Abstract
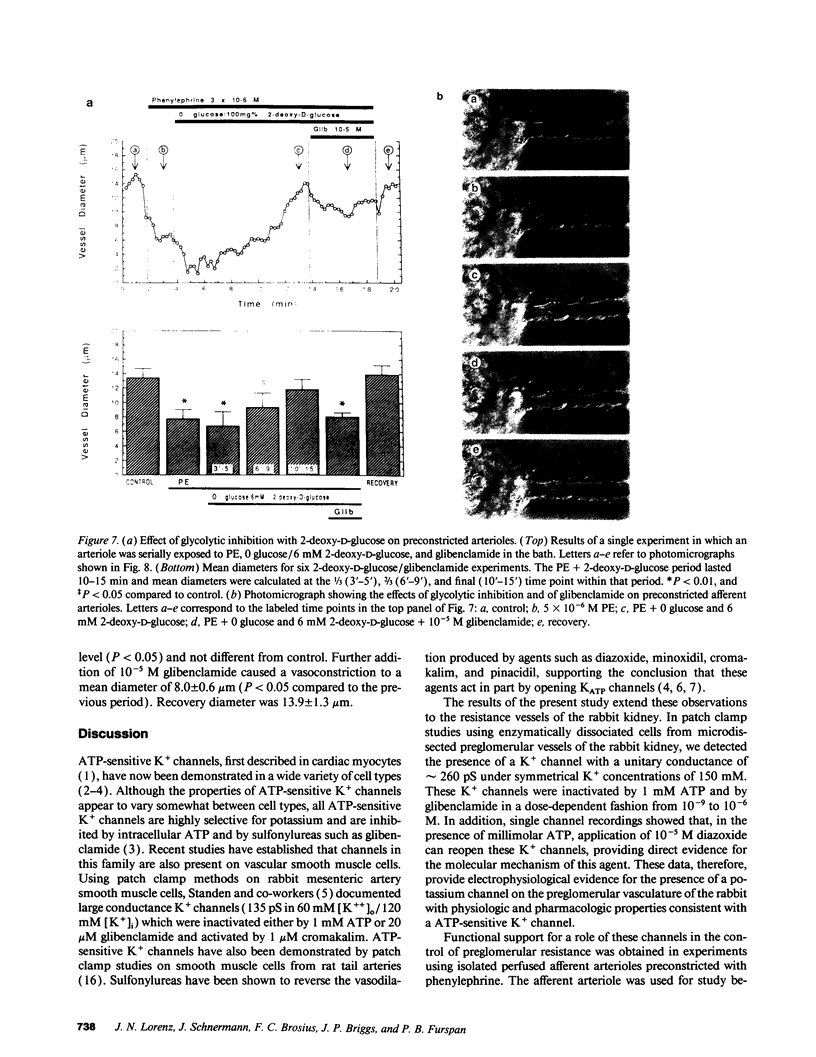
Studies were performed to assess whether ATP-sensitive K+ (KATP) channels on rabbit preglomerular vessels can influence afferent arteriolar (AA) tone. K+ channels with a slope conductance of 258 +/- 13 (n = 7) pS and pronounced voltage dependence were demonstrated in excised patches from vascular smooth muscle cells of microdissected preglomerular segments. Channel activity was markedly reduced by 1 mM ATP and in a dose-dependent fashion by glibenclamide (10(-9) M to 10(-6) M), a specific antagonist of KATP channels. 10(-5) M diazoxide, a K+ channel opener, activated these channels in the presence of ATP, and this effect was also blocked by glibenclamide. To determine the role of these KATP channels in the control of vascular tone, diazoxide was tested on isolated perfused AA. After preconstriction from a control diameter of 13.1 +/- 1.1 to 3.5 +/- 2.1 microns with phenylephrine (PE), addition of 10(-5) M diazoxide dilated vessels to 11.2 +/- 0.7 microns, which was not different from control. Further addition of 10(-5) M glibenclamide reconstricted the vessels to 5.8 +/- 1.5 microns (n = 5; P less than 0.03). In support of its specificity for KATP channels, glibenclamide did not reverse verapamil induced dilation in a separate series of experiments. To determine whether intracellular ATP levels can effect AA tone, studies were conducted to test the effect of the glycolytic inhibitor 2-deoxy-D-glucose. After preconstriction from 13.4 +/- 3.2 to 7.7 +/- 1.3 microns with PE, bath glucose was replaced with 6 mM 2-deoxy-D-glucose. Within 10 min, the arteriole dilated to a mean value of 11.8 +/- 1.4 microns (n = 6; NS compared to control). Subsequent addition of 10(-5) M glibenclamide significantly reconstricted the vessels to a diameter of 8.6 +/- 0.5 micron (P less than 0.04). These data demonstrate that KATP channels are present on the preglomerular vasculature and that changes in intracellular ATP can directly influence afferent arteriolar tone via these channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Barron J. T., Bárány K., Bárány M., Kopp S. J. Effects of ATP reduction on the pattern of force development and myosin light chain phosphorylation in intact arterial smooth muscle. Biochim Biophys Acta. 1989 Feb 9;1010(2):278–282. doi: 10.1016/0167-4889(89)90174-2. [DOI] [PubMed] [Google Scholar]
- Barron J. T., Kopp S. J., Tow J. P., Messer J. V. Effects of altering carbohydrate metabolism on energy status and contractile function of vascular smooth muscle. Biochim Biophys Acta. 1989 Aug 17;976(1):42–52. doi: 10.1016/s0005-2728(89)80187-2. [DOI] [PubMed] [Google Scholar]
- Brayden J. E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990 Sep;259(3 Pt 2):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Cremer-Lacuara M. G., Lacuara J. L., Fiol de Cuneo M., Ruiz R. D. Substrate supply and function of isolated venous smooth muscle under anoxia and metabolic inhibition. Can J Physiol Pharmacol. 1980 Jun;58(6):723–730. doi: 10.1139/y80-115. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Davies N. W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature. 1990 Jan 25;343(6256):375–377. doi: 10.1038/343375a0. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of ADP upon the ATP-sensitive K+ channel in rat ventricular myocytes. J Membr Biol. 1988;101(1):83–92. doi: 10.1007/BF01872823. [DOI] [PubMed] [Google Scholar]
