Abstract
Objectives. Compare the Plan B levonorgestrel (LNG) area under the concentration- time curve (AUC12) prior to and with efavirenz (EFV). Design. Prospective, open-label, single-arm, equivalence study. Methods. Healthy HIV-negative subjects underwent 12 hr intensive pharmacokinetic (PK) sampling following single dose LNG alone and after 14 days of EFV. Geometric means, Geometric Mean Ratios, and 90% confidence intervals (CI) are reported for PK Parameters. T-tests were utilized. Clinical parameters and liver function tests (LFTs) were assessed. Results. 24 women enrolled and 21 completed the study. With EFV, LNG AUC12 was reduced 56% (95% CI: 49%, 62%) from 42.9 to 17.8 ng∗hr/mL, and maximum concentration (Cmax) was reduced 41% (95% CI: 33%, 50%) from 8.4 to 4.6 ng/mL. LNG was well tolerated with no grade 3 or 4 treatment-related toxicities. Conclusions. EFV significantly reduced LNG exposures. Higher LNG doses may be required with EFV. These results reinforce the importance of effective contraception in women taking EFV.
1. Introduction
The majority of women with human immunodeficiency virus −1 (HIV) are of reproductive age and may use an efavirenz- (EFV-) containing antiretroviral (ARV) regimen [1, 2]. EFV is a nonnucleoside reverse transcriptase inhibitor (NNRTI) indicated in combination with other antiretroviral agents for the treatment of HIV [3]. EFV is an FDA pregnancy category D drug based on animal studies and human case reports of fetal neural tube defects [3–6]. Thus, preventing pregnancy is critical in HIV-infected women receiving EFV.
Pregnancy rates for HIV-infected women range from 6.0 to 8.2 pregnancies per 100 person-years, and in 2001, 49% of all pregnancies in the United States were unintended [7–9]. Women with HIV not desiring pregnancy are advised to use dual methods of contraception to prevent pregnancy and HIV transmission to their partners. Some women use emergency hormonal contraception to prevent pregnancy after unprotected sex or contraceptive failure (condom breakage).
Plan B is a levonorgestrel- (LNG-) containing emergency contraceptive pill indicated for pregnancy prevention following unprotected intercourse or a known or suspected contraceptive failure [10]. It is taken as soon as possible within 72 hours after unprotected intercourse either as a single dose (LNG 1.5 mg) or as two doses (0.75 mg) taken twelve hours apart. LNG use for emergency hormonal contraception has been shown to reduce pregnancy rates by 85% [11]. The mechanism of action of Plan B is not fully elucidated. It may inhibit ovulation, fertilization, or implantation [10, 11]. The minimum effective LNG plasma concentration is unknown.
Few data are available on the pharmacokinetics (PK) of progesterone-based contraceptives with NNRTIs. A study of depomedroxyprogesterone acetate (DMPA) depot injections in HIV-infected women on antiretroviral therapy revealed no significant change in plasma levels of MPA or EFV, nevirapine, or nelfinavir [12]. However, in a study of EFV and the combination oral contraceptive pill OrthoTriCyclen-Lo and OrthoCyclen (25 mcg ethinyl estradiol plus 0.18–0.25 mg norgestimate), LNG area under the concentration time curve from 0 to 12 hours (AUC12), maximum concentration (Cmax), and minimum concentration (Cmin) were decreased by 80%, 83%, and 86%, respectively [13]. A case of contraceptive failure with ectopic pregnancy in an HIV-infected woman occurred with the etonogestrel contraceptive implant and EFV [14]. No PK interaction studies of Plan B and concomitant efavirenz have been performed [14].
2. Methods
2.1. Subjects
Subjects were HIV-seronegative women ages 18–45 years with normal body mass index and no recent use of hormonal contraceptive agents (oral or vaginal hormonal contraception use within 60 days or injectable hormonal contraception use within 180 days of study entry; subjects with Mirena IUD were excluded) or other medications/therapies known to interact with EFV. Subjects who had not undergone surgical sterilization used 2 nonhormonal types of contraception throughout the study period and for 2 weeks following study completion.
The protocol was approved by the institutional review board at participating sites, and informed consent was obtained from each woman before participation.
