Abstract
Fanconi anemia is a recessively inherited disease that is characterized by congenital abnormalities, bone marrow failure, and a predisposition to develop cancer, particularly squamous cell carcinomas (SCCs) in the head and neck and anogenital regions. Previous studies of Fanconi anemia SCCs, mainly from US patients, revealed the presence of high-risk human papillomavirus (HPV) DNA in 21 (84%) of 25 tumors analyzed. We examined a panel of 21 SCCs mainly from European Fanconi anemia patients (n = 19 FA patients; 16 head and neck squamous cell carcinomas [HNSCCs], 2 esophageal SCCs, and 3 anogenital SCCs) for their clinical and molecular characteristics, including patterns of allelic loss, TP53 mutations, and the presence of HPV DNA by GP5+/6+ polymerase chain reaction. HPV DNA was detected in only two (10%) of 21 tumors (both anogenital SCCs) but in none of the 16 HNSCCs. Of the 18 tumors analyzed, 10 contained a TP53 mutation. The patterns of allelic loss were comparable to those generally found in sporadic SCCs. Our data show that HPV does not play a major role in squamous cell carcinogenesis in this cohort of Fanconi anemia patients and that the Fanconi anemia SCCs are genetically similar to sporadic SCCs despite having a different etiology.
CONTEXT AND CAVEATS
Prior knowledge
Fanconi anemia is a rare recessively inherited disease that is characterized by congenital abnormalities, bone marrow failure, and a predisposition to develop cancer, particularly squamous cell carcinomas (SCCs) in the head and neck (HNSCCs) and anogenital regions. In previous studies of mostly US Fanconi anemia patients, high-risk human papillomavirus (HPV) DNA was detected in the majority of the SCCs analyzed.
Study design
An examination of the clinical and molecular characteristics of 21 SCCs (16 HNSCCs, 2 esophageal SCCs, and 3 anogenital SCCs) from 19 mostly European Fanconi anemia patients, including the presence of HPV DNA, allelic loss patterns, and TP53 mutations.
Contribution
HPV DNA was detected in only two of 21 tumors (both anogenital SCCs) but in none of the 16 HNSCCs. Ten of 18 SCCs analyzed contained a TP53 mutation. The patterns of allelic loss for the Fanconi anemia SCCs were similar to those found in sporadic SCCs, despite the differing etiologies of these tumors.
Implications
The role of HPV infection in squamous cell carcinogenesis may differ among cohorts of Fanconi anemia patients.
Limitations
The number of tumor samples analyzed was small.
From the Editors
Fanconi anemia is a recessively inherited disease that is characterized by congenital abnormalities, aplastic anemia, and a predisposition for the development of cancer. The disease is caused by defects in the Fanconi anemia–BRCA pathway, a response network involved in DNA damage repair (1). Cells from Fanconi anemia patients are hypersensitive to DNA cross-linking agents, such as cisplatin and mitomycin C, and display chromosome instability (2,3). The major life-threatening symptoms of Fanconi anemia—aplastic anemia and the development of acute myeloid leukemia—can be managed by treatments that include bone marrow transplantation. However, Fanconi anemia patients also have a 500- to 1000-fold increased risk of developing squamous cell carcinoma (SCC) (4), particularly squamous cell carcinoma of the mucosal linings of the head and neck region (HNSCC), which is now rapidly becoming a major cause of mortality in these patients. Bone marrow transplantation and graft vs host disease further increase the risk of developing SCC in Fanconi anemia patients (5).
