Abstract
Early intensive care for severe acute pancreatitis is essential for improving SAP mortality rates. However, intensive therapies for SAP are often delayed because there is no ideal way to accurately evaluate severity in the early stages. Currently, perfusion CT has been shown useful to predict prognosis of SAP in the early stage. In this presented paper, we would like to review the clinical usefulness and limitations of perfusion CT for evaluation of local and systemic complications in early stage of SAP.
1. Introduction
Severe acute pancreatitis (SAP) is a fatal disease [1]. The Atlanta Symposium criteria for the severity of acute pancreatitis define SAP as acute pancreatitis with local complications (pancreatic necrosis, abscess, and pseudocysts) and/or with systemic complications (organ failure, disseminated intravascular coagulation, and severe metabolic disturbances) [2] (Figure 1). Both acute necrotizing pancreatitis (ANP) and multiple-organ failure (MOF) have been shown to be significant prognostic factors [3–6]. Mortality rates for SAP patients developing ANP and MOF are 32% and 30%, respectively [7]. Early intensive care for SAP is essential for improving SAP mortality rates [8–10]. However, intensive therapies for SAP are often delayed because there is no ideal way to accurately evaluate severity in the early stages [11–13].
Figure 1.
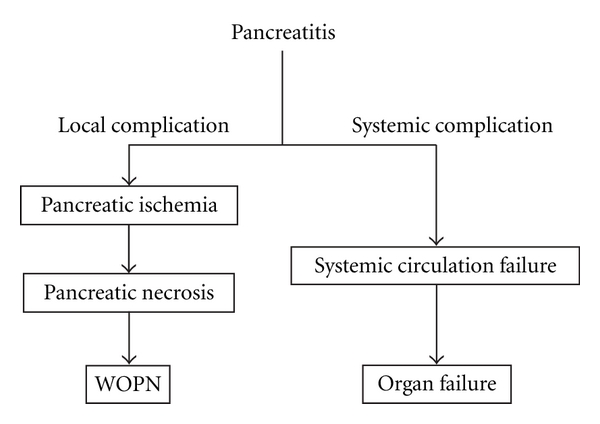
Schema of complications of severe acute pancreatitis. WOPN: walled-off pancreatic necrosis.
Perfusion CT has been used for evaluation of various pancreatic diseases [14–22]. Perfusion CT has been shown useful to predict prognosis of SAP in the early stage [17, 18]. In this presented paper, we would like to review the clinical usefulness and limitations of perfusion CT for evaluation of local and systemic complications in early stage of SAP.
2. Perfusion CT Technique
Previously published perfusion CT protocols are summarized in Table 1. Multidetector CT (MDCT) is essential for performing perfusion CT of pancreas. With a 4–64 slice MDCT scanner, perfusion CT scans are obtained with the patient in a stationary position. The craniocaudal CT scan coverage is limited to 20 to 32 mm (4 slices of 5 to 8 mm thickness). Therefore, scan location must be carefully chosen to cover as much of the pancreas as possible as it is often difficult to cover the entire pancreas. Since most pancreatic necrosis occurs in the neck region, it is probably uncommon to exclude the area of necrosis due to the scanning coverage limitation. With the use of a recently developed 256–320-slice MDCT scanners [19], craniocaudal coverage has increased to 80–160 mm. Alternative way to increase the craniocaudal coverage is by using the so-called shuttle or toggle mode. In this mode, similar to conventional CT scans, patient table moves back and forth as the multiple scans are performed.
Table 1.
Scanning protocols of pancreatic perfusion CT.
| Authors | Disease | CT | The number of detector | kv | mA | Images | Contrast matter | Duration time (sec) | Algorithm | |
|---|---|---|---|---|---|---|---|---|---|---|
| Injection rate (mL/sec) | Amount | |||||||||
| Miles [25] | — | — | — | — | 50–100 | 60 | 4–7 | 40 mL | 60 | Deconvolution |
| 100–250 | 15 | 7–10 | 50 mL | 45 | Compartment | |||||
| 100–250 | 6 | 4 | 100 mL | 120 | Patlak plot | |||||
| Tsushima and kusano [15] | Normal | S | 1 | — | — | 19 | 5 | 40 mL | 85 | Maximum slope |
| Abe et al. [16] | PC | G | 1 | 120 | 60 | — | 5 | 0.5 mL/kg | 40 | Deconvolution |
| Bize et al. [17] | AP | P | 16 | 90 | 100 | 40 | 5 | 40 mL | 40 | Maximum slope |
| Tsuji et al. [18] | AP | T | 16/64 | 120 | 30–50 | 30–48 | 4 | 40 mL | 33–48 | Deconvolution |
| Tsuji et al. [27] | AP/NET | T | 64 | 80 | 40 | 106 | 4 | 40 mL | 54 | Deconvolution |
| Sheiman and Stick [28] | Normal | G | 64 | 100 | 80 | 30 | 4 | 40 mL | 90 | Compartment |
| d'Assignies et al. [20] | NET | G | 64 | 100 | 100 | 70 | 4 | 40 mL | 150 | Compartment |
| Park et al. [21] | PC | S | 64 | 100 | 100 | 30 | 5 | 50 mL | 30 | Patlak plot |
| Kandel et al. [19] | PC | T | 320 | 100 | 45 | 19 | 8 | 60 mL | 80 | Maximum slope |
PC: pancreatic cancer; AP: acute pancreatitis; NET: neuroendocrine tumor; S: Siemens; G: GE Health care; P: Philipse; T: Toshiba.
