):299–302. [PubMed] [Google Scholar]
- 20.Barranger E, Grahek D, Antoine M, Montravers F, Talbot JN, Uzan S. Evaluation of fluorodeoxyglucose positron emission tomography in the detection of axillary lymph node metastases in patients with early-stage breast cancer. Ann Surg Oncol. 2003;10(6):622–627. doi: 10.1245/aso.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 21.McCready DR, Yong WS, Ng AK, Miller N, Done S, Youngson B. Influence of the new AJCC breast cancer staging system on sentinel lymph node positivity and false-negative rates. J Natl Cancer Inst. 2004;96(11):873–875. doi: 10.1093/jnci/djh142. [DOI] [PubMed] [Google Scholar]
- 22.Dabbs DJ, Fung M, Landsittel D, McManus K, Johnson R. Sentinel lymph node micrometastasis as a predictor of axillary tumor burden. Breast J. 2004;10(2):101–105. doi: 10.1111/j.1075-122x.2004.21280.x. [DOI] [PubMed] [Google Scholar]
- 23.Zgajnar J, Besic N, Podkrajsek M, Hertl K, Frkovic-Grazio S, Hocevar M. Minimal risk of macrometastases in the non-sentinel axillary lymph nodes in breast cancer patients with micrometastatic sentinel lymph nodes and preoperatively ultrasonically uninvolved axillary lymph nodes. Eur J Cancer. 2005;41(2):244–248. doi: 10.1016/j.ejca.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Changsri C, Prakash S, Sandweiss L, Bose S. Prediction of additional axillary metastasis of breast cancer following sentinel lymph node surgery. Breast J. 2004;10(5):392–397. doi: 10.1111/j.1075-122X.2004.21446.x. [DOI] [PubMed] [Google Scholar]
- 25.Leidenius MH, Vironen JH, Riihela MS, et al. The prevalence of non-sentinel node metastases in breast cancer patients with sentinel node micrometastases. Eur J Surg Oncol. 2005;31(1):13–18. doi: 10.1016/j.ejso.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241(2):319–325. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrenk P, Konstantiniuk P, Wolfl S, et al. Prediction of non-sentinel lymph node status in breast cancer with a micrometastatic sentinel node. Br J Surg. 2005;92(6):707–713. doi: 10.1002/bjs.4937. [DOI] [PubMed] [Google Scholar]
- 28.de Widt-Levert L, Tjan-Heijnen V, Bult P, Ruers T, Wobbes T. Stage migration in breast cancer: surgical decisions concerning isolated tumour cells and micro-metastases in the sentinel lymph node. Eur J Surg Oncol. 2003;29(3):216–220. doi: 10.1053/ejso.2002.1401. [DOI] [PubMed] [Google Scholar]
- 29.Klevesath MB, Bobrow LG, Pinder SE, Purushotham AD. The value of immunohistochemistry in sentinel lymph node histopathology in breast cancer. Br J Cancer. 2005;92(12):2201–2205. doi: 10.1038/sj.bjc.6602641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoun KE, Hansen NM, Turner RR, Giuliano AE. Nonsentinel node metastases in breast cancer patients with isolated tumor cells in the sentinel node: implications for completion axillary node dissection. Am J Surg. 2005;190(4):588–591. doi: 10.1016/j.amjsurg.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Krauth JS, Charitansky H, Isaac S, Bobin JY. Clinical implications of axillary sentinel lymph node ‘micrometastases’ in breast cancer. Eur J Surg Oncol. 2006;32(4):400–404. doi: 10.1016/j.ejso.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Choi YJ, Yun HR, Yoo KE, et al. Intraoperative examination of sentinel lymph nodes by ultrarapid immunohistochemistry in breast cancer. Jpn J Clin Oncol. 2006;36(8):489–493. doi: 10.1093/jjco/hyl045. [DOI] [PubMed] [Google Scholar]
- 33.van Rijk MC, Peterse JL, Nieweg OE, Oldenburg HS, Rutgers EJ, Kroon BB. Additional axillary metastases and stage migration in breast cancer patients with micrometastases or submicrometastases in sentinel lymph nodes. Cancer. 2006;107(3):467–471. doi: 10.1002/cncr.22069. [DOI] [PubMed] [Google Scholar]
- 34.Amin BD, Hoda SA. Minimal metastatic disease in sentinel lymph nodes in breast carcinoma: some modest proposals to refine criteria for “isolated tumor cells. Adv Anat Pathol. 2006;13(4):185–189. doi: 10.1097/00125480-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Falconieri G, Pizzolitto S, Gentile G. Comprehensive examination of sentinel lymph node in breast cancer: a solution without a problem? Int J Surg Pathol. 2006;14(1):1–8. doi: 10.1177/106689690601400101. [DOI] [PubMed] [Google Scholar]
- 36.Ryden L, Chebil G, Sjostrom L, Pawlowski R, Jonsson PE. Determination of sentinel lymph node (SLN) status in primary breast cancer by prospective use of immunohistochemistry increases the rate of micrometastases and isolated tumour cells: analysis of 174 patients after SLN biopsy. Eur J Surg Oncol. 2007;33(1):33–38. doi: 10.1016/j.ejso.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Bolster MJ, Peer PG, Bult P, et al. Risk factors for non-sentinel lymph node metastases in patients with breast cancer. The outcome of a multi-institutional study. Ann Surg Oncol. 2007;14(1):181–189. doi: 10.1245/s10434-006-9065-1. [DOI] [PubMed] [Google Scholar]