The reason for the extremely high frequency of SCC in Fanconi anemia patients is unclear. In the general population, the most common risk factors for the development of SCC, particularly HNSCC, are tobacco smoking and alcohol consumption. However, in recent years, it has become clear that infection with high-risk human papillomaviruses (HPVs), which are known to cause cervical cancer, is also associated with a proportion of HNSCCs. For example, in the Netherlands, HPV is involved in approximately 10% of sporadic SCCs in the oral cavity and oropharynx (6). HPV involvement is usually assessed by a polymerase chain reaction (PCR) assay to detect the viral DNA in the tumor. However, because this method can lead to false-positive results, measurement of the actual transcriptional activity of the viral oncogenes by reverse transcription–polymerase chain reaction (RT-PCR) is currently considered the most reliable test for HPV involvement in HNSCC. However, RT-PCR can only be used to amplify RNA of frozen specimens, whereas in many studies, only formalin-fixed paraffin-embedded specimens are available. As an alternative, surrogate markers that can be analyzed on formalin-fixed paraffin-embedded specimens could be used, in addition to HPV DNA PCR, to detect HPV infection more reliably. For example, the genetic profiles of HNSCCs with transcriptionally active HPV differ from those of HNSCC without HPV and could be used to confirm HPV infection in a tumor (6). Absence of mutations in the TP53 gene could also be used as a surrogate marker for HPV infection: Because the HPV E6 oncoprotein inactivates p53, mutations in the TP53 gene are usually not observed in HPV-infected tumors, but are found in 50% of HNSCCs without HPV (6,7). Overexpression of CDKN2A, the gene that encodes p16Ink4, has also been used as a surrogate marker for HPV infection. In particular, p16Ink4 immunostaining followed by HPV DNA PCR on the p16Ink4-positive cases has been shown to be a highly sensitive and specific assay system to assess the presence of transcriptionally active HPV in tumors, including formalin-fixed paraffin-embedded specimens (8).
Kutler et al. (9) reported that 21 (84%) of 25 SCCs from US Fanconi anemia patients had detectable levels of HPV DNA. However, in a pilot study, we failed to detect HPV DNA in four Fanconi anemia SCC cell lines or the corresponding primary tumors from which they were derived (10,11). Here, we examined the clinical and molecular characteristics of SCCs from a larger cohort of mostly European Fanconi anemia patients.
Via a network of mostly European head and neck surgeons and Fanconi anemia patient support groups, we obtained clinical information and resected specimens of 21 SCCs from 19 Fanconi anemia patients. Tumors were staged according to International Union Against Cancer guidelines (12). This study was approved by the Institutional Review Board of the Vrije Universiteit Medical Center and carried out according to Dutch guidelines for the analysis of human samples. Relevant clinical data are listed in Supplementary Table 1 (available online). Of the 21 specimens that we received, 19 were formalin fixed and paraffin embedded and 2 were frozen. In one case, we received only a few sections of formalin-fixed paraffin-embedded tumor tissue. In all cases except the latter, 5-µm thick sections were stained with hematoxylin–eosin and evaluated by an experienced pathologist (E. Bloemena) for histological examination and to delineate areas of neoplastic and normal tissue for microdissection, as previously described (13). Before we cut sections of each specimen, we cleaned the microtome and changed the knife to avoid cross contamination between samples. DNA was isolated from microdissected normal and tumor tissue using proteinase K treatment, phenol–chloroform extraction, and ethanol precipitation and used for genetic analysis and HPV DNA detection. The allelic loss profiles were determined using 23 microsatellite markers at chromosomes 3p, 8p, 9p, 13q, 17q, and 18q, as previously described (10). Immunostaining for p53 and TP53 mutation analysis was performed as previously described (14,15). For TP53 mutation analysis, we analyzed exons 5–9 because the large majority of mutations occur there. For p16 immunohistochemistry, we used a CINtec p16Ink 4a histology kit (DakoCytomation BV, Heverlee, Belgium). Both positive and negative control specimens were included in every immunostaining run. Immunostaining was evaluated by the study pathologist (E. Bloemena). Immunostaining in more than 50% of the tumor cells was considered positive (8). HPV DNA detection was performed by using a GP5+/6+ PCR enzyme immunoassay that included an oligoprobe cocktail for 14 high-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as previously described (16). Samples were considered HPV DNA positive in the GP5+/6+ PCR enzyme assay when the optical density value was at least three times the value of the blanks without DNA template measured in triplicate. PCR amplification of the β-globin (HBB) gene was carried out in a separate assay to control for the integrity of the template DNA. Negative controls (solvent instead of DNA) and positive controls (serial dilution of DNA isolated from SiHa, a cervical carcinoma cell line that contains 1–2 copies of HPV16 DNA) were run in parallel in each assay. GP5+/6+ PCR enzyme immunoassay–positive cases were subsequently typed for HPV by reverse line blot genotyping and subjected to viral load analysis, as previously described (16).