First, noncontrast transaxial images of the upper abdomen are obtained using low-dose technique. This scan is performed to localize the pancreas, and it determines the scan range of the perfusion CT.
Perfusion CT is performed after a bolus injection of intravenous contrast material. Unlike conventional CT, the perfusion CT requires smaller dose (40–50 mL) of contrast material injected at a high rate (4–10 mL/sec). Higher concentration of contrast material (350–370 mgI/kg) is preferred [23–25].
Perfusion CT images are obtained multiple times through the pancreas. In most of previous reports, scan interval ranges from 0.5 to 1.5 second, and the scan duration ranges from 30 to 150 seconds, respectively (Table 1). Total scan duration necessary for calculation of perfusion parameters may depend on the algorithm used. For example, maximum slope method needs shorter duration scan time than the deconvolution method [14, 19, 26]. Because the scan duration is long, the scans are usually performed under free breathing.
Perfusion CT scan is obtained at a low tube current (mAs) to reduce radiation dose. At 120 kV, mAs of 100 is commonly used. There is increased interest in the use of low tube voltage setting, as it reduces radiation dose and improves iodine contrast material conspicuity. In a smaller patient, the use of 100 kV or 80 kV is recommended. In a larger patient, the use of low-kV scan may result in noisy images due to photon deficiency.
2.1. Radiation Dose and Scan Parameters
Radiation dose is dependent on the tube current (mAs), tube voltage (kV), number of scans, and scan coverage [29]. Tube current (mAs) and tube voltage (kV) are largely dictated by the patient size to maintain adequate image quality. Radiation dose should be kept as low as reasonably achievable (ALARA) by reducing the scanning parameter settings but achieving image dataset adequate for calculating CT perfusion parameters [27, 30]. Further study is necessary to optimize the scanning protocol.
From a European study, the effective dose of pancreatic perfusion CT was 3.54 mSv with 90 KV, 100 mAs, and 40 scans [17]. A study from Japan reported that mean radiation dose of pancreatic perfusion CT was approximately 204.8 mGy·cm (dose-length product (DLPw)), 3.07 mSv (effective dose), and 64 mGy (CT dose index volume (CTDIvol)) with 80 kV, 60 mAs, and 106 scans [27]. In the national survey, the radiation exposure of a single-phase abdominal CT was 13–25 mGy (CTDIvol) [31]. Therefore, the radiation dose of perfusion CT is slightly higher than that of biphasic (two phase), which is commonly used for pancreatic or liver imaging. Average abdominal transverse diameter of the Japanese patients in our experience was 32 cm, while transverse diameters of patients in the Unites States are usually larger [32]. Therefore, the radiation dose will likely be higher in the western countries
2.2. Perfusion CT Data Analysis
Pancreatic perfusion CT image data are analyzed by using perfusion CT analysis software. There are various perfusion algorithms to calculate perfusion parameters. Maximum slope method, deconvolution method, single-compartment method, and the Patlak method are commonly used perfusion algorithms. Which perfusion best suits in the evaluation of SAP is yet to be determined. As different perfusion algorithms are suited for different disease processes and require different scanning protocol, determination of scanning protocol and perfusion algorithms should be considered together. For example, maximum slope method may require shorter scanning duration, but higher rate of contrast injection is required, while deconvolution method may require longer scanning duration but slower rate of contrast injection rate [14, 26].
The software requires placement of small regions of interest (ROI) on an artery to generate arterial input function. Venous outflow function is required in deconvolution method. This process is required because the computer algorithm compares the shape and height of the time-density curve of each pixel of the pancreatic CT time series with shape and height of the arterial and/or venous time-density curves to calculate pancreatic perfusion parameters. Calculated pancreatic perfusion parameters are displayed using color maps [14].