- Furspan P. B., Webb R. C. Potassium channels and vascular reactivity in genetically hypertensive rats. Hypertension. 1990 Jun;15(6 Pt 2):687–691. doi: 10.1161/01.hyp.15.6.687. [DOI] [PubMed] [Google Scholar]
- Gaposchkin C. G., Tornheim K., Sussman I., Ruderman N. B., McCall A. L. Glucose is required to maintain ATP/ADP ratio of isolated bovine cerebral microvessels. Am J Physiol. 1990 Mar;258(3 Pt 1):E543–E547. doi: 10.1152/ajpendo.1990.258.3.E543. [DOI] [PubMed] [Google Scholar]
- Greger R., Hampel W. A modified system for in vitro perfusion of isolated renal tubules. Pflugers Arch. 1981 Jan;389(2):175–176. doi: 10.1007/BF00582110. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Codina J., Birnbaumer L., Brown A. M. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Nelson M. T., Huang Y., Standen N. B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991 Mar;260(3 Pt 2):H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. M., Paul R. J. Compartmentation of glycolytic and glycogenolytic metabolism in vascular smooth muscle. Science. 1983 Dec 23;222(4630):1344–1346. doi: 10.1126/science.6658455. [DOI] [PubMed] [Google Scholar]
- Lynch R. M., Paul R. J. Glucose uptake in porcine carotid artery: relation to alterations in active Na+-K+ transport. Am J Physiol. 1984 Nov;247(5 Pt 1):C433–C440. doi: 10.1152/ajpcell.1984.247.5.C433. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990 Apr 19;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Ripoll C., Lederer W. J. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. ATP regulation of the slow calcium channels in vascular smooth muscle cells of guinea pig mesenteric artery. Circ Res. 1989 Jan;64(1):145–154. doi: 10.1161/01.res.64.1.145. [DOI] [PubMed] [Google Scholar]
- Paul R. J. Smooth muscle energetics. Annu Rev Physiol. 1989;51:331–349. doi: 10.1146/annurev.ph.51.030189.001555. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. In vitro and in vivo comparison of two K+ channel openers, diazoxide and cromakalim, and their inhibition by glibenclamide. J Pharmacol Exp Ther. 1989 Jul;250(1):261–271. [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Weihprecht H., Lorenz J. N., Briggs J. P., Schnermann J. Vasoconstrictor effect of angiotensin and vasopressin in isolated rabbit afferent arterioles. Am J Physiol. 1991 Aug;261(2 Pt 2):F273–F282. doi: 10.1152/ajprenal.1991.261.2.F273. [DOI] [PubMed] [Google Scholar]
- Weir C. J., Gibson I. F., Martin W. Effects of metabolic inhibitors on endothelium-dependent and endothelium-independent vasodilatation of rat and rabbit aorta. Br J Pharmacol. 1991 Jan;102(1):162–166. doi: 10.1111/j.1476-5381.1991.tb12147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. N., Lamp S. T. Glycolysis preferentially inhibits ATP-sensitive K+ channels in isolated guinea pig cardiac myocytes. Science. 1987 Oct 2;238(4823):67–69. doi: 10.1126/science.2443972. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Heaney L. A., Wallace A. A., Baskin E. P., Stein R. B., Garcia M. L., Kaczorowski G. J. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jan;248(1):149–156. [PubMed] [Google Scholar]
- de Weille J. R., Fosset M., Mourre C., Schmid-Antomarchi H., Bernardi H., Lazdunski M. Pharmacology and regulation of ATP-sensitive K+ channels. Pflugers Arch. 1989;414 (Suppl 1):S80–S87. doi: 10.1007/BF00582253. [DOI] [PubMed] [Google Scholar]