Our panel of tumors consisted of 16 HNSCCs, 2 esophageal SCCs, and 3 anogenital SCCs. Of the 19 Fanconi anemia patients in this study, 10 had received a bone marrow transplant; these patients were approximately 6 years younger at SCC diagnosis than those who had not received a bone marrow transplant (median age at SCC diagnosis, bone marrow transplant vs no bone marrow transplant: 24.9 vs 30.7 years, log-rank P = .009; Supplementary Figure 1, available online). All 21 SCCs were tested for the presence of HPV DNA by GP5+/6+ PCR, and only two—both anogenital SCCs—were positive; one HPV DNA–positive tumor had HPV16 (237 copies per cell) and the other had HPV33 (22 copies per cell). In parallel, we subjected 18 SCCs with sufficient material for analysis to p16 and p53 immunostaining and TP53 mutation analysis. Of the 18 SCCs tested, five were positive for p16 immunostaining, including the two that were HPV DNA positive, and eight were positive for p53 immunostaining. The two HPV DNA–positive SCCs were negative for p53 immunostaining. TP53 mutations were detected in 10 of 18 SCCs. As expected, both of the HPV DNA–positive SCCs had wild-type TP53 (Table 1 and Supplementary Table 2, available online).
Table 1.
Human papillomavirus (HPV) DNA status, p16 and p53 immunohistochemistry (IHC), and TP53 mutation status of Fanconi anemia squamous cell carcinomas by anatomical region
| Anatomical region | n | No. HPV DNA–positive/No. tested | No. p16 IHC–positive/No. tested | No. p53 IHC–positive/No. tested | No. with TP53 mutation/No. tested |
| Head and neck | 16 | 0/16 | 2/13 | 6/13 | 8/13 |
| Esophagus | 2 | 0/2 | 0/2 | 1/2 | 1/2 |
| Anogenital | 3 | 2/3 | 3/3 | 1/3 | 1/3 |
| Total | 21 | 2/21 | 5/18 | 8/18 | 10/18 |
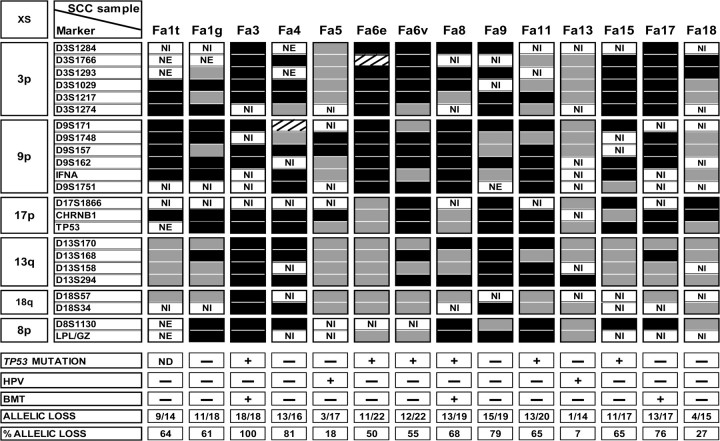
As an additional surrogate marker for HPV infection and to compare the genetic profiles of SCCs in Fanconi anemia patients with those of sporadic tumors in the general population, we further analyzed allelic loss profiles using microsatellite markers located at chromosome arms that frequently display allelic losses in HPV DNA–negative sporadic HNSCCs (ie, chromosome arms 3p, 8p, 9p, 13q, 17q, and 18q) (15,17). We were also able to assess allelic loss patterns for three SCCs from patients who had received a bone marrow transplant (Fa3, Fa8, and Fa17). SCCs from the other seven patients who had undergone bone marrow transplantation could not be analyzed due to the presence of donor-derived tumor-infiltrating lymphocytes that caused mixed allelic loss patterns (Supplementary Figure 2, available online). All of the HPV DNA–negative SCCs except one (Fa18) showed many allelic losses (more than 50% of the informative markers; Figure 1), which is a pattern that is typical for HPV DNA–negative sporadic SCC. By contrast, the two HPV DNA–positive tumors—Fa5 and Fa13—showed fewer allelic losses (fewer than 20% of the informative markers in each), which is characteristic of sporadic HPV-infected tumors in the general population (6,7). The HPV DNA–negative SCC Fa18 also showed relatively few allelic losses, which might suggest that the HPV DNA PCR result for this sample was a false negative. However, the negative HPV DNA status of SCC Fa18 was confirmed by its lack of p16 immunostaining (Supplementary Table 2, available online). Such genetically normal tumors also occur in sporadic SCC (18). Hence, all immunostaining and genetic data support our conclusion that the large majority of SCCs in this panel of Fanconi anemia patients were not induced by HPV.