3. Perfusion CT for Predicting Development of Pancreatic Necrosis in the Early Stage of Severe Acute Pancreatitis
Development of pancreatic necrosis is the critical event of acute pancreatitis that determines the prognosis of the patients. The overall mortality rate of acute pancreatitis is reported to be between 2.1% and 9.2% worldwide [1]. Pancreatic necrosis occurs in 10–15% of patients with SAP, with a mortality rate of 23% [1]. This rate is nearly twice that for patients with SAP who do not develop pancreatic necrosis (i.e., 11%) [1].
There is a report that dynamic contrast-enhanced CT is more accurate than either the Ranson criteria for pancreatitis mortality or the APACHE II scoring system in predicting the development of pancreatic necrosis [33]. However, the accuracy of contrast-enhanced CT in predicting necrosis at an early stage of SAP is not satisfactory [34]. The United Kingdom guidelines for the management of acute pancreatitis, the most popular clinical guideline of acute pancreatitis, recommends that contrast-enhanced CT should be performed at day 3 or later after onset of SAP because of its low sensitivity of CT [11].
In our experience, perfusion CT performed within 3 days of onset of symptoms had a sensitivity and specificity of 100% and 95.3% for predicting development of pancreatic necrosis [18]. The area of necrosis was depicted as area of pancreatic blood flow decreased by more than 19.3% of surrounding pancreatic parenchyma. The area of perfusion defect was commonly diagnosed by using pancreatic blood flow. The perfusion defects detected by perfusion CT reflected ischemia which was produced by vasospasms of the intrapancreatic arteries [35, 36].
4. Perfusion CT for Evaluating Systemic Blood Flow
Perfusion CT could be a useful tool to evaluate abnormal systemic circulation in early stage of SAP. Recent study by Whitcomb et al. showed that elevated serum angiopoietin-2 (Ang-2) on admission is predictive of persistent organ failure in patients with sap [37]. Ang-2 is produced by damaged vessels and increases vascular permeability [38]. In our study, elevated serum Ang-2 is related with hyperdynamic state of systemic circulation [22]. In this study, perfusion CT parameter (τ) was calculated using single-compartment model [28, 39]. τ is a measure of the mean transit time of contrast material from upper abdominal aorta to pancreas; thus, this could be considered a surrogate of systemic circulation with a lower value indicating hyperdynamic state of systemic circulation [28]. In the result, significant correlation was found between τ and serum Ang-2 levels (P < 0.05); higher serum Ang-2 levels were associated with lower τ values (hyperdynamic state of systemic circulation).
Hepatic circulation abnormality has been reported in patients with SAP using Perfusion CT [40]. They reported that hepatic arterial perfusion is increased in the early stage of SAP as measured on dual-input maximum slope method.
5. Clinical Utility of Pancreatic Perfusion CT
Early diagnosis of pancreatic necrosis is very important in the treatment of patients with SAP. Current methods to predict early pancreatic necrosis or SAP is not satisfactory [11–13]. Perfusion CT is a promising technique that allows accurate diagnosis of pancreatic necrosis. Early diagnosis allows prompt clinical decision such as transferring patients to ICU or institution of aggressive treatment such as anticoagulation therapy [41], continuous regional arterial infusion of antiprothrombin agent [8, 9], early fluid resuscitation [10], and molecular targeted therapy [42, 43].