- 38.Groen RS, Oosterhuis AW, Boers JE. Pathologic examination of sentinel lymph nodes in breast cancer by a single haematoxylin-eosin slide versus serial sectioning and immunocytokeratin staining: clinical implications. Breast Cancer Res Treat. 2007;105(1):1–5. doi: 10.1007/s10549-007-9728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245(3):452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fait V, Chrenko V. Sentinel node biopsy in breast cancer: short time results show appropriate regional control. Neoplasma. 2007;54(3):256–261. [PubMed] [Google Scholar]
- 41.Herbert GS, Sohn VY, Brown TA. The impact of nodal isolated tumor cells on survival of breast cancer patients. Am J Surg. 2007;193(5):571–573. doi: 10.1016/j.amjsurg.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Cserni G, Burzykowski T, Vinh-Hung V, et al. Axillary sentinel node and tumour-related factors associated with non-sentinel node involvement in breast cancer. Jpn J Clin Oncol. 2004;34(9):519–524. doi: 10.1093/jjco/hyh090. [DOI] [PubMed] [Google Scholar]
- 43.Cserni G, Bianchi S, Vezzosi V, et al. The value of cytokeratin immunohistochemistry in the evaluation of axillary sentinel lymph nodes in patients with lobular breast carcinoma. J Clin Pathol. 2006;59(5):518–522. doi: 10.1136/jcp.2005.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cserni G, Bianchi S, Vezzosi V, et al. Sentinel lymph node biopsy in staging small (up to 15 mm) breast carcinomas. Results from a European multi-institutional study. Pathol Oncol Res. 2007;13(1):5–14. doi: 10.1007/BF02893435. [DOI] [PubMed] [Google Scholar]
- 45.van Deurzen CH, van Hillegersberg R, Hobbelink MG, Seldenrijk CA, Koelemij R, van Diest PJ. Predictive value of tumor load in breast cancer sentinel lymph nodes for second echelon lymph node metastases. Cell Oncol. 2007;29(6):497–505. doi: 10.1155/2007/570683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu KU, Turner RR, Hansen NM, Brennan MB, Giuliano AE. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel node metastasis. Ann Surg Oncol. 1999;6(8):756–761. doi: 10.1007/s10434-999-0756-2. [DOI] [PubMed] [Google Scholar]
- 47.Cserni G, Bianchi S, Boecker W, et al. Improving the reproducibility of diagnosing micrometastases and isolated tumor cells. Cancer. 2005;103(2):358–367. doi: 10.1002/cncr.20760. [DOI] [PubMed] [Google Scholar]
- 48.Turner RR, Weaver DL, Cserni G, et al. Nodal stage classification for breast carcinoma: improving interobserver reproducibility through standardized histologic criteria and image-based training. (2):63. doi: 10.1200/JCO.2007.13.0179. [DOI] [PubMed] [Google Scholar]
- 49.Cserni G, Amendoeira I, Apostolikas N, et al. Discrepancies in current practice of pathological evaluation of sentinel lymph nodes in breast cancer. Results of a questionnaire based survey by the European Working Group for Breast Screening Pathology. J Clin Pathol. 2004;57(7):695–701. doi: 10.1136/jcp.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degnim AC, Griffith KA, Sabel MS, et al. Clinicopathologic features of metastasis in nonsentinel lymph nodes of breast carcinoma patients. Cancer. 2003;98(11):2307–2315. doi: 10.1002/cncr.11803. [DOI] [PubMed] [Google Scholar]
- 51.Wada N, Imoto S. Clinical evidence of breast cancer micrometastasis in the era of sentinel node biopsy. Int J Clin Oncol. 2008;13(1):24–32. doi: 10.1007/s10147-007-0736-0. [DOI] [PubMed] [Google Scholar]
- 52.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Goyal A, Newcombe RG, Chhabra A, Mansel RE on behalf of the ALMANAC-Trialists-Group. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer—results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99:203–208. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 54.Guenther JM, Hansen NM, Difronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138(1):52–56. doi: 10.1001/archsurg.138.1.52. [DOI] [PubMed] [Google Scholar]
- 55.Langer I, Marti WR, Guller U, et al. Axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases: prospective analysis of 150 patients after SLN biopsy. Ann Surg. 2005;241(1):152–158. doi: 10.1097/01.sla.0000149305.23322.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110(4):723–730. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 57.Vecchi M, Confalonieri S, Nuciforo P, et al. Breast cancer metastases are molecularly distinct from their primary tumors. Oncogene. 2007 doi: 10.1038/sj.onc.1210858. [DOI] [PubMed] [Google Scholar]