Figure 1.
Patterns of allelic loss in Fanconi anemia squamous cell carcinomas. All tumors that could be analyzed for allelic imbalances are depicted, including tumors from patients who underwent bone marrow transplantation that could be microdissected without contamination by donor cells (Fa3, Fa8, and Fa17). Also indicated are TP53 mutations, presence of HPV DNA, bone marrow transplantation status, and allelic loss frequency (%: number of markers with allelic loss divided by the total number of informative markers). Black boxes indicate allelic loss (a change in the microsatellite allele ratios of more than 50% in the tumor DNA compared with genetically normal DNA isolated from the stroma), gray boxes indicate no allelic loss, and boxes with diagonal lines indicate microsatellite instability. Presence and absence of a TP53 mutation or HPV DNA in the tumor genome or of receipt of bone marrow transplantation are indicated with + and −, respectively. The specific TP53 mutations are listed in Supplementary Table 2 (available online). XS = chromosome; SCC = squamous cell carcinoma; NI = not informative; NE = not evaluable; ND = not determined; HPV = human papillomavirus; BMT = bone marrow transplant.
Several lines of evidence suggest that donor stem cells in patients who received bone marrow or stem cell transplants as part of the treatment for leukemia or other clinical indications might differentiate and become part of the squamous tissues of the transplanted patient (19). There has also been an indication that such donor cells could develop into skin cancer in transplanted patients (20). We therefore used DNA fingerprinting to examine whether SCCs that had developed in the Fanconi anemia patients who had undergone bone marrow transplantation were derived from the donor's or the patient's cells. Each of the seven tumors that could be analyzed (Fa3, Fa8, Fa10, Fa16, Fa17, Fa19, and Fa21) was clearly derived from patient's cells (an example of this analysis is shown in Supplementary Table 2, available online).
The most prominent finding in this study is that HPV DNA was detected in only two (10%) of 21 Fanconi anemia SCCs. This finding was based on HPV DNA detection and supported by assessment of surrogate markers of HPV infection, such as p16 immunostaining, p53 immunostaining, TP53 mutation analysis, and allelic loss profiling of the tumors (6,7). This observation is in distinct contrast to a previous report, in which 21 (84%) of 25 Fanconi anemia SSCs were positive for HPV DNA (9). Further comparison of the data from this study and the previous report (9) revealed similar proportions of HPV DNA–positive anogenital SCCs (two of three anogenital SCCs in this study were HPV DNA positive vs six of seven in the previous study) (9). However, there was a clear and statistically significant difference between these studies in the proportion of HPV DNA–positive HNSCCs: 0 (0%) of 16 in our series vs 15 (83%) of 18 in the previous series (P < .001) (9). How can we explain this striking difference? Similar methods for HPV DNA detection were used in the two studies. It is noteworthy that some of the laser-dissected specimens in the previous report had a relatively low viral load (range = 0.16–249.3 copies per cell) (9), suggesting that in at least some cases, the HPV infections might not have been clinically relevant. Results of a previous study in microdissected HNSCCs suggested that viral loads of at least 0.5–1 HPV genome per cell should be expected to represent a biologically relevant infection of transcriptionally active HPV (8). However, it is unlikely that the difference in HPV prevalence between our series and the previous series can be explained completely by a few cases with low viral load in the earlier study (9). The high HPV prevalence in the earlier report was supported by the absence of TP53 mutations in the Fanconi anemia SCCs (9). It is therefore likely that the difference between the two studies may also be explained, at least in part, by geographic differences in the prevalence of HPV infection (21). For example, in a case–control study in Baltimore, D’Souza et al. (22) reported that 72% of a cohort of sporadic oropharyngeal tumors were HPV infected; by contrast, only 16% of oropharyngeal SCCs from patients in the Amsterdam region were HPV infected, a puzzling observation (6). The previously reported series (9) included tumors that were mainly from US Fanconi anemia patients, whereas most of the SCCs in our series (89%) were from European Fanconi anemia patients.