6. Conclusion
Perfusion CT is a promising technique for diagnosis of local and systemic complications of SAP at an early stage.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Sekimoto M, Takada T, Kawarada Y, et al. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. Journal of Hepato-Biliary-Pancreatic Surgery. 2006;13(1):10–24. doi: 10.1007/s00534-005-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley EL. A clinically based classification system for acute pancreatitis. Annales de Chirurgie. 1993;47(6):537–541. [PubMed] [Google Scholar]
- 3.Banks PA, Freeman ML, Fass R, et al. Practice guidelines in acute pancreatitis. American Journal of Gastroenterology. 2006;101(10):2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Tenner S, Sica G, Hughes M, et al. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology. 1997;113(3):899–903. doi: 10.1016/s0016-5085(97)70185-9. [DOI] [PubMed] [Google Scholar]
- 5.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53(9):1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi N, Papachristou GI, Schmit GD, et al. CT findings of walled-off pancreatic necrosis (WOPN): differentiation from pseudocyst and prediction of outcome after endoscopic therapy. European Radiology. 2008;18(11):2522–2529. doi: 10.1007/s00330-008-1039-1. [DOI] [PubMed] [Google Scholar]
- 7.Petrov MS, Shanbhag S, Chakraborty M, Phillips ARJ, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Matsuno S, Sunamura M, Kakugawa Y. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. American Journal of Surgery. 1996;171(4):394–398. doi: 10.1016/S0002-9610(97)89617-1. [DOI] [PubMed] [Google Scholar]
- 9.Piaścik M, Rydzewska G, Milewski J, et al. The results of severe acute pancreatitis treatment with continuous regional arterial infusion of protease inhibitor and antibiotic: a randomized controlled study. Pancreas. 2010;39(6):863–867. doi: 10.1097/MPA.0b013e3181d37239. [DOI] [PubMed] [Google Scholar]
- 10.Warndorf MG, Kurtzman JT, Bartel MJ, et al. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clinical Gastroenterology and Hepatology. 2011;9(8):705–709. doi: 10.1016/j.cgh.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson CD. UK guidelines for the management of acute pancreatitis. Gut. 2005;54(3):iii1–iii9. doi: 10.1136/gut.2004.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollen TL, Van Santvoort HC, Besselink MG, et al. The Atlanta Classification of acute pancreatitis revisited. British Journal of Surgery. 2008;95(1):6–21. doi: 10.1002/bjs.6010. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan S, Forsmark CE. The difficulty in predicting outcome in acute pancreatitis. American Journal of Gastroenterology. 2010;105(2):443–445. doi: 10.1038/ajg.2009.623. [DOI] [PubMed] [Google Scholar]
- 14.Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? British Journal of Radiology. 2003;76(904):220–231. doi: 10.1259/bjr/13564625. [DOI] [PubMed] [Google Scholar]
- 15.Tsushima Y, Kusano S. Age-dependent decline in parenchymal perfusion in the normal human pancreas: measurement by dynamic computed tomography. Pancreas. 1998;17(2):148–152. doi: 10.1097/00006676-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Abe H, Murakami T, Kubota M, et al. Quantitative tissue blood flow evaluation of pancreatic tumor: comparison between xenon CT technique and perfusion CT technique based on deconvolution analysis. Radiation Medicine. 2005;23(5):364–370. [PubMed] [Google Scholar]
- 17.Bize PE, Platon A, Becker CD, Poletti PA. Perfusion measurement in acute pancreatitis using dynamic perfusion MDCT. American Journal of Roentgenology. 2006;186(1):114–118. doi: 10.2214/AJR.04.1416. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji Y, Yamamoto H, Yazumi S, et al. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clinical Gastroenterology and Hepatology. 2007;5(12):1484–1492. doi: 10.1016/j.cgh.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Kandel S, Kloeters C, Meyer H, Hein P, Hilbig A, Rogalla P. Whole-organ perfusion of the pancreas using dynamic volume CT in patients with primary pancreas carcinoma: acquisition technique, post-processing and initial results. European Radiology. 2009;19(11):2641–2646. doi: 10.1007/s00330-009-1453-z. [DOI] [PubMed] [Google Scholar]
- 20.d’Assignies G, Couvelard A, Bahrami S, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250(2):407–416. doi: 10.1148/radiol.2501080291. [DOI] [PubMed] [Google Scholar]
- 21.Park MS, Klotz E, Kim MJ, et al. Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo-And radiation therapy. Radiology. 2009;250(1):110–117. doi: 10.1148/radiol.2493080226. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T, Tsuji Y, Kodama Y, Isoda H, Yamamoto H, Chiba T. Relationship between serum angiopoietin-2 level and perfusion CT parameters in severe acute pancreatitis. American Journal of Gastroenterology. 2011;106(10):1859–1861. doi: 10.1038/ajg.2011.175. [DOI] [PubMed] [Google Scholar]
- 23.Tsushima Y, Miyazaki M, Taketomi-Takahashi A, Endo K. Feasibility of measuring human pancreatic perfusion in vivo using imaging techniques. Pancreas. 2011;40(5):747–752. doi: 10.1097/MPA.0b013e318215ac22. [DOI] [PubMed] [Google Scholar]
- 24.Sahani DV, Holalkere NS, Kambadakone A, Matthes K, Mino-Kenudson M, Brugge WR. Role of computed tomography perfusion in the evaluation of pancreatic necrosis and pancreatitis after endoscopic ultrasound-guided ablation of the pancreas in a porcine model. Pancreas. 2009;38(7):775–781. doi: 10.1097/MPA.0b013e3181a66fa6. [DOI] [PubMed] [Google Scholar]
- 25.Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? British Journal of Radiology. 2003;76(1):S36–S42. doi: 10.1259/bjr/18486642. [DOI] [PubMed] [Google Scholar]
- 26.Kishimoto M, Tsuji Y, Katabami N, et al. Measurement of canine pancreatic perfusion using dynamic computed tomography: influence of input-output vessels on deconvolution and maximum slope methods. European Journal of Radiology. 2009;77:175–181. doi: 10.1016/j.ejrad.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Tsuji Y, Koizumi K, Isoda H, et al. The radiological exposure of pancreatic perfusion computed tomography. Pancreas. 2010;39(4):p. 541. doi: 10.1097/MPA.0b013e3181c16037. [DOI] [PubMed] [Google Scholar]
- 28.Sheiman RG, Sitek A. Feasibility of measurement of pancreatic perfusion parameters with single-compartment kinetic model applied to dynamic contrast-enhanced CT images. Radiology. 2008;249(3):878–882. doi: 10.1148/radiol.2492080026. [DOI] [PubMed] [Google Scholar]
- 29.Goh V, Dattani M, Farwell J, et al. Radiation dose from volumetric helical perfusion CT of the thorax, abdomen or pelvis. European Radiology. 2011;21:974–981. doi: 10.1007/s00330-010-1997-y. [DOI] [PubMed] [Google Scholar]
- 30.Kambadakone AR, Sharma A, Catalano OA, Hahn PF, Sahani DV. Protocol modifications for CT perfusion (CTp) examinations of abdomen-pelvic tumors: impact on radiation dose and data processing time. European Radiology. 2011;21(6):1293–1300. doi: 10.1007/s00330-010-2048-4. [DOI] [PubMed] [Google Scholar]
- 31.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. British Journal of Radiology. 2006;79(948):968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 32.Yu L, Li H, Fletcher JG, McCollough CH. Automatic selection of tube potential for radiation dose reduction in CT: a general strategy. Medical Physics. 2010;37(1):234–243. doi: 10.1118/1.3264614. [DOI] [PubMed] [Google Scholar]
- 33.Leung TK, Lee CM, Lin SY, et al. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II scoring system in predicting acute pancreatitis outcome. World Journal of Gastroenterology. 2005;11(38):6049–6052. doi: 10.3748/wjg.v11.i38.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. The Lancet. 2000;355(9219):1955–1960. doi: 10.1016/s0140-6736(00)02327-8. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Mikami Y, Fukuyama S, et al. Pancreatic ischemia associated with vasospasm in the early phase of human acute necrotizing pancreatitis. Pancreas. 2005;30(1):40–49. [PubMed] [Google Scholar]
- 36.Tsuji Y, Hamaguchi K, Watanabe Y, et al. Perfusion CT is superior to angiography in predicting pancreatic necrosis in patients with severe acute pancreatitis. Journal of Gastroenterology. 2010;45:1155–1162. doi: 10.1007/s00535-010-0267-8. [DOI] [PubMed] [Google Scholar]
- 37.Whitcomb DC, Muddana V, Langmead CJ, et al. Angiopoietin-2, a regulator of vascular permeability in inflammation, is associated with persistent organ failure in patients with acute pancreatitis from the United States and Germany. American Journal of Gastroenterology. 2010;105(10):2287–2292. doi: 10.1038/ajg.2010.183. [DOI] [PubMed] [Google Scholar]
- 38.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nature Medicine. 2006;12(2):235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 39.Sitek A, Sheiman RG. Small-bowel perfusion measurement: feasibility with single-compartment kinetic model applied to dynamic contrast-enhanced CT. Radiology. 2005;237(2):670–674. doi: 10.1148/radiol.2372041403. [DOI] [PubMed] [Google Scholar]
- 40.Koyasu S, Isoda H, Tsuji Y, et al. Hepatic arterial perfusion increases in the early stage of severe acute pancreatitis patients: evaluation by perfusion computed tomography. European Journal of Radiology. 2012;81(1):43–46. doi: 10.1016/j.ejrad.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Hackert T, Werner J, Uhl W, Gebhard MM, Büchler MW, Schmidt J. Reduction of ischemia/reperfusion injury by antithrombin III after experimental pancreas transplantation. American Journal of Surgery. 2005;189(1):92–97. doi: 10.1016/j.amjsurg.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Li W, Niu C, Pan L, Li N, Li J. Thymosin alpha 1 is associated with improved cellular immunity and reduced infection rate in severe acute pancreatitis patients in a double-blind randomized control study. Inflammation. 2011;34:198–202. doi: 10.1007/s10753-010-9224-1. [DOI] [PubMed] [Google Scholar]
- 43.Tidswell M, Tillis W, Larosa SP, et al. Phase 2 trial of eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis. Critical Care Medicine. 2010;38(1):72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]