2.1.1. Study Design
This was a prospective, open-label, single-arm, two-period, PK equivalence study. The primary objective was to compare Plan B LNG AUC12 prior to and during steady-state EFV. Secondary objectives included (1) characterization of other LNG plasma PK parameters, (2) assessment of the safety and tolerability of coadministration of Plan B and EFV, and (3) evaluation of potential effects of LNG on EFV AUC24 with comparison to previous data in HIV+ women. Study participants received LNG 0.75 mg at time 0 and 12 hours at baseline (visit 1-day 0) and after steady state EFV dosing (visit 2-day 17). Subjects were begun on EFV 600 mg at bedtime on empty stomach 72 hours after visit 1 for a total duration of 14 days. Participants fasted at least 12 hours prior to the PK study visits and ate a standardized breakfast with LNG dosing (600 kcal; 15% protein, 30% fat, and 55% carbohydrates). Serial blood (plasma) sampling for LNG PK analysis was performed after LNG dosing at 0 (predose), 2, 3, 4, 6, 8, 10, and 12 hours at Visit 1 and 2. Blood (plasma) sampling for EFV PK analysis was performed prior to LNG dose, 6 and 12 hours after LNG dose at visit 2 only (corresponding to approximately 10, 16, and 22 hours from EFV dosing). Relevant clinical adverse events were assessed at study and 4 telephone visits (study days 4, 11, 16, and 20–28). Safety and laboratory profiles and pregnancy testing were performed at screening and visits 1, 2 (LFT's only), and 3. EFV adherence was assessed by subject self-report at telephone visits approximately 7 and 17 days after EFV was initiated.
2.2. Bioanalyses
LNG plasma concentrations were determined with a liquid chromographic assay with MS/MS detection linear in the range of 50–25000 pg/mL. Accuracy and precision were within ±11% using a 0.5 mL plasma sample. EFV plasma concentrations were determined using a validated HPLV/UC method linear in the range of 20–20,000 ng/mL. Accuracy and precision were within ±15% with 0.2 mL plasma. Samples were frozen and shipped to PPD, Inc. for LNG analysis and University of Colorado Pharmacology lab for EFV analysis.
2.3. Data Analyses
Sample size calculations assumed that expected LNG AUC12 was 123.1 ng∗hr/mL with a standard deviation of 50.1 [15]. Assuming equal variances and a modest correlation of 0.5, the standard deviation of the difference is also 50.1. 18 subjects were required to detect a difference of 49.2 (a 40% change) in LNG AUC12 using a two-sided, paired t-test with a significance level of 0.05 and 97.5% power. To account for drop-outs, we enrolled 24 participants.
LNG PK was determined by noncompartmental methods (WinNonLin V5.2.1, Pharsight Corporation, Mountain View, CA). LNG AUC12 was calculated with the linear-log trapezoidal rule and LNG Cmin, Cmax, and time to Cmax (Tmax) determined visually. LNG half-lives (t1/2) were calculated as 0.693 divided by λz, where λz was the terminal elimination rate constant. LNG total apparent oral clearance (CL/F) was determined as dose divided by AUC12. Apparent volume of distribution (V/F) was determined by CL/F divided by λz.
A post hoc Bayesian approach (NONMEM vVI) was used to estimate each subject's EFV AUC24 based on the three measured EFV levels. The estimated AUC24 was compared to data from a previous PK study of HIV+ women using a 2-sample t-test [16].
For the primary hypothesis, equivalence was defined as a decrease of less than 40% LNG AUC12 after addition of EFV based on previous studies utilizing a 40% difference in contraceptive steroid hormone AUC as that which is clinically relevant [12]. Percent change was calculated from the raw (untransformed) data. The null hypothesis of equivalence was rejected if the corresponding 95% confidence interval included values ≤40%.
PK data were log transformed. Point estimates and 90% confidence intervals for geometric means of LNG AUC12, Cmax, Cmin, V/F and CL/F, and t1/2 were determined for LNG dosed alone and with EFV. Geometric mean ratios (GMR) for LNG AUC12, Cmax, and Cmin with versus without EFV were calculated. Relevant clinical adverse events and liver function test elevations were summarized. Paired t-tests were used.
3. Results
3.1. Demographics
Twenty-four women enrolled, and 23 subjects commenced study visits and treatments. Three subjects discontinued; 2 for adverse events and 1 for personal reasons. Evaluable PK data was generated for 21 women who had a mean age of 31 years (range 21–45) and BMI of 27 (range 21–35). The majority of subjects were white (62%), and 24% were Latina and 10% Black. Contraception use included condoms, spermicide, diaphragm, abstinence, and intrauterine device.
3.2. LNG and EFV Pharmacokinetics
The estimated percent decrease in LNG AUC12 with EFV was 56% (Table 1), and the corresponding 95% confidence interval (49%, 62%) excluded a change of ≤40% (P < 0.0001), such that the equivalence hypothesis was rejected. A decrease in LNG AUC12 of >40% was observed in 90.5% (95% CI: 0.70%, 0.99%) of women. LNG Cmax and Cmin GMR were 0.55 and 0.31, respectively. LNG concentration time curves are shown in Figure 1. The geometric mean EFV AUC24 in combination with LNG was 69597 ng∗hr/mL. (90% CI 27629, 175316 ng∗hr/mL). This value was compared to a previous study of EFV PK in HIV-infected females which demonstrated an EFV geometric mean AUC24 of 61361 ng∗hr/mL (90% CI 19076, 197379 ng∗hr/mL) (P value = 0.35) [15]. Study participants had a >95% adherence with EFV dosing, and all had detectable EFV levels.
Table 1.
Estimated LNG PK parameters.
| PK parameter | Percent change raw scale (95% CI) | LNG GM (90% CI) | LNG + EFV GM (90% CI) | P value | GMR (90% CI) |
|---|---|---|---|---|---|
| AUC12 (ng∗hr/mL) | −56% (−49%, −62%) | 42.9 (38.0, 48.5) | 17.8 (15.5, 20.5) | <0.0001 | 0.42 (0.36, 0.48) |
| Cmax (ng/mL) | −41% (−33%, −50%) | 8.4 (7.6, 9.3) | 4.6 (4.0, 5.4) | <0.0001 | 0.55 (0.49, 0.63) |
| Cmin (ng/mL) | −67% (−59%, −74%) | 2.04 (1.7, 2.3) | 0.6 (0.5, 0.7) | <0.0001 | 0.31 (0.26, 0.36) |
| V/F (L) | 110% (−155%, 176%) | 144 (120, 173) | 256 (217, 301) | 0.0001 | — |
| CL/F (L/hr) | 260% (159%, 364%) | 9.7 (8.0, 11.6) | 32.1 (27.6, 37.3) | <0.0001 | — |
| t1/2 (hr) | −34% (−17%, −55%) | 10.3 (8.1, 13.2) | 5.5 (4.6, 6.7) | 0.0001 | — |
Figure 1.
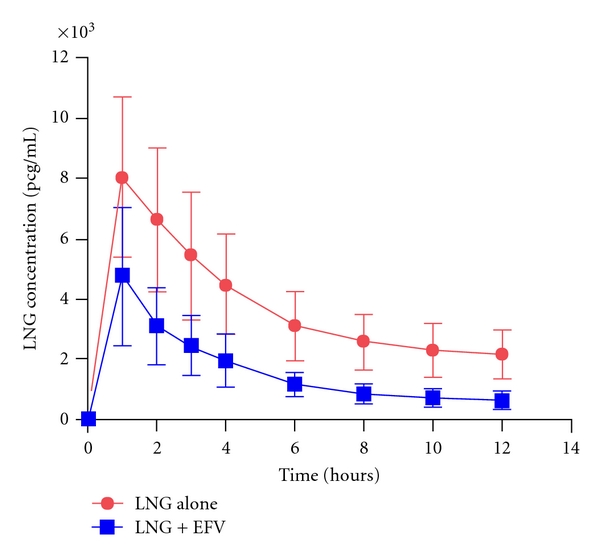
Mean plasma concentration versus time profile for LNG. Mean (±SD) levonorgestrel concentration-time profile in 21 healthy volunteers administered alone (red) and after 14 days of pretreatment with efavirenz (blue).
3.3. Safety and Tolerability
Headache, abdominal pain, diarrhea, and menstrual cycle changes were the most common adverse events occurring in >10% of subjects after Plan B dosing alone. The incidence of abdominal pain, diarrhea, and menstrual cycle changes was decreased, while incidence of fatigue was increased with EFV. The occurrence of rash, pruritus, abnormal dreams, and insomnia was similar to previous studies of EFV [3]. Changes in LFT's were rare and resolved with discontinuation of EFV. Adverse events were mild (Grade 1) to moderate (Grade 2) in majority and were resolved at follow-up visits. Two subjects discontinued study secondary to adverse events. One subject had a grade 2 rash likely related to study treatment (EFV) and resolved at follow-up visits. One subject had grade 3 syncope not related to study treatment and attributed to a vasovagal reaction with phlebotomy.
4. Discussion
Data are limited regarding the use of hormonal contraception in HIV-infected women on antiretroviral therapy. Interactions have been described between steroid hormones and both protease inhibitors and NNRTI which could lead to decreased protection from pregnancy or increased contraceptive side effects [17]. Previous studies have focused predominantly on combined oral contraceptive pills, injectable DMPA, and one small study evaluated PK with the transdermal contraceptive patch [12, 13, 18]. Women taking EFV are specifically advised to avoid pregnancy due to this agents' potential role in fetal neural tube defects [3, 19]. Thus, emergency hormonal contraception, like Plan B, may be even more important for these women.
We sought to evaluate the effect of EFV on plasma concentration of LNG in Plan B in healthy HIV-negative women. We found that pretreatment with EFV for 14 days was associated with a 56%, 41%, and 67% decrease in LNG AUC12, Cmax, and Cmin, respectively.
The mechanism for this interaction is likely EFV induction of LNG metabolism. EFV is an inducer of CYP3A and uridine-diphosphate glucuronosyl transferases (UGTs) in vivo [3]. LNG exposures are reduced approximately 40% with the anticonvulsants phenytoin and carbamazepine (inducers of CYP3A) and 19% with lamotrigine (an inducer of glucuronidation enzymes) [20, 21]. A study of rifampin and oral contraception demonstrated considerable reduction in contraceptive hormone levels; however, ovulation suppression persisted [22].
These findings may have important ramifications with regard to the efficacy of Plan B when taken with this ARV. However, the clinical relevance of this finding is unclear as the minimal effective LNG plasma concentration is unknown. We did not monitor for ovulation which may signal failed contraception. It is possible the alternate Plan B single dosing of LNG 1.5 mg would mitigate this effect; however, this is unlikely given the magnitude of our observed difference. Further clinical studies of Plan B and EFV are thus needed to inform providers of potential need for Plan B dosing adjustments for these women. HIV providers' role in providing family planning services including contraception and preconception counseling is significant given the inherent complexities with HIV and antiretroviral therapy.
Conflict of Interests
Drs. M. L. Carten, J. J. Kiser, A. Kwara, and S. Cu-Uvin have no conflict of interests to report.
Acknowledgments
This study was supported by an investigator-initiated study grant from Bristol-Meyers Squibb Co, the Colorado Translational Research Center (1UL1 RR025780), the Colorado Center for AIDS Research P30AI054907 (J. Kiser), and Lifespan/Tufts/Brown University Center for AIDS Research P30AI4285 (S. Cu-Uvin), K24A1066884 (S. Cu-Uvin). The study team would like to thank Michelle Ray with the Colorado Antiretroviral Pharmacology Laboratory for quantifying the EFV levels, the Miriam Hospital HIV Research Unit, and all of the study subjects. This data was presented as abstract at the 17th Conference on Retroviruses and Opportunistic Infections in San Francisco, CA, February 27-March 2, 2010 (ref).
References
- 1.HIV/AIDS Surveillance Report. Cases of HIV and AIDS in the United States and Dependent Areas. 2007. [Google Scholar]
- 2.UNAIDS. AIDS Epidemic Update: December 2005.
- 3.Sustiva (Efavirenz) Capsule and Tablets.US Prescribing Information. Princeton, NJ, USA: Bristol-Myers-Squibb; 2010. [Google Scholar]
- 4.Saitoh A, Hull AD, Franklin P, Spector SA. Myelomeningocele in an infant with intrauterine exposure to efavirenz. Journal of Perinatology. 2005;25(8):555–556. doi: 10.1038/sj.jp.7211343. [DOI] [PubMed] [Google Scholar]
- 5.Fundarò C, Genovese O, Rendeli C, Tamburrini E, Salvaggio E. Myelomeningocele in a child with intrauterine exposure to efavirenz. AIDS. 2002;16(2):299–300. doi: 10.1097/00002030-200201250-00025. [DOI] [PubMed] [Google Scholar]
- 6.De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G. Periconceptional exposure to efavirenz and neural tube defects. Archives of Internal Medicine. 2002;162(3):p. 355. doi: 10.1001/archinte.162.3.355. [DOI] [PubMed] [Google Scholar]
- 7.Massad LS, Springer G, Jacobson L, et al. Pregnancy rates and predictors of conception, miscarriage and abortion in US women with HIV. AIDS. 2004;18(2):281–286. doi: 10.1097/00002030-200401230-00018. [DOI] [PubMed] [Google Scholar]
- 8.Van Benthem BH, De Vincenzi I, Delmas MC, et al. Pregnancy bfore and after HIV diagnosis in a European cohort of HIV-infected women. European study on the natural history of HIV infection in women. AIDS. 2000;14(14):2171–2178. doi: 10.1097/00002030-200009290-00014. [DOI] [PubMed] [Google Scholar]
- 9.Finner LB, Henshw SK. Disparities in rates of unintended pregnancy in the United States, 1994–2001. Perspect Sex Reprod Health. 2006;38(2):9–96. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 10.Plan B (LNG0.75) Tablets. US Prescribing Information. Pomona, NY, US: Barr Pharmaceuticals Inc.; 2006. [Google Scholar]
- 11.Task Force on Postovulatory Methods of Fertility Regulation. Randomized controlled trial of levonorgestrel versus the yuzpe regimen of combined oral contraceptives for emergency contraception. The Lancet. 1998;352(9126):428–433. [PubMed] [Google Scholar]
- 12.Cohn SE, Park JG, Watts DH, et al. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clinical Pharmacology and Therapeutics. 2007;81(2):222–227. doi: 10.1038/sj.clpt.6100040. [DOI] [PubMed] [Google Scholar]
- 13.Sevinsky H, Eley T, He A, Persson D, et al. Effect of efavirenz on the pharmacokinetics of ethinyl estradiol and norgestimate in healthy female subjects. In: Proceedings of the 48th Annual ICAAC/IDSA 46th Annual Meeting; October 2008; Washington, DC, USA; [Google Scholar]
- 14.Matiluko A, Soundararjan L, Hodston P, et al. Early contraceptive failure of implanon in an HIV-seropositive patient on triple antiretroviral therapy with zidovudine, lamivudine and efavirenz. Journal of Family Planning and Reproductive Health Care. 2007;33(4):277–278. doi: 10.1783/147118907782101724. [DOI] [PubMed] [Google Scholar]
- 15.Kook K, Gabelnick H, Duncan G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception. 2002;66(1):73–76. doi: 10.1016/s0010-7824(02)00321-9. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi M, Benet L, Bacchetti P, et al. Nonnucleoside reverse transcriptase inhibitor pharmacokinetics in a large unselected cohort of HIV-infected women. Journal of Acquired Immune Deficiency Syndromes. 2009;50(5):482–491. doi: 10.1097/qai.0b013e31819c3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Ibiary S, Cocohoba J. Effects of HIV antiretrovirals on the pharmacokinetics of hormonal contraceptives. European Journal of Contraception and Reproductive Health Care. 2008;13(2):123–132. doi: 10.1080/13625180701829952. [DOI] [PubMed] [Google Scholar]
- 18.Vogler M, Patterson K, Kamemoto L, et al. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of ACTG trial A5188. Journal of Acquired Immune Deficiency Syndromes. 2010;55(4):473–482. doi: 10.1097/QAI.0b013e3181eb5ff5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. US Department of Health and Human Services. 2010, http://www.aidsinfo.nih.gov/guidelines/adult/AA 100606.pdf.
- 20.Crawford P, Chadwick DJ, Martin C, Tjia J, Back DJ, Orme M. The interaction of phenytoin and carbamazepine with combined oral contraceptive steroids. British Journal of Clinical Pharmacology. 1990;30(6):892–896. doi: 10.1111/j.1365-2125.1990.tb05457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sidhu J, Job S, Singh S, Philipson R. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. British Journal of Clinical Pharmacology. 2006;61(2):191–199. doi: 10.1111/j.1365-2125.2005.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barditch-Crovo P, Trapnell CB, Ette E, et al. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clinical Pharmacology and Therapeutics. 1999;65(4):428–438. doi: 10.1016/S0009-9236(99)70138-4. [DOI] [PubMed] [Google Scholar]