Our observation that HPV does not seem to play a role in head and neck SCCs in Fanconi anemia patients, at least in Europe, might have important clinical consequences. Vaccination against some HPV subtypes has been shown to prevent HPV-associated anogenital diseases (23). Our data indicate that HPV vaccination of Fanconi anemia patients might prevent only a proportion of anogenital SCCs, and no HNSCCs, at least among European patients. Frequent surveillance of the mucosal linings, particularly in the head and neck region, therefore remains indicated for Fanconi anemia patients in all countries. Dutch pediatric oncology guidelines recommend that all Fanconi anemia patients undergo inspection of the head and neck region every 3 months beginning at 10 years of age, as well as a yearly anogenital inspection for female patients beginning at the age of menarche. Screening and monitoring of precancerous lesions remain the most important approach to detect SCCs at the earliest possible stage regardless of HPV vaccination, particularly because the treatment options for SCCs in Fanconi anemia patients are limited.
The patterns of allelic loss we found suggest that the chromosomes involved in Fanconi anemia SCCs are similar to those involved in sporadic SCCs. Although the etiology of Fanconi anemia HNSCCs (a DNA repair deficiency causing genetic alterations) differs from that of most sporadic SCCs (tobacco smoking and alcohol consumption), the same cancer genes and chromosomal locations seem to be targeted in both types of SCCs. Our finding that SCCs of Fanconi anemia patients and SCCs in the general patient population have identical patterns of allelic loss might be exploited for the early diagnosis of cancer and precancerous lesions in Fanconi anemia patients. Analysis of the allelic loss pattern of biopsy specimens facilitates accurate diagnosis and assessment of the risk of malignant transformation of visible oral lesions in the general population, and the same approach could be followed for Fanconi anemia patients to improve the clinical management of such lesions (24). Unfortunately, most preneoplastic changes in the oral mucosa are not visible by eye, which limits early detection of mucosal regions that are at increased risk of malignant transformation. Noninvasive genetic screening to detect these mucosal regions might therefore be of value to improve the early detection of cancer and precancerous lesions in Fanconi anemia patients (25).
A limitation of this work is our small sample size of 21 tumors from only 19 patients. A larger cohort of samples might substantiate the data. However, Fanconi anemia is a relatively rare disease, and it takes a major effort to collect samples and clinical information for these patients. In future studies, it will be important to exchange samples between studies to formally and definitively rule out that minor differences in methodologies might influence the findings.
Funding
This work was supported by the Fanconi Anemia Research Fund Inc (Eugene, OR) and the Fanconi-Anämie Hilfe e.V. (Unna-Siddinghausen, Germany).
Supplementary Material
Footnotes
The authors thank the following investigators for providing tumor material and clinical information for Fanconi anemia patients: Harald Reinhard (Pediatric Oncology and Hematology Clinic, University Hospital Homburg, Germany); Frank Autsbach and Jorgen Wacker (Institute of Pathology/Department of Dermatology, University of Heidelberg, Germany); Johannes Rischewski (Department of Pediatric Hematology/Oncology, Universitätsklinikum Hamburg-Eppendorf, Germany); Malcolm Little (Department of Pediatrics, University College Galway, Ireland); Beatriz Arribalaga (Hematology Service, Hospital de Cruces, Vizcaya, Spain); Maria Angeles Dasí (Pediatric Hematology Unit, Hospital La Fé, Valencia, Spain); Teresa Olivé (Unit of Pediatric Hemato-Oncology, Hospital Vall d’Hebrón, Barcelona, Spain); Pedro Gómez (Hematology Service, Hospital de Córdoba, Córdoba, Spain); and María Tapia (Hematology Service, Hospital General La Palma, Santa Cruz de la Palma, Spain). The authors also thank Bart Hesselink (Department of Pathology) and Hinke Dekter (Department of Otolaryngology/Head-Neck Surgery) VU University Medical Center for their contributions to the molecular analyses. The sponsors helped to collect the samples and clinical information, but had no role in the design, analysis, or interpretation of the data.
References
- 1.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8(10):735–748. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 2.Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25(43):5875–5884. doi: 10.1038/sj.onc.1209878. [DOI] [PubMed] [Google Scholar]
- 3.Levitus M, Joenje H, de Winter JP. The Fanconi anemia pathway of genomic maintenance. Cell Oncol. 2006;28(1–2):3–29. doi: 10.1155/2006/974975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129(1):106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105(1):67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 6.Braakhuis BJM, Snijders PJF, Keune W-JH, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2004;96(13):998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 7.Smeets SJ, Braakhuis BJM, Abbas S, et al. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006;25(17):2558–2564. doi: 10.1038/sj.onc.1209275. [DOI] [PubMed] [Google Scholar]
- 8.Smeets SJ, Hesselink AT, Speel E-JM, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121(11):2465–2472. doi: 10.1002/ijc.22980. [DOI] [PubMed] [Google Scholar]
- 9.Kutler DI, Wreesmann VB, Goberdhan A, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95(22):1718–1721. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- 10.van Zeeburg HJT, Snijders PJF, Pals G, et al. Generation and molecular characterization of head and neck squamous cell lines of Fanconi anemia patients. Cancer Res. 2005;65(4):1271–1276. doi: 10.1158/0008-5472.CAN-04-3665. [DOI] [PubMed] [Google Scholar]
- 11.van Zeeburg HJT, Snijders PJF, Joenje H, Brakenhoff RH. Re: Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2004;96(12):968. doi: 10.1093/jnci/djh178. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumors. 6th ed. New York, NY: Whiley-Liss; 2002. [Google Scholar]
- 13.Tabor MP, Brakenhoff RH, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7(6):1523–1532. [PubMed] [Google Scholar]
- 14.Cruz IB, Snijders PJF, Meijer CJ, et al. P53 expression above the basal cell layer in oral mucosa is an early event of malignant transformation and has predictive value for developing oral squamous cell carcinoma. J Pathol. 1998;184(4):360–368. doi: 10.1002/(SICI)1096-9896(199804)184:4<360::AID-PATH1263>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 15.Tabor MP, Van Houten VMM, Kummer JA, et al. Discordance of genetic alterations between primary head and neck tumors and corresponding metastases associated with mutational status of the TP53 gene. Genes Chromosomes Cancer. 2002;33(2):168–177. doi: 10.1002/gcc.10019. [DOI] [PubMed] [Google Scholar]
- 16.Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110(1):94–101. doi: 10.1002/ijc.20076. [DOI] [PubMed] [Google Scholar]
- 17.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56(11):2488–2492. [PubMed] [Google Scholar]
- 18.Perrone F, Suardi S, Pastore E, et al. Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2006;12(22):6643–6651. doi: 10.1158/1078-0432.CCR-06-1759. [DOI] [PubMed] [Google Scholar]
- 19.Tran SD, Pillemer SR, Dutra A, et al. Differentiation of human bone marrow-derived cells into buccal epithelial cells in vivo: a molecular analytical study. Lancet. 2003;361(9363):1084–1088. doi: 10.1016/S0140-6736(03)12894-2. [DOI] [PubMed] [Google Scholar]
- 20.Aractingi S, Kanitakis J, Euvrard S, et al. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005;65(5):1755–1760. doi: 10.1158/0008-5472.CAN-04-2783. [DOI] [PubMed] [Google Scholar]
- 21.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 23.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 24.Rosin MP, Cheng X, Poh C, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6(2):357–362. [PubMed] [Google Scholar]
- 25.Bremmer JF, Braakhuis BJM, Ruijter-Schippers HJ, et al. A noninvasive genetic screening test to detect oral preneoplastic lesions. Lab Invest. 2005;85(12):1481–1488. doi: 10.1038/labinvest.3700342